Self-Healing Bio-Concrete Using Bacillus subtilis Encapsulated in Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
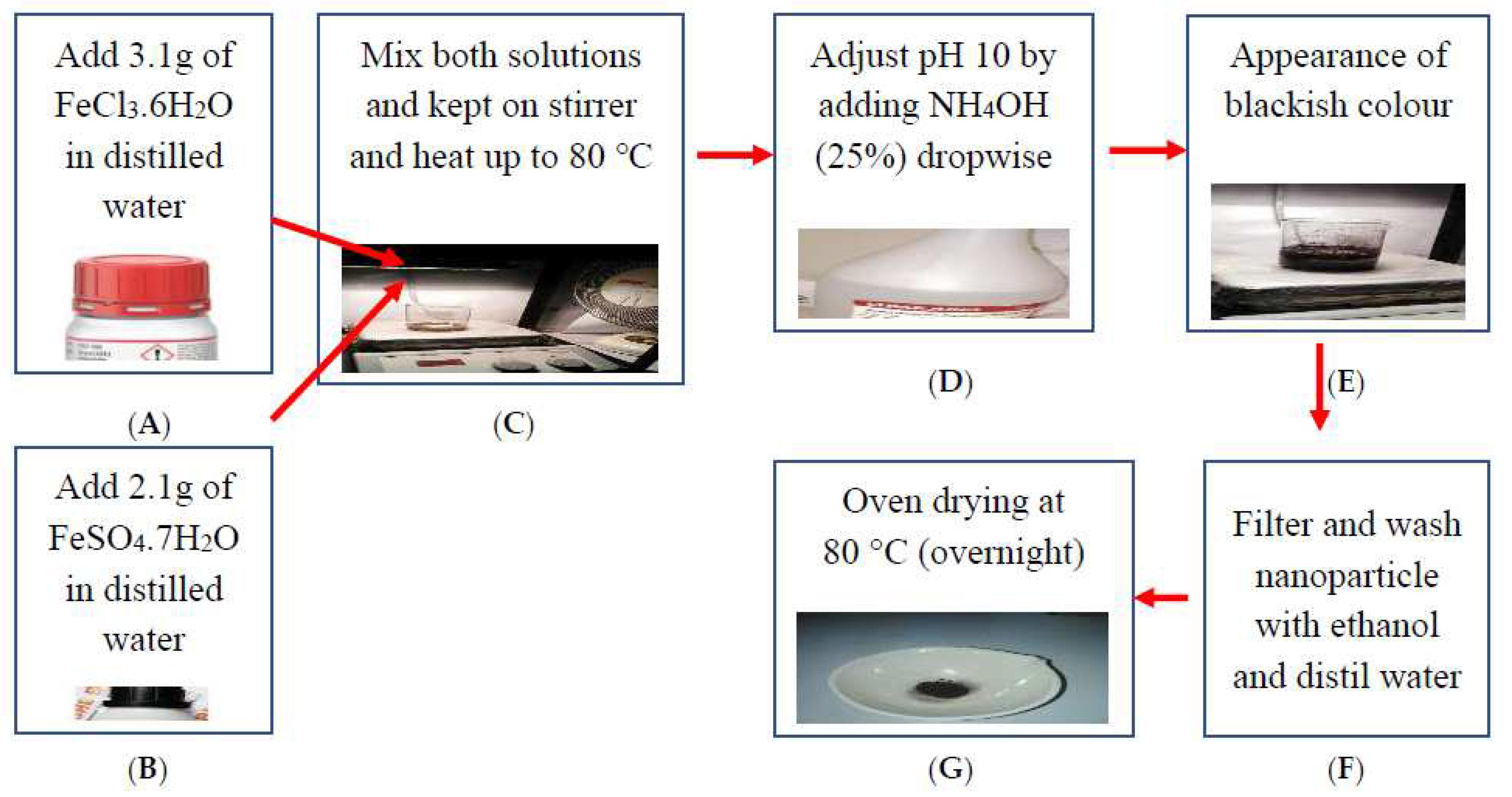
2.2. Synthesis and Characterization of Iron Oxide Nanoparticles (IONPs)
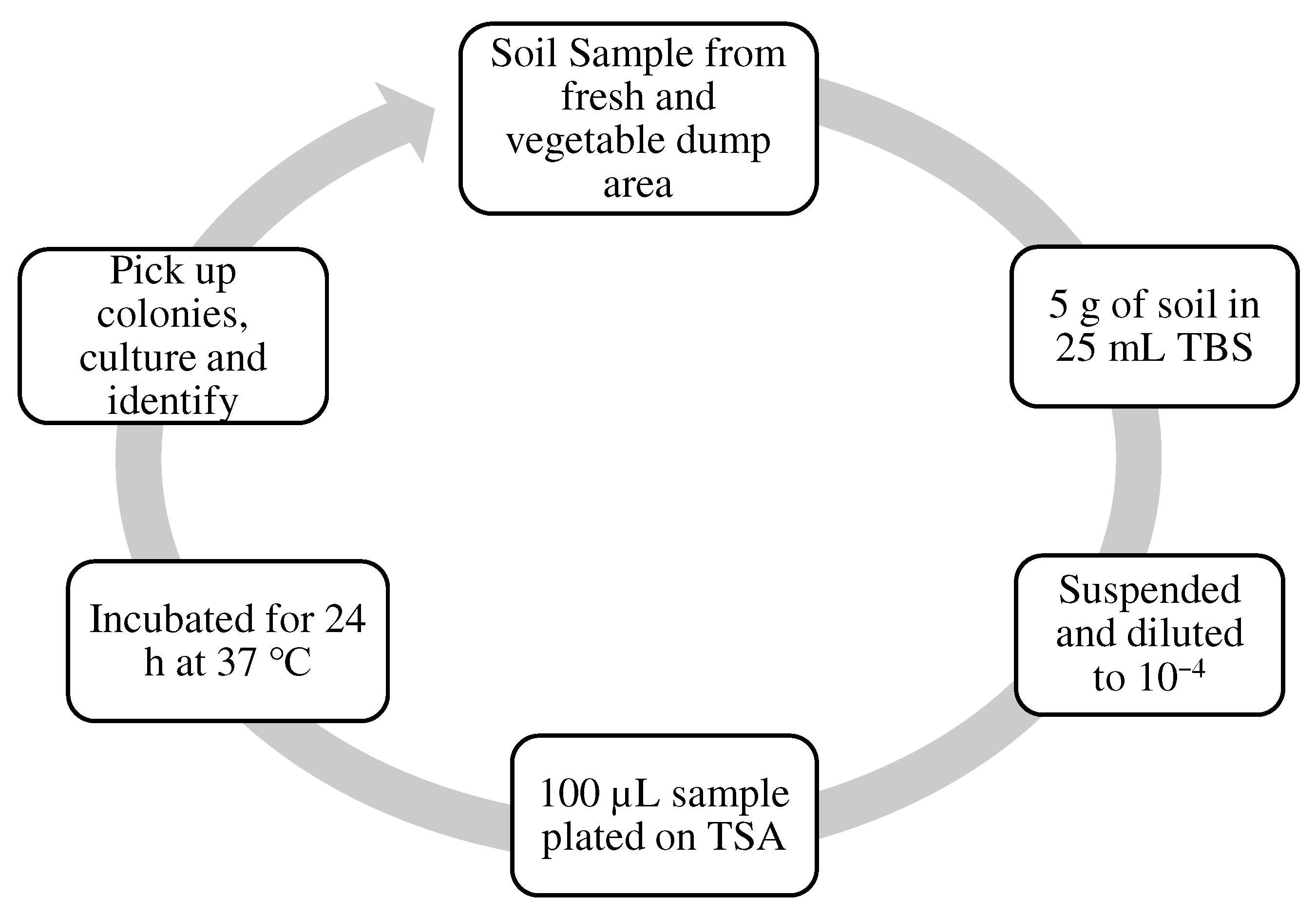
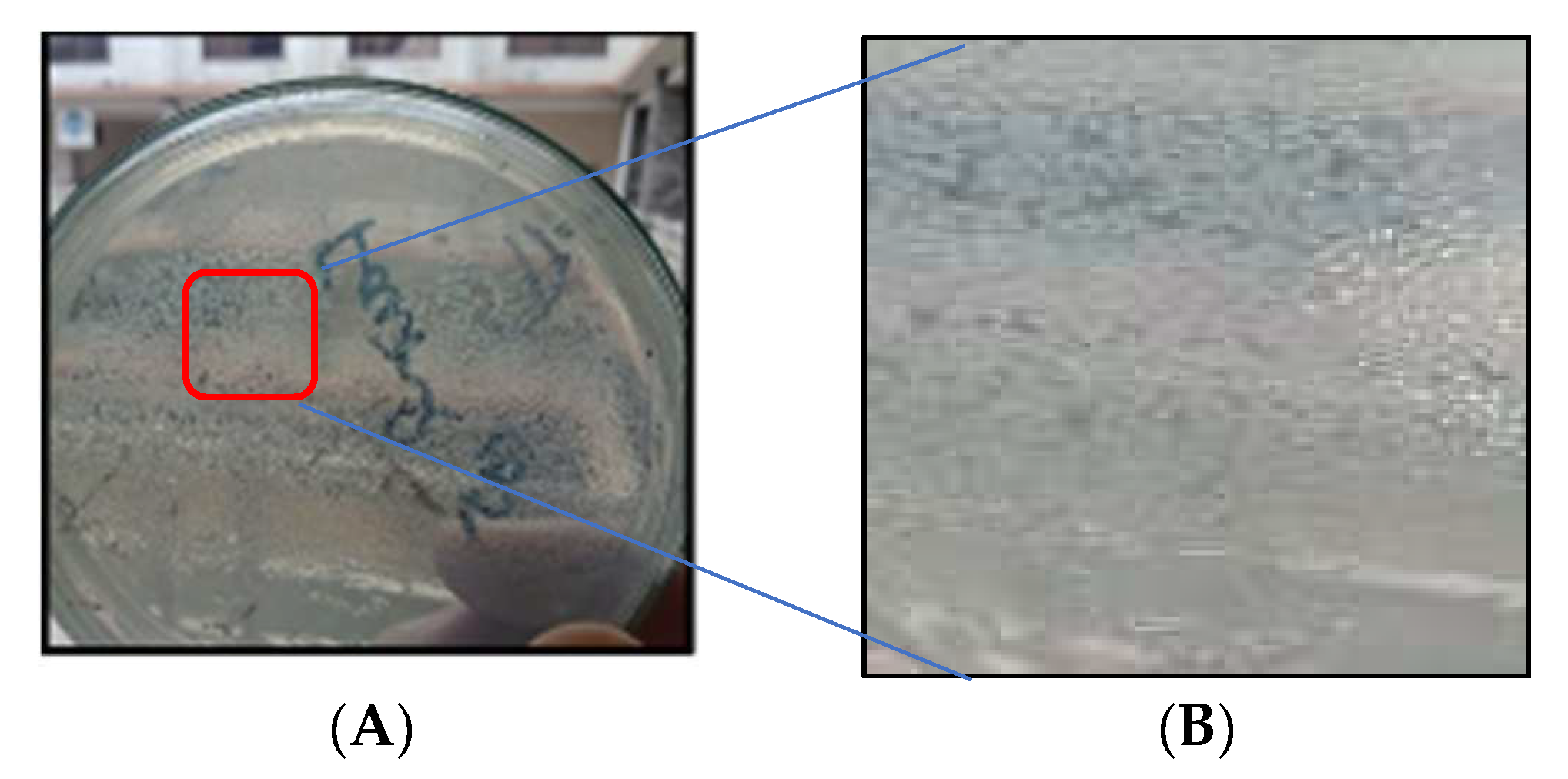
2.3. Microorganisms’ Isolation, Identification, and Growth Medium
2.4. Iron Oxide Nanoparticle Immobilization Procedure
2.5. Casting of the Concrete Specimens
2.6. Experimental Procedure
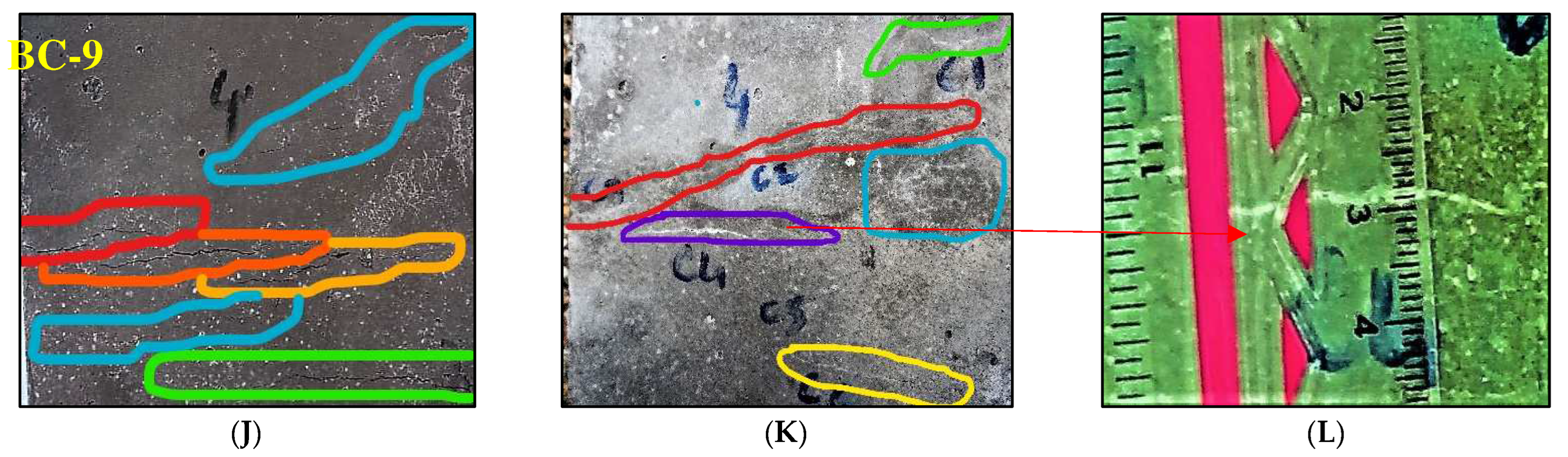
2.7. Preparation of Cracks and Quantification of Crack Healing by Bacterium
2.8. Mix Proportions
3. Results and Discussion
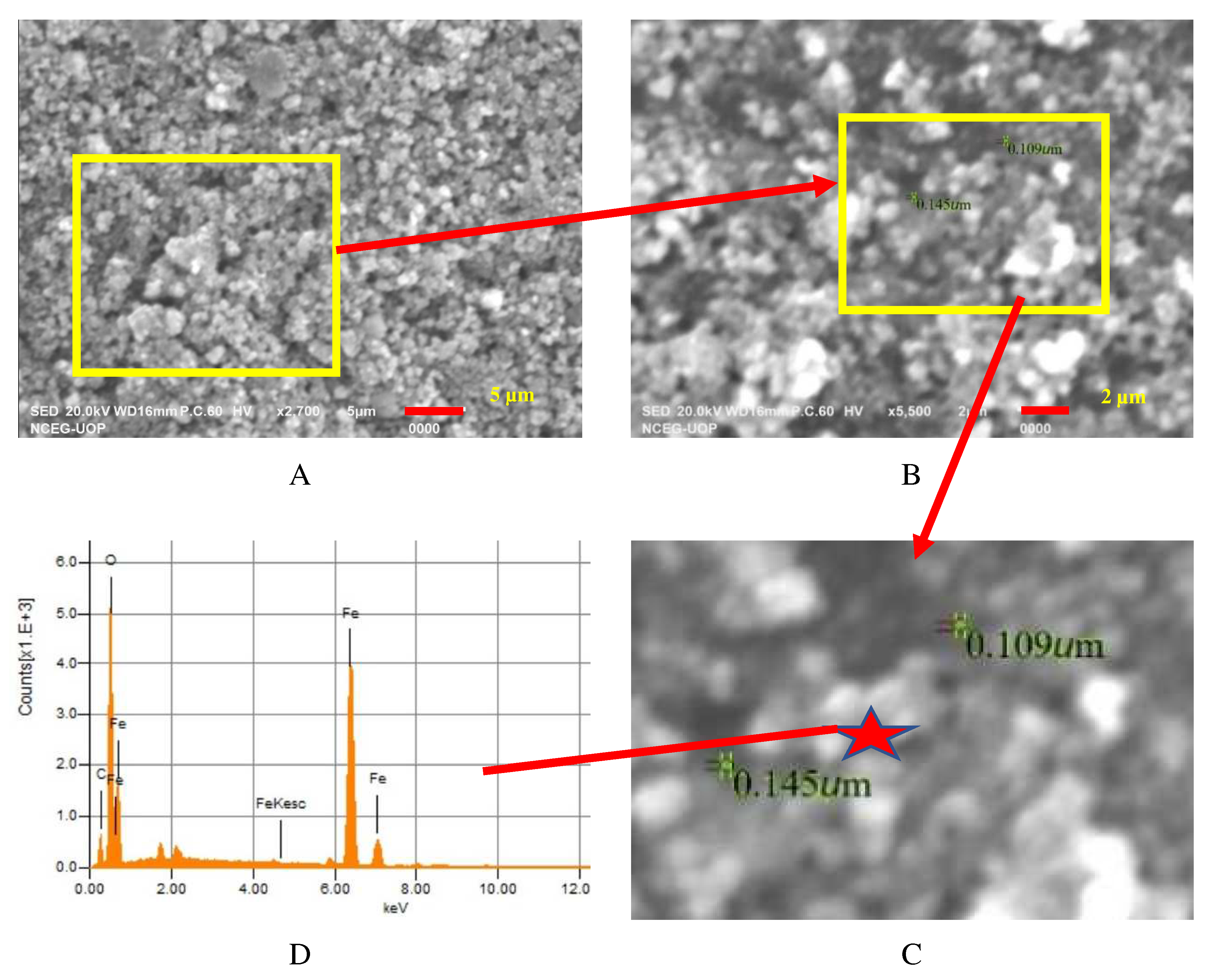
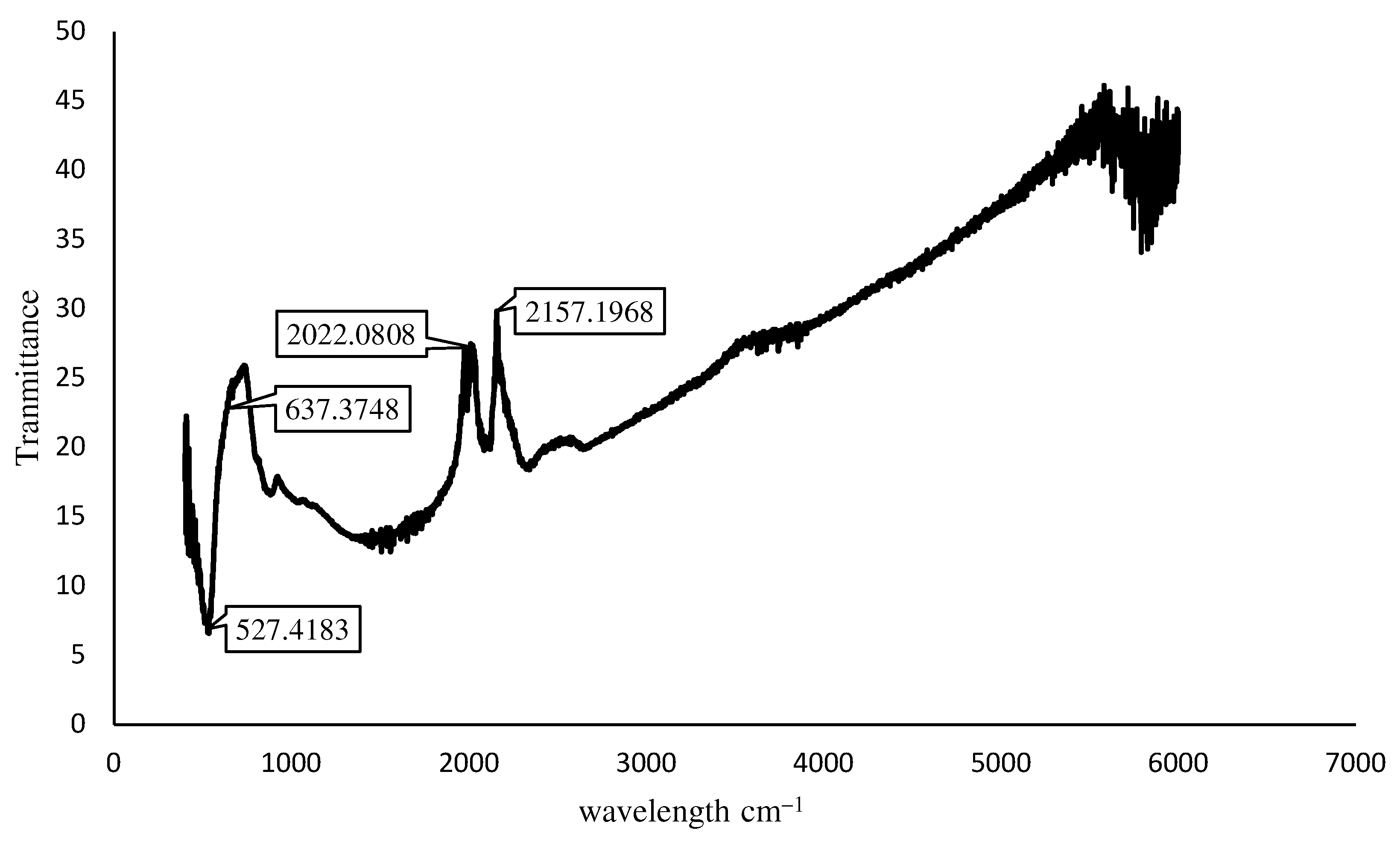
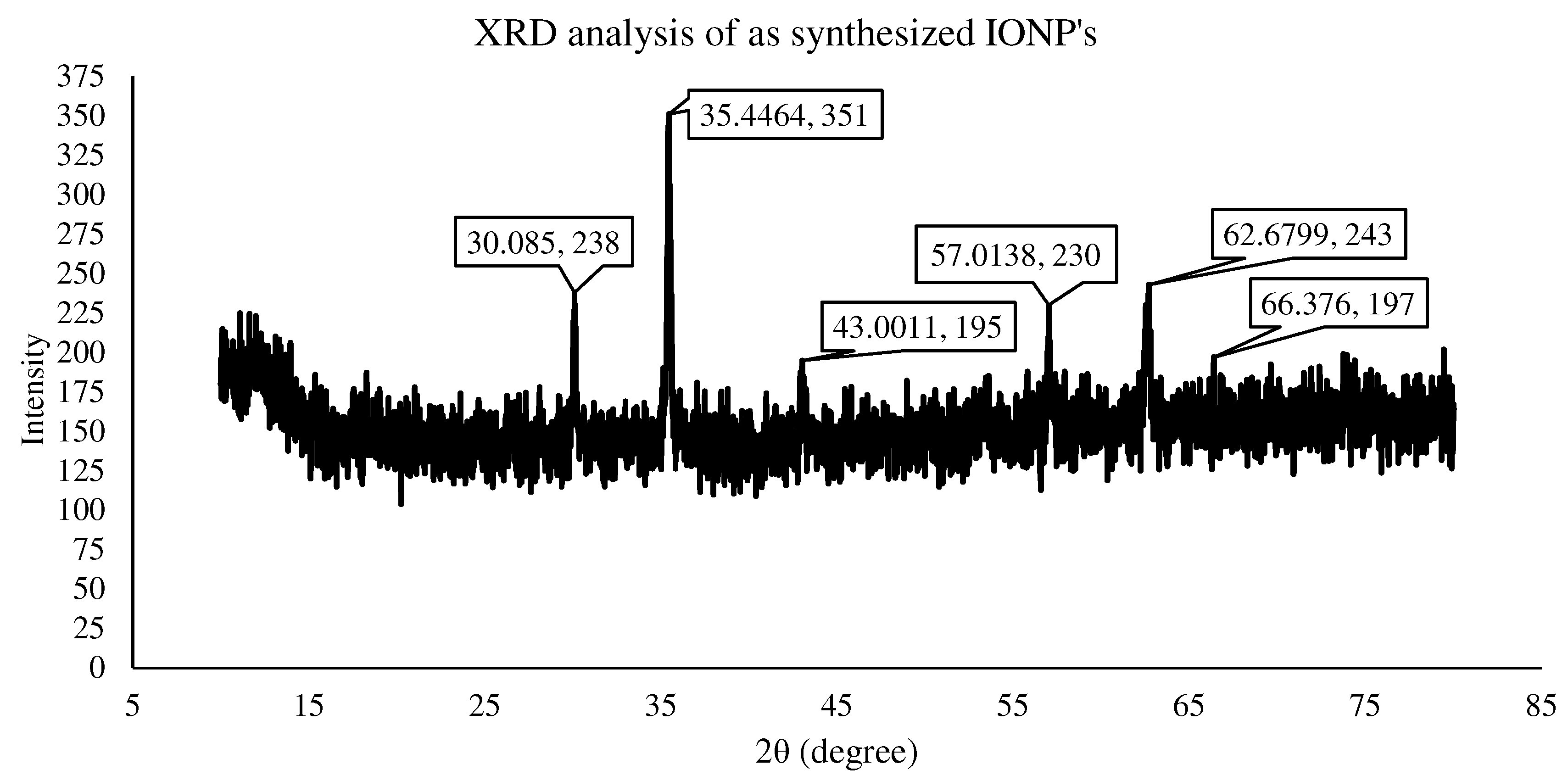
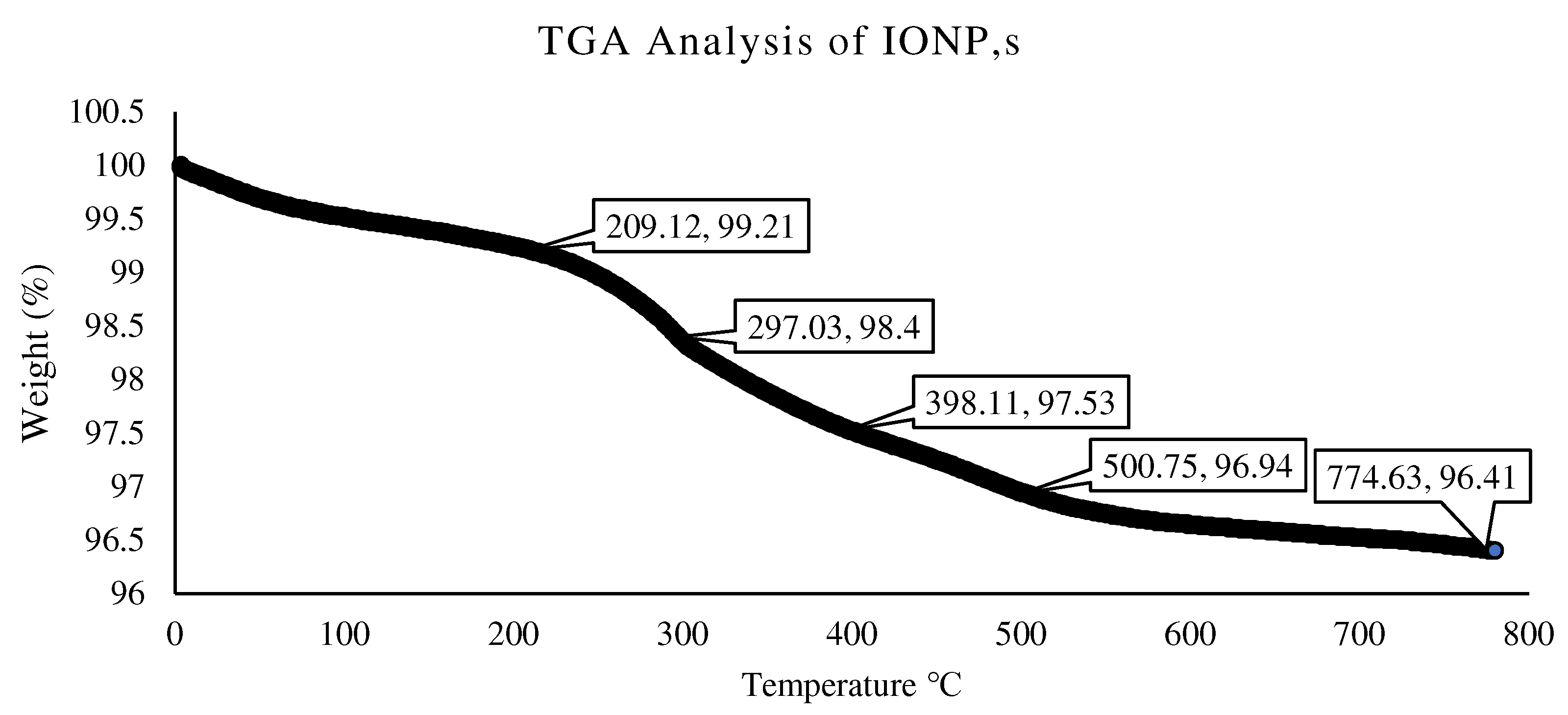
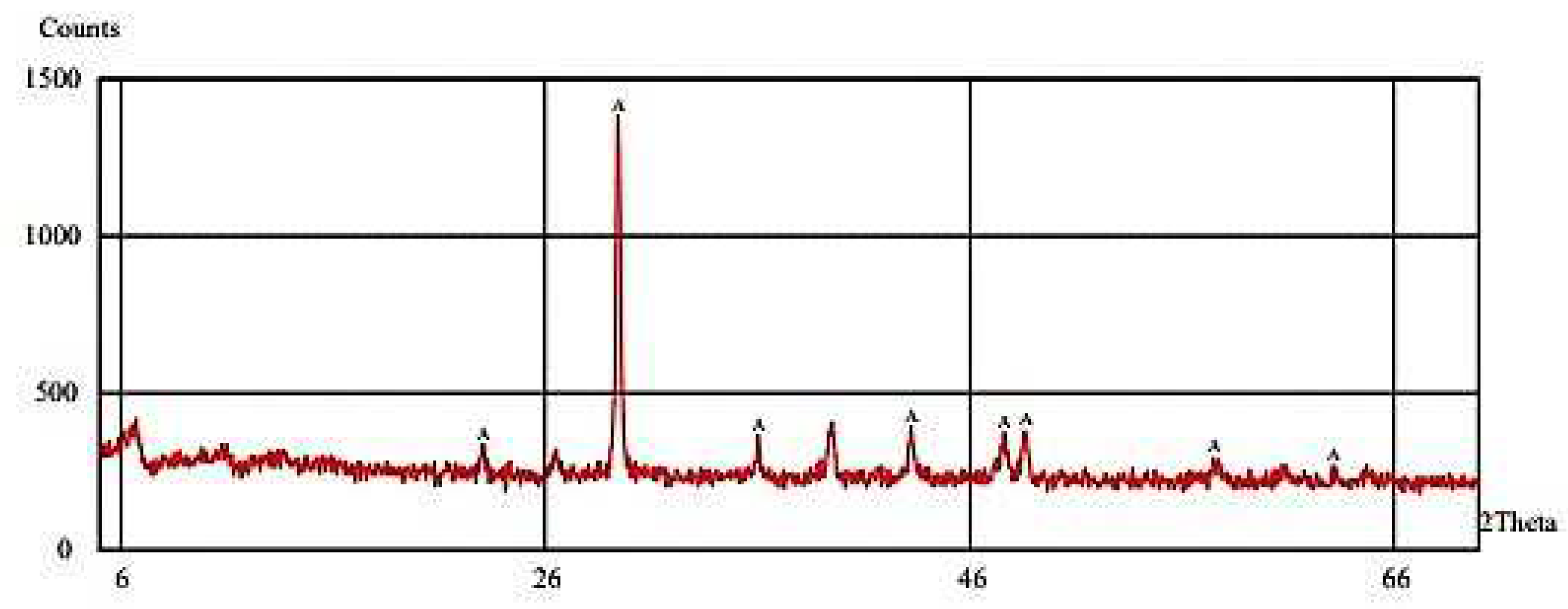
3.1. Characterization of IONPs
3.2. Compressive Strength
3.3. Self-Healing Capability of Bacteria Bacillus Subtilis Immobilized with IONPs
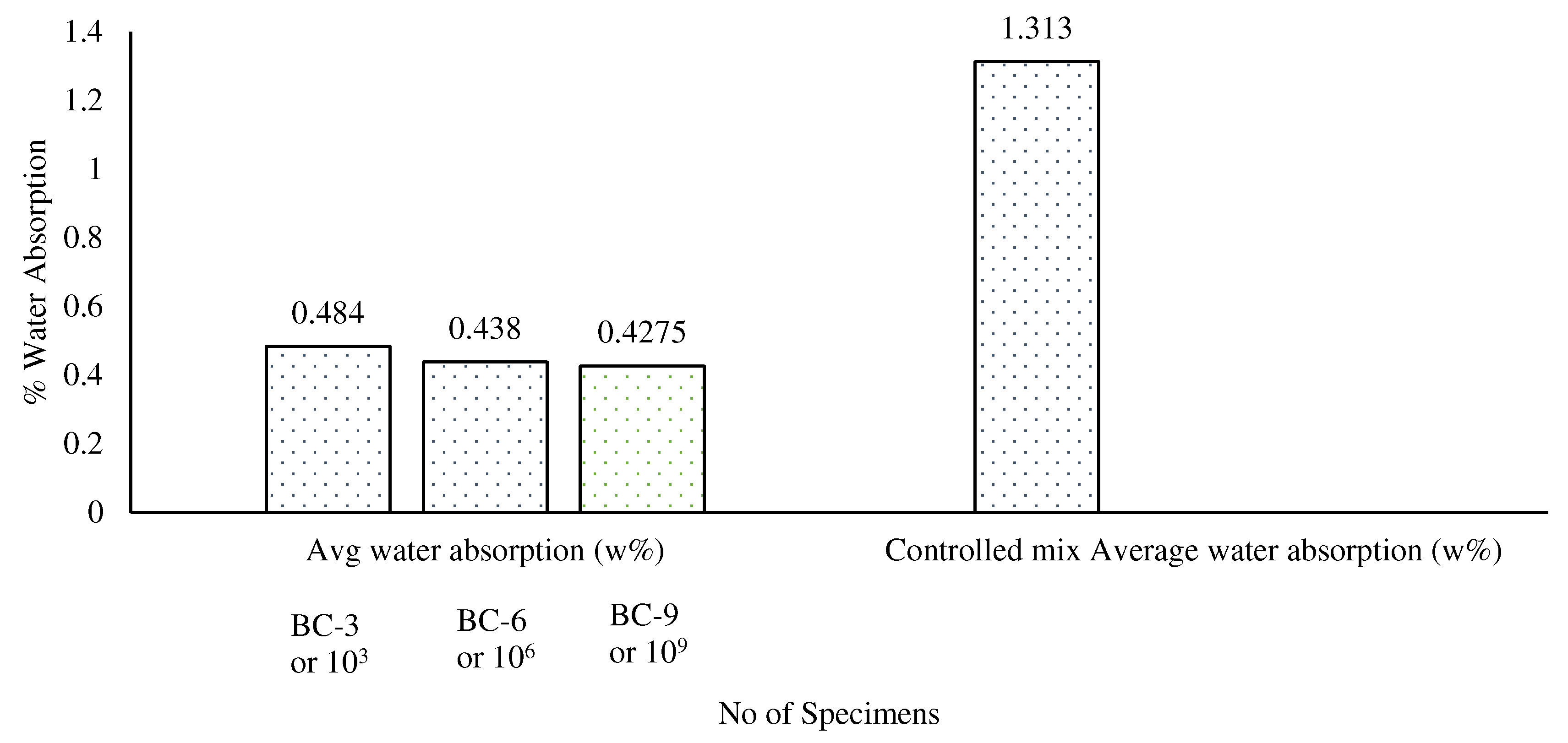
3.4. Water Absorption
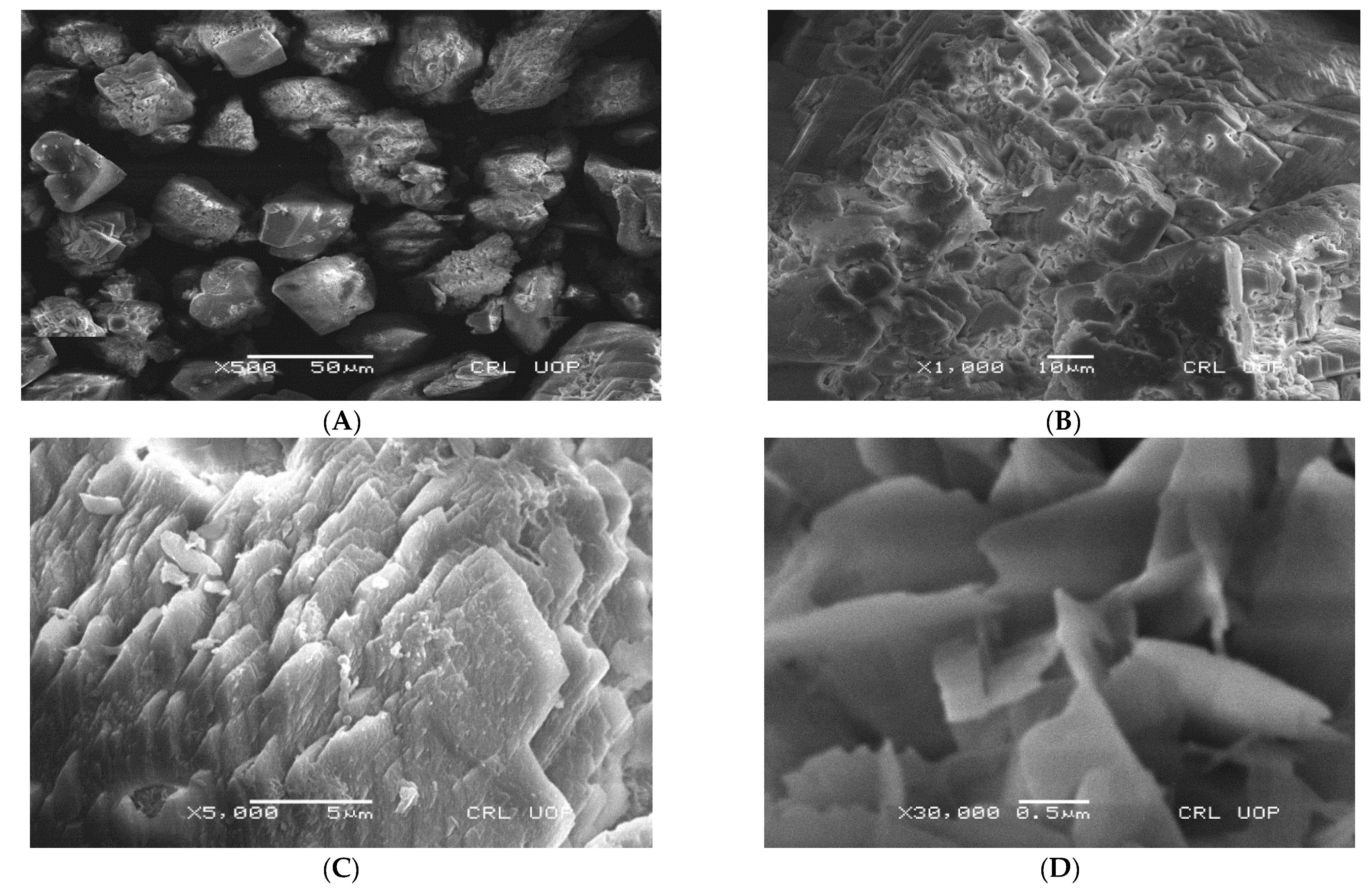
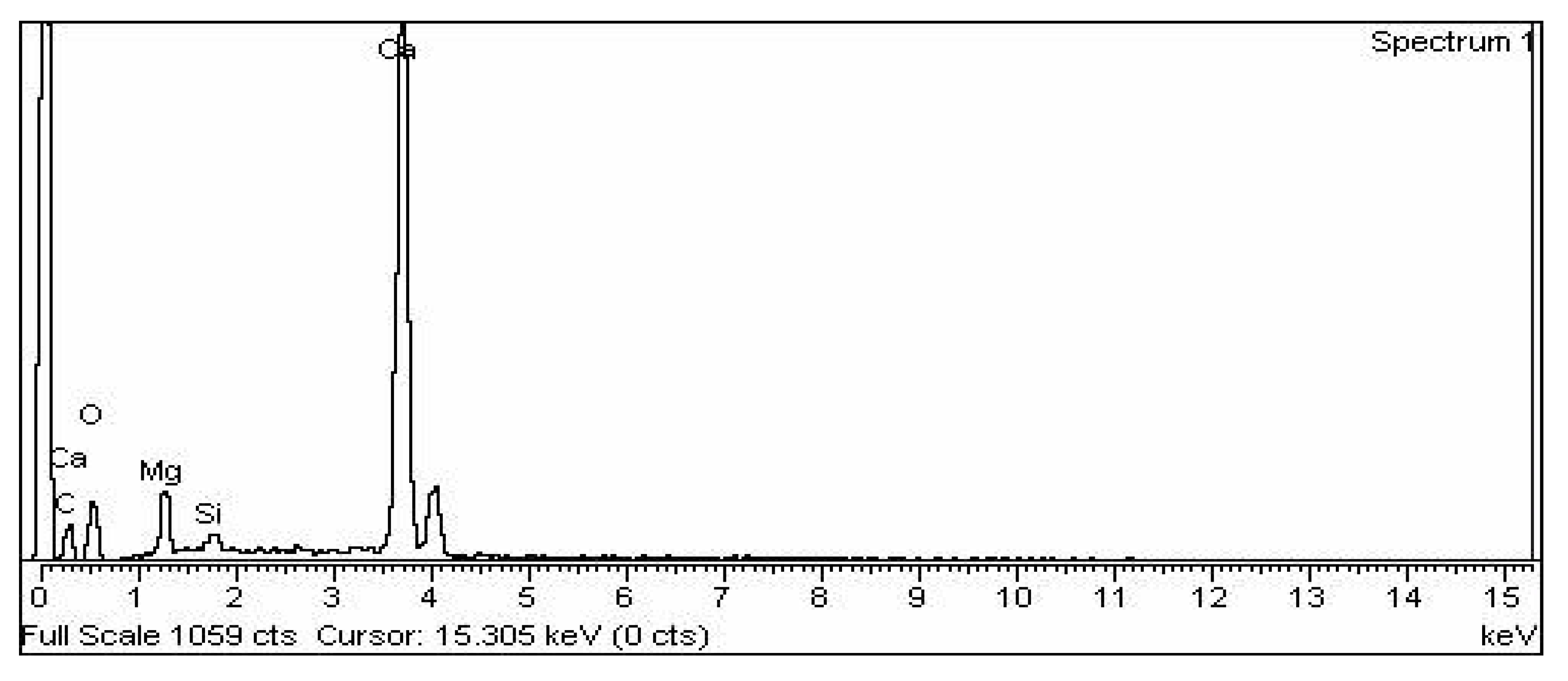
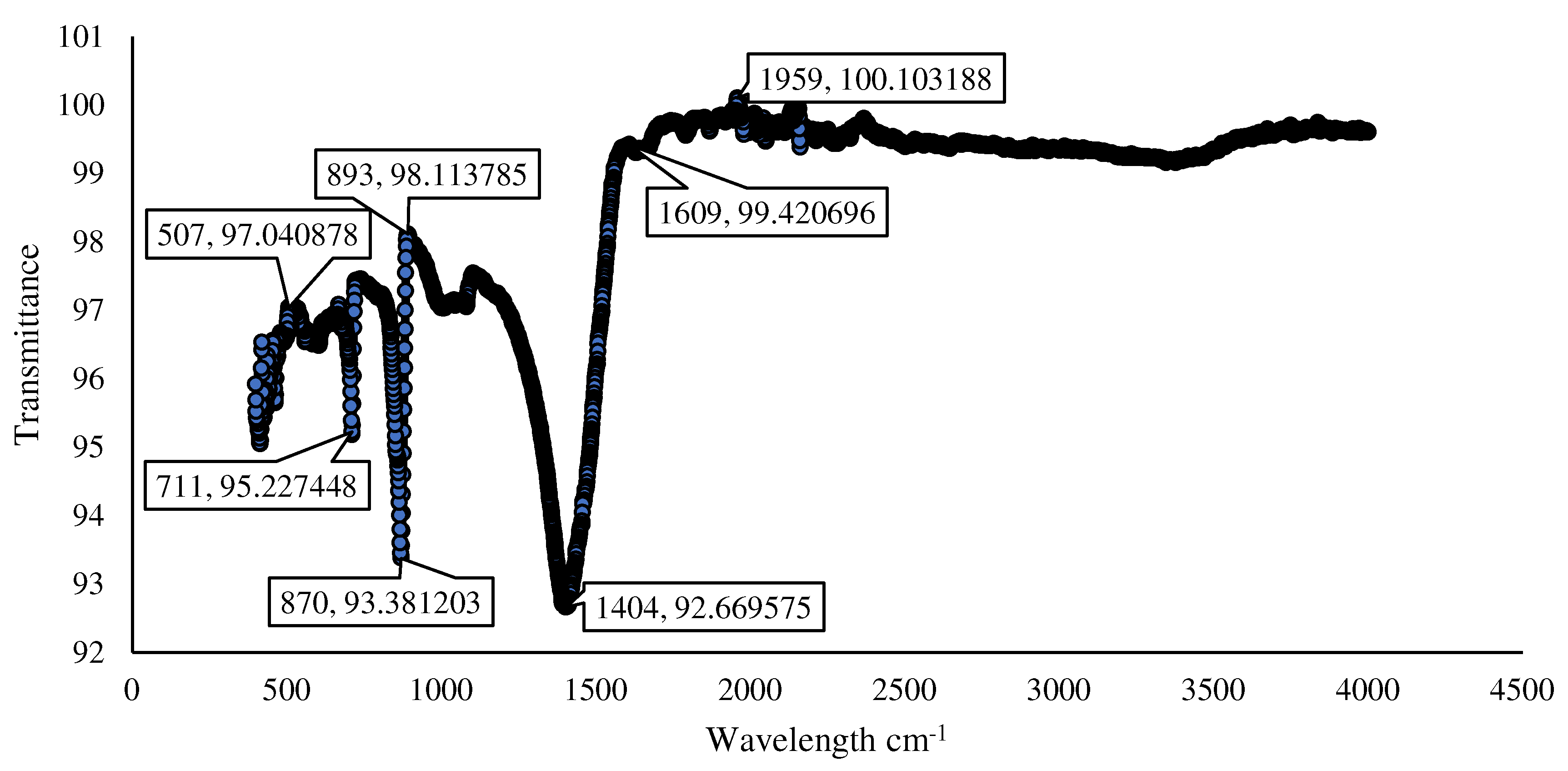
3.5. Characterization of Microbially Induced CaCO3
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seifan, M.; Samani, A.K.; Berenjian, A. Induced calcium carbonate precipitation using Bacillus species. Appl. Microbiol. Biotechnol. 2016, 100, 9895–9906. [Google Scholar] [CrossRef] [Green Version]
