Hemolysis-Inspired, Highly Sensitive, Label-Free IgM Detection Using Erythrocyte Membrane-Functionalized Nanomechanical Resonators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus and Measurements
2.3. EM Collection
2.4. EM Functionalization
2.5. Theory
2.6. Measurement of Immunoglobulin by EMMC
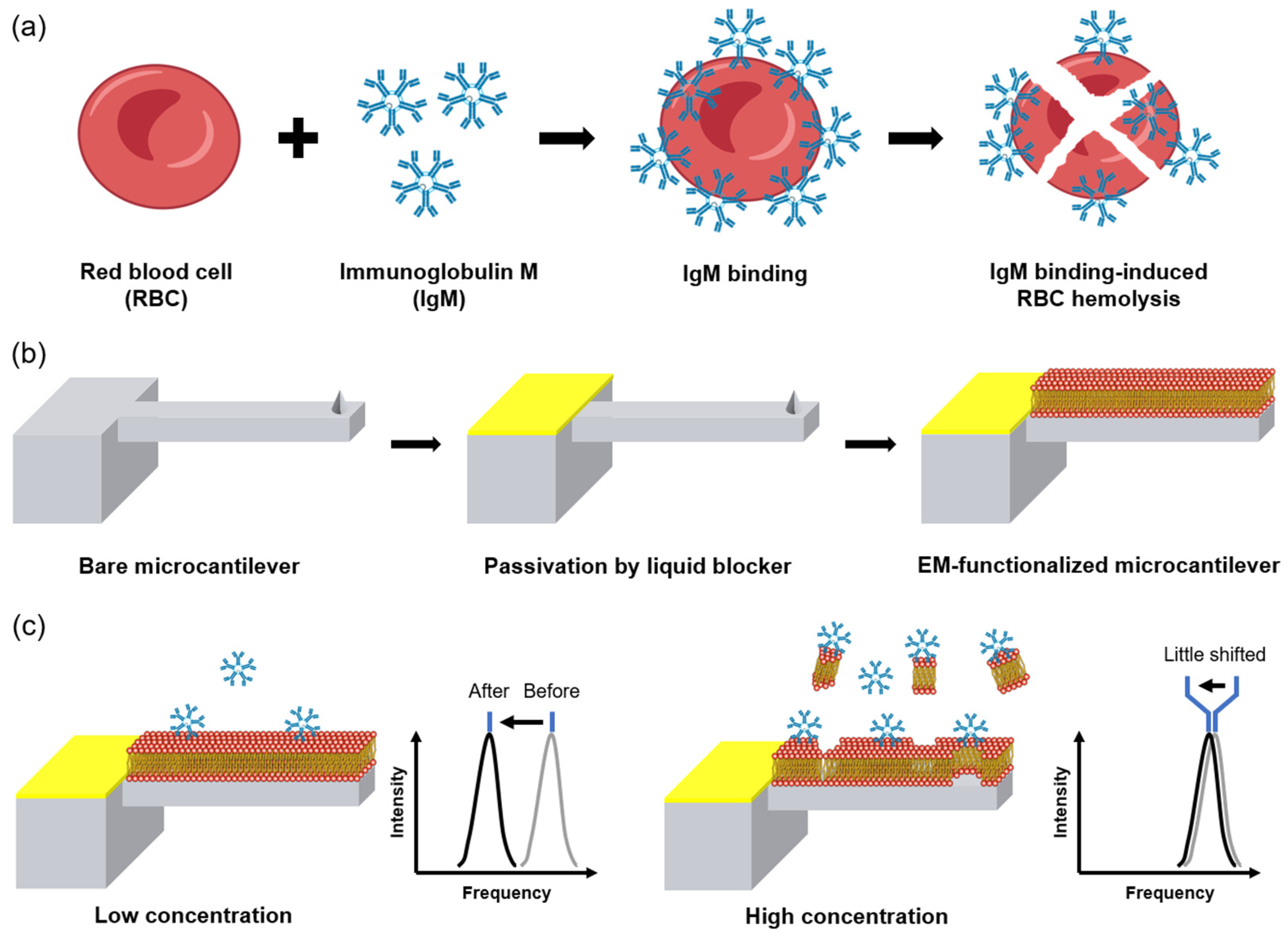
3. Results and Discussion
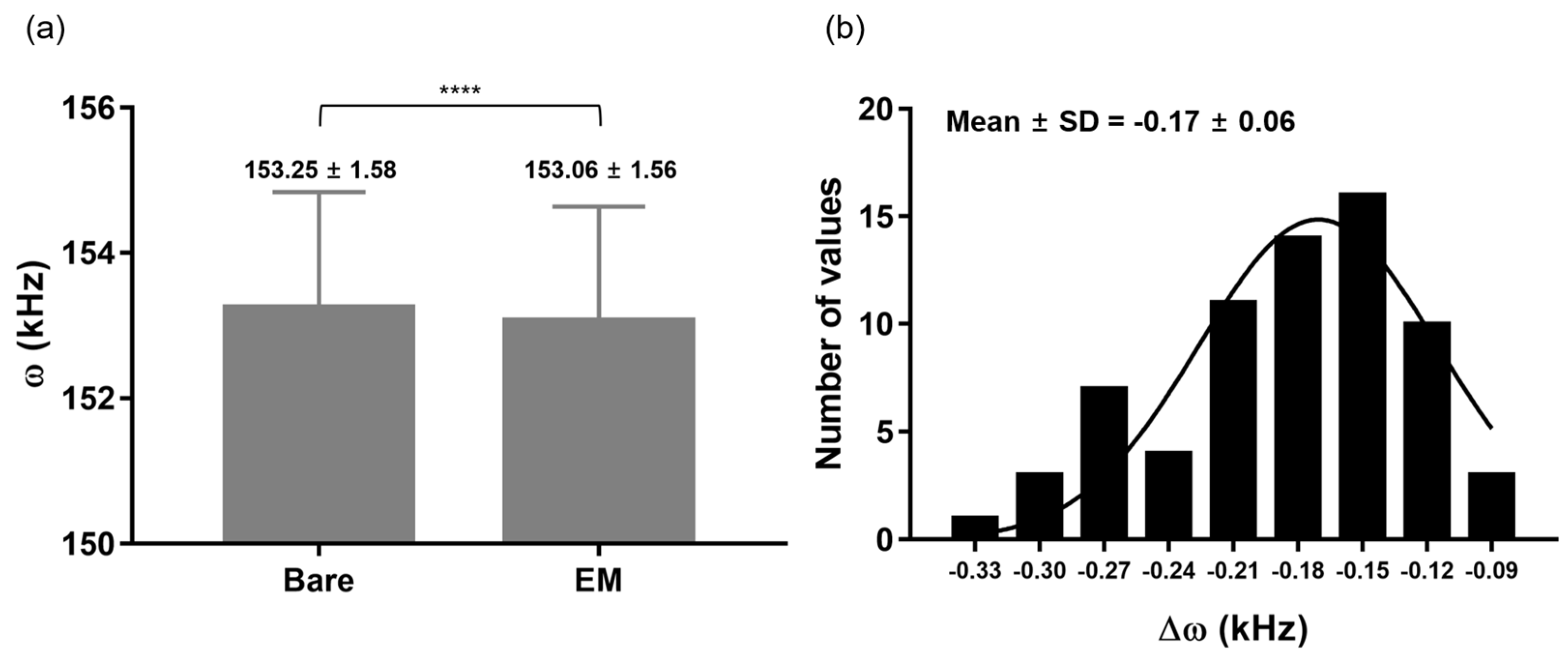
3.1. EMMC Fabrication
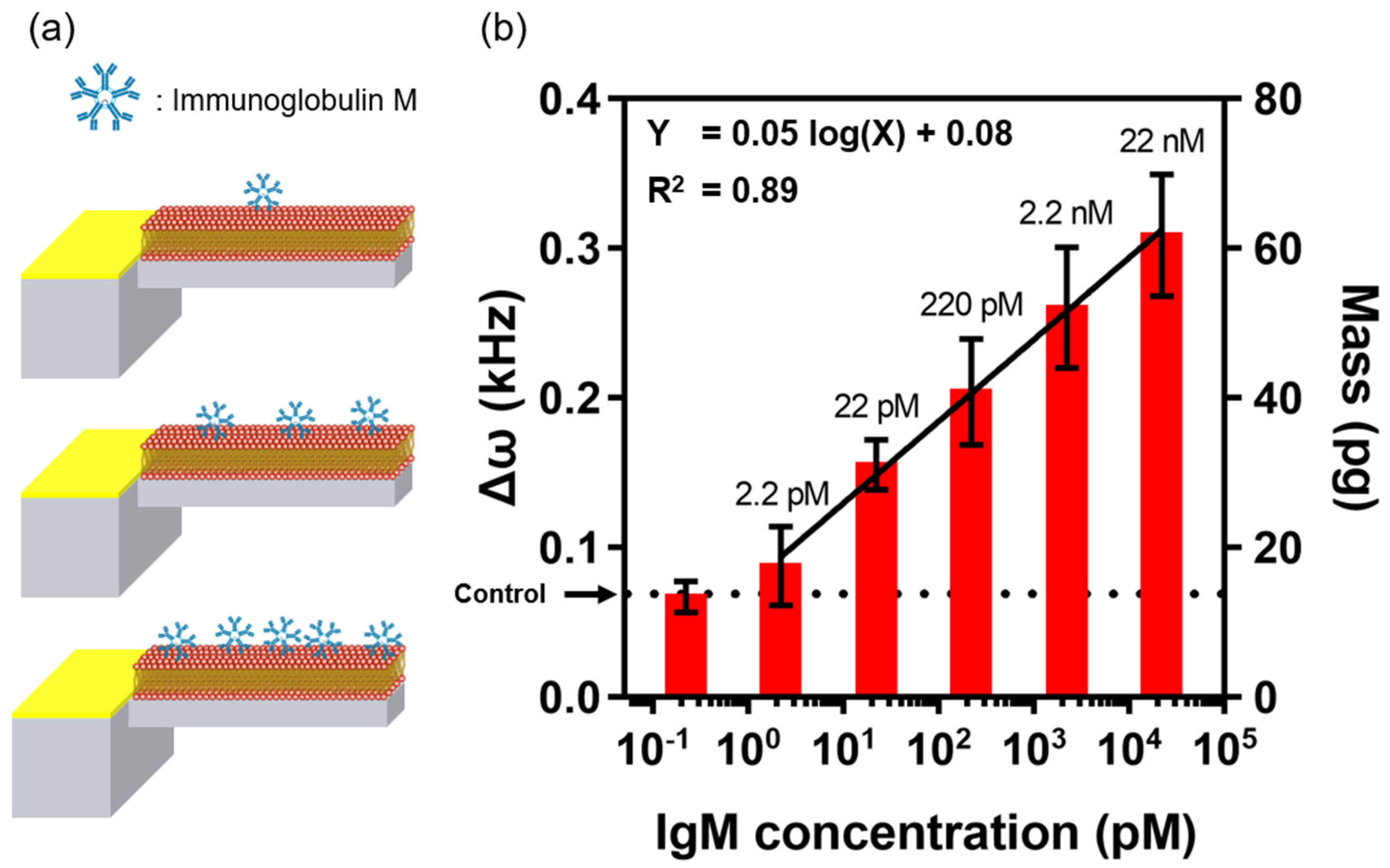
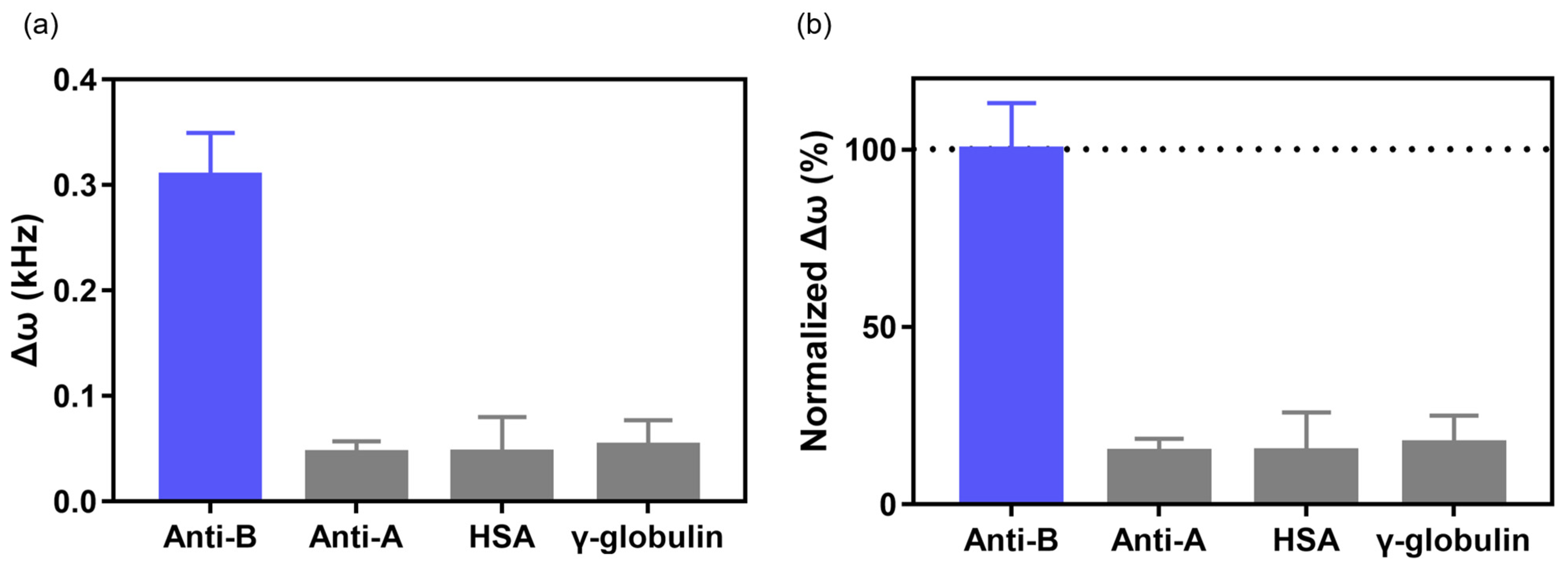
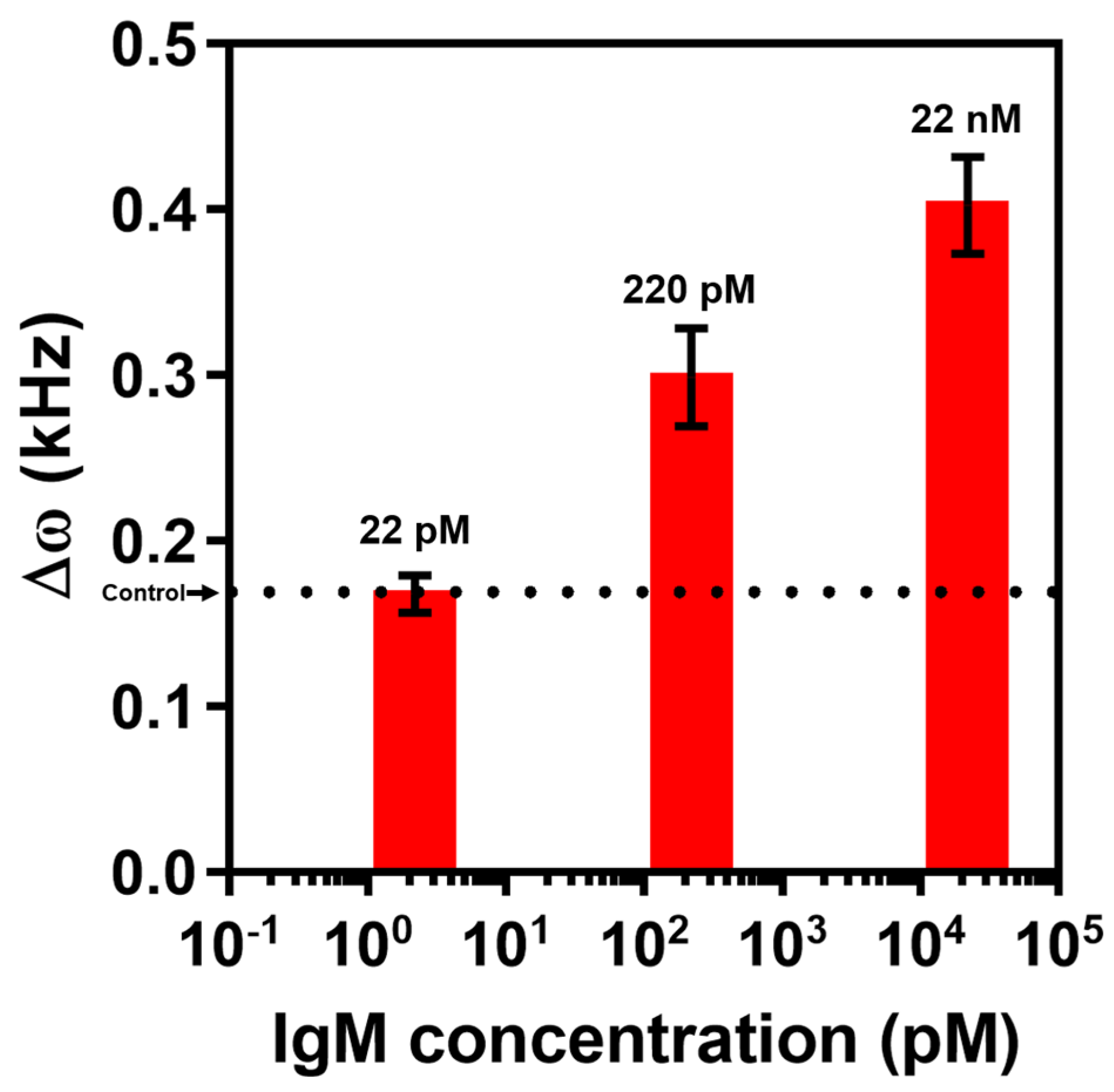
3.2. Immunoglobulin Detection and Selectivity Test Using EMMC Sensor
3.3. EM Exfoliation at High IgM Concentration
3.4. IgM Detection in Human Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burton, D.R. Immunoglobulin G: Functional sites. Mol. Immunol. 1985, 22, 161–206. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.M.; Kerr, M.A. The function of immunoglobulin A in immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Cerutti, A. The function and regulation of immunoglobulin D. Curr. Opin. Immunol. 2011, 23, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mix, E.; Goertsches, R.; Zett, U.K. Immunoglobulins—Basic considerations. J. Neurol. 2006, 253, v9–v17. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125 (Suppl. 2), S41–S52. [Google Scholar] [CrossRef] [Green Version]
- Chothia, C.; Lesk, A.M.; Tramontano, A.; Levitt, M.; Smith-Gill, S.J.; Air, G.; Sheriff, S.; Padlan, E.A.; Davies, D.; Tulip, W.R.; et al. Conformations of immunoglobulin hypervariable regions. Nature 1989, 342, 877–883. [Google Scholar] [CrossRef]
- Grönwall, C.; Vas, J.; Silverman, G. Protective Roles of Natural IgM Antibodies. Front. Immunol. 2012, 3, 66. [Google Scholar] [CrossRef] [Green Version]
- Grönwall, C.; Silverman, G.J. Natural IgM: Beneficial Autoantibodies for the Control of Inflammatory and Autoimmune Disease. J. Clin. Immunol. 2014, 34, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Lin, Q.; Wen, D.; Wu, J.; Liu, L.; Wu, W.; Fang, X.; Kong, J. Microfluidic Immunoassays for Sensitive and Simultaneous Detection of IgG/IgM/Antigen of SARS-CoV-2 within 15 min. Anal. Chem. 2020, 92, 9454–9458. [Google Scholar] [CrossRef]
- Griffin, I.; Martin, S.W.; Fischer, M.; Chambers, T.V.; Kosoy, O.; Falise, A.; Ponomareva, O.; Gillis, L.D.; Blackmore, C.; Jean, R. Zika Virus IgM Detection and Neutralizing Antibody Profiles 12–19 Months after Illness Onset. Emerg. Infect. Dis. 2019, 25, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Løset, G.