- Luhar, S.; Luhar, I.; Shaikh, F.U.A. A Review on the Performance Evaluation of Autonomous Self-Healing Bacterial Concrete: Mechanisms, Strength, Durability, and Microstructural Properties. J. Compos. Sci. 2022, 6, 23. [Google Scholar] [CrossRef]
- Biondini, F.; Camnasio, E.; Palermo, A. Lifetime seismic performance of concrete bridges exposed to corrosion. Struct. Infrastruct. Eng. 2014, 10, 880–900. [Google Scholar] [CrossRef]
- Müller, H.S.; Haist, M.; Vogel, M. Assessment of the sustainability potential of concrete and concrete structures considering their environmental impact, performance and lifetime. Constr. Build. Mater. 2014, 67, 321–337. [Google Scholar] [CrossRef]
- Roels, E.; Terryn, S.; Iida, F.; Bosman, A.W.; Norvez, S.; Clemens, F.; Van Assche, G.; Vanderborght, B.; Brancart, J. Processing of Self-Healing Polymers for Soft Robotics. Adv. Mater. 2022, 34, 2104798. [Google Scholar] [CrossRef]
- Palmer, F. Using Emergent Technologies to Develop Sustainable Architectural Composites; Auckland University of Technology: Auckland, New Zealand, 2009. [Google Scholar]
- Virmani, Y.P.; Clemena, G.G. Corrosion Protection: Concrete Bridges; Federal Highway Administration: Washington, DC, USA, 1998. [Google Scholar]
- Tannous, F. Durability of AR Non-Metallic Reinforcement Bars and Prestressing Tendons. Ph.D Thesis, The University of Arizona, Tucson, AZ, USA, 1997. [Google Scholar]
- Mancio, M.; Zhang, J.; Monteiro, P.; Engineer, C.C. Nondestructive surface measurement of corrosion of reinforcing steel in concrete. Can. Civ. Eng. 2004, 21, 12–14. [Google Scholar]
- Nkurunziza, G.; Benmokrane, B.; Debaiky, A.S.; Masmoudi, R. Effect of sustained load and environment on long-term tensile properties of glass fiber-reinforced polymer reinforcing bars. ACI Struct. J. 2005, 102, 615–621. [Google Scholar]
- Van Tittelboom, K.; De Belie, N.; De Muynck, W.; Verstraete, W. Use of bacteria to repair cracks in concrete. Cem. Concrete Res. 2010, 40, 157–166. [Google Scholar] [CrossRef]
- Van der Zwaag, S.; Van Dijk, N.H.; Jonkers, H.M.; Mookhoek, S.D.; Sloof, W.G. Self-healing behaviour in man-made engineering materials: Bioinspired but taking into account their intrinsic character. Philos. Trans. R. Soc. A: Math. Phys. Eng. Sci. 2009, 367, 1689–1704. [Google Scholar] [CrossRef]
- Algaifi, H.A.; Bakar, S.A.; Sam, A.R.M.; Ismail, M.; Abidin, A.R.Z.; Shahir, S.; Altowayti, W.A.H. Insight into the role of microbial calcium carbonate and the factors involved in self-healing concrete. Constr. Build. Mater. 2020, 254, 119258. [Google Scholar] [CrossRef]
- Jogi, P.K.; Lakshmi, T.V. Self healing concrete based on different bacteria: A review. Mater. Today Proc. 2020, 43, 1246–1252. [Google Scholar] [CrossRef]
- Ganesh, A.C.; Muthukannan, M.; Malathy, R.; Ramesh Babu, C. An experimental study on effects of bacterial strain combination in fibre concrete and self-healing efficiency. KSCE J. Civ. Eng. 2019, 23, 4368–4377. [Google Scholar] [CrossRef]
- De Muynck, W.; Debrouwer, D.; De Belie, N.; Verstraete, W. Bacterial carbonate precipitation improves the durability of cementitious materials. Cem. Concrete Res. 2008, 38, 1005–1014. [Google Scholar] [CrossRef]
- Reddy, S.V.; Satya, A.K.; Rao, S.M.; Azmatunnisa, M. A biological approach to enhance strength and durability in concrete structures. Int. J. Adv. Eng. Technol. 2012, 4, 392. [Google Scholar]
- Ramachandran, S.K.; Ramakrishnan, V.; Bang, S.S. Remediation of concrete using micro-organisms. ACI Mater. J.-Am. Concrete Inst. 2001, 98, 3–9. [Google Scholar]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Ganendra, G.; De Muynck, W.; Ho, A.; Arvanti, E.C.; Hosseinkhani, B.; Ramos, J.A.; Rahier, H.; Boon, N. Formate oxidation-driven calcium carbonate precipitation by Methylocystis parvus OBBP. Appl. Environ. Microbiol. 2014, 80, 4659–4667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seifan, M.; Samani, A.K.; Berenjian, A. Bioconcrete: Next generation of self-healing concrete. Appl. Microbiol. Biotechnol. 2016, 100, 2591–2602. [Google Scholar] [CrossRef] [Green Version]
- Rao, M.S.; Srinivasa Reddy, V.; Hafsa, M.; Veena, P.; Anusha, P. Bioengineered Concrete-A Sustainable Self-Healing Construction Material. Res. J. Eng. Sci. 2013, 2, 45–51. [Google Scholar]
- Wang, J.-Y.; De Belie, N.; Verstraete, W. Diatomaceous earth as a protective vehicle for bacteria applied for self-healing concrete. J. Ind. Microbiol. Biotechnol. 2012, 39, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Algaifi, H.A.; Bakar, S.A.; Sam, A.R.M.; Abidin, A.R.Z.; Shahir, S.; AL-Towayti, W.A.H. Numerical modeling for crack self-healing concrete by microbial calcium carbonate. Constr. Build. Mater. 2018, 189, 816–824. [Google Scholar] [CrossRef]
- Cheong, W.; Gaskell, P.; Neville, A. Substrate effect on surface adhesion/crystallisation of calcium carbonate. J. Cryst. Growth 2013, 363, 7–21. [Google Scholar] [CrossRef]
- Meldrum, F. Calcium carbonate in biomineralisation and biomimetic chemistry. Int. Mater. Rev. 2003, 48, 187–224. [Google Scholar] [CrossRef]
- Chithambaram, S.J.; Kumar, S.; Prasad, M.M.; Adak, D. Effect of parameters on the compressive strength of fly ash based geopolymer concrete. Struct. Concrete 2018, 19, 1202–1209. [Google Scholar] [CrossRef]
- Riaz, N.; Chong, F.K.; Dutta, B.K.; Man, Z.B.; Khan, M.S.; Nurlaela, E. Photodegradation of Orange II under visible light using Cu–Ni/TiO2: Effect of calcination temperature. Chem. Eng. J. 2012, 185, 108–119. [Google Scholar] [CrossRef]
- Seifan, M.; Sarmah, A.K.; Samani, A.K.; Ebrahiminezhad, A.; Ghasemi, Y.; Berenjian, A. Mechanical properties of bio self-healing concrete containing immobilized bacteria with iron oxide nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4489–4498. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; de Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2014, 6, 133–146. [Google Scholar] [CrossRef]
- Galano, M.; van den Dungen, M.W.; van Rij, T.; Abbas, H.E. Safety evaluation of food enzymes produced by a safe strain lineage of Bacillus subtilis. Regul. Toxicol. Pharmacol. 2021, 126, 105030. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.K.; Sidhu, N.; Agnihotri, S.; Mukherjee, A.; Reddy, M.S. Role of nanomaterials in protecting building materials from degradation and deterioration. In Biodegradation and Biodeterioration at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2022; pp. 405–475. [Google Scholar]
- Hariani, P.L.; Muhammad, F.; Ridwan, R.; Marsi, M.; Setiabudidaya, D. Synthesis and properties of Fe3O4 nanoparticles by co-precipitation method to removal procion dye. Int. J. Environ. Sci. Dev. 2013, 4, 336–340. [Google Scholar] [CrossRef]
- Lemine, O.; Omri, K.; Zhang, B.; El Mir, L.; Sajieddine, M.; Alyamani, A.; Bououdina, M. Sol–gel synthesis of 8 nm magnetite (Fe3O4) nanoparticles and their magnetic properties. Superlattices Microstruct. 2012, 52, 793–799. [Google Scholar] [CrossRef]
- Wei, Y.; Han, B.; Hu, X.; Lin, Y.; Wang, X.; Deng, X. Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Eng. 2012, 27, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Tadic, M.; Trpkov, D.; Kopanja, L.; Vojnovic, S.; Panjan, M. Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: Synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J. Alloys Compounds 2019, 792, 599–609. [Google Scholar] [CrossRef]
- Wild, S.; Khatib, J.M.; Jones, A. Relative strength, pozzolanic activity and cement hydration in superplasticised metakaolin concrete. Cem. Concrete Res. 1996, 26, 1537–1544. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G.J.N. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef]
- El Kahoui, R.; Adolphe, J.-P.; Daudon, M. Identification of Early bacillus-induced crystals in vitro using Fourier transform infrared spectroscopy. Microbes Environ. 2000, 15, 161–171. [Google Scholar] [CrossRef]
- Gayathri, S.; Lakshminarayanan, R.; Weaver, J.C.; Morse, D.E.; Kini, M.; Valiyaveettil, S. In vitro study of magnesium-calcite biomineralization in the skeletal materials of the seastar Pisaster giganteus. Chem.—Eur. J. 2007, 13, 3262–3268. [Google Scholar] [CrossRef]



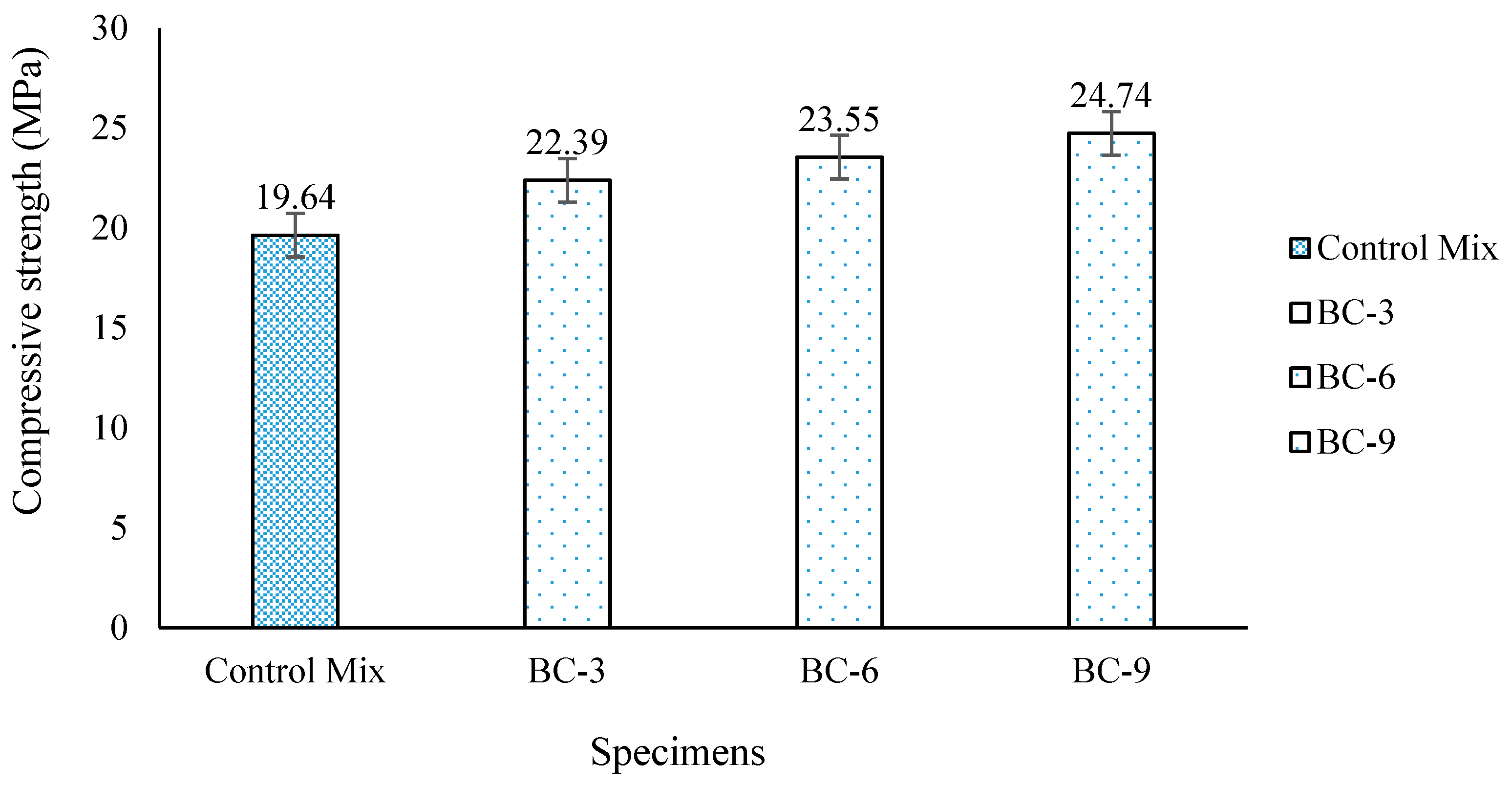

| Encapsulation Material | Application | Precursor | Temperature (°C) | Type of Bacteria |
|---|---|---|---|---|
| Iron oxide nanoparticles | As a crack healer | Urea | Room temperature (27 °C) | Bacillus subtilis |
| Chemical Composition | Content (%) | Physical Properties | Contents (%) |
|---|---|---|---|
| CaO | 65.81 | Insoluble residue (% mass) | 0.55 |
| SiO2 | 18.83 | Specific gravity (g/cm3) | 3.15 |
| Al2O3 | 6.94 | Specific surface area (m2/g) | 0.83 |
| Fe2O3 | 3.47 | Particle size (d50) (µm) | 16.58 |
| MgO | 1.94 | Loss on ignition (% mass) | 2.21 |
| SO3 | 1.32 | ||
| Na2O + K2O | 1.20 |
| Sample | Fine Aggregate(kg/m3) | Coarse Aggregate(kg/m3) | Bacillus subtilis (Cells/mL) | Urea (g/L) | Yeast Extract (g/L) | Calcium Chloride Anhydrous (g/L) | IONPs (µg/L) | Portland Cement (kg/m3) | Super-Plasticizer (kg/m3) | w/c Ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| Control mix | 945.87 | 1316.6 | - | - | - | - | - | 574.2 | 5.80 | 0.42 |
| BC-3 | 945.87 | 1316.6 | 2.8 × 103 | 65 | 2 | 40 | 250 | 574.2 | 5.80 | 0.42 |
| BC-6 | 945.87 | 1316.6 | 2.8 × 106 | 65 | 2 | 40 | 250 | 574.2 | 5.80 | 0.42 |
| BC-9 | 945.87 | 1316.6 | 2.8 × 109 | 65 | 2 | 40 | 250 | 574.2 | 5.80 | 0.42 |
| Sample | 80% Applied Load (Mpa) after 28 Days of Curing | Crack Healing Strength (Mpa) After 28 Days | UPV | % (w) Absorption (gm) | Density (kg/m3) | |
|---|---|---|---|---|---|---|
| Value | Remarks | |||||
| Control mix | 19.45 | - | 4.43 | v. good | 1.345 | 2428.53 |
| 23.76 | 4.51 | excellent | 1.057 | 2383.34 | ||
| 15.72 | 4.13 | v. good | 1.36 | 2487.76 | ||
| BC-3 | 10.64 | 14.68 | 4.12 | v. good | 0.412 | 2423.43 |
| 16.37 | 27.70 | 4.39 | v. good | 0.556 | 2520.20 | |
| 21.03 | 24.79 | 4.41 | v. good | 0.434 | 2408.16 | |
| BC-6 | 13.99 | 29.06 | 4.46 | v. good | 0.352 | 2532.62 |
| 9.35 | 14.61 | 3.98 | good | 0.524 | 2496.53 | |
| 23.92 | 26.98 | 4.15 | v. good | 0.374 | 2438.7 | |
| BC-9 | 11.75 | 23.09 | 4.29 | v. good | 0.468 | 2425.21 |
| 8.45 | 17.58 | 3.68 | good | 0.387 | 2408.32 | |
| 20.6 | 33.56 | 4.13 | v. good | 0.362 | 2413.14 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, F.; Kashif Ur Rehman, S.; Jameel, M.; Riaz, N.; Javed, M.F.; Salmi, A.; Awad, Y.A. Self-Healing Bio-Concrete Using Bacillus subtilis Encapsulated in Iron Oxide Nanoparticles. Materials 2022, 15, 7731. https://doi.org/10.3390/ma15217731
Mahmood F, Kashif Ur Rehman S, Jameel M, Riaz N, Javed MF, Salmi A, Awad YA. Self-Healing Bio-Concrete Using Bacillus subtilis Encapsulated in Iron Oxide Nanoparticles. Materials. 2022; 15(21):7731. https://doi.org/10.3390/ma15217731
Chicago/Turabian StyleMahmood, Faisal, Sardar Kashif Ur Rehman, Mohammed Jameel, Nadia Riaz, Muhammad Faisal Javed, Abdelatif Salmi, and Youssef Ahmed Awad. 2022. "Self-Healing Bio-Concrete Using Bacillus subtilis Encapsulated in Iron Oxide Nanoparticles" Materials 15, no. 21: 7731. https://doi.org/10.3390/ma15217731