Å.; Roux, K.H.; Zhu, P.; Michaelsen, T.E.; Sandlie, I. Differential Segmental Flexibility and Reach Dictate the Antigen Binding Mode of Chimeric IgD and IgM: Implications for the Function of the B Cell Receptor. J. Immunol. 2004, 172, 2925–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stussi, G.; Huggel, K.; Lutz, H.U.; Schanz, U.; Rieben, R.; Seebach, J.D. Isotype-specific detection of ABO blood group antibodies using a novel flow cytometric method. Br. J. Haematol. 2005, 130, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.; Ward, G. Interferences in immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]
- Schuurs, A.H.W.M.; Van Weemen, B.K. Enzyme-immunoassay. Clin. Chim. Acta 1977, 81, 1–40. [Google Scholar] [CrossRef]
- Banerjee, R.; Jaiswal, A. Recent advances in nanoparticle-based lateral flow immunoassay as a point-of-care diagnostic tool for infectious agents and diseases. Analyst 2018, 143, 1970–1996. [Google Scholar] [CrossRef]
- Pettersson Bergstrand, M.; Helander, A.; Hansson, T.; Beck, O. Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays. Drug Test. Anal. 2017, 9, 640–645. [Google Scholar] [CrossRef]
- Yadav, S.; Sadique, M.A.; Ranjan, P.; Kumar, N.; Singhal, A.; Srivastava, A.K.; Khan, R. SERS Based Lateral Flow Immunoassay for Point-of-Care Detection of SARS-CoV-2 in Clinical Samples. ACS Appl. Bio Mater. 2021, 4, 2974–2995. [Google Scholar] [CrossRef]
- Mahmood, T.; Yang, P.C. Western blot: Technique, theory, and trouble shooting. N. Am. J. Med. Sci. 2012, 4, 429–434. [Google Scholar]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef] [Green Version]
- Sugimura, T.; Kajimoto, S.; Nakabayashi, T. Label-Free Imaging of Intracellular Temperature by Using the O−H Stretching Raman Band of Water. Angew. Chem. Int. Ed. 2020, 59, 7755–7760. [Google Scholar] [CrossRef] [PubMed]
- Durnez, L.; Van Bortel, W.; Denis, L.; Roelants, P.; Veracx, A.; Trung, H.D.; Sochantha, T.; Coosemans, M. False positive circumsporozoite protein ELISA: A challenge for the estimation of the entomological inoculation rate of malaria and for vector incrimination. Malar. J. 2011, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Garrote, B.L.; Santos, A.; Bueno, P.R. Perspectives on and Precautions for the Uses of Electric Spectroscopic Methods in Label-free Biosensing Applications. ACS Sens. 2019, 4, 2216–2227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, Y.; Guo, T.; Zhou, L.; Offenhäusser, A.; Mayer, D. Label-Free Split Aptamer Sensor for Femtomolar Detection of Dopamine by Means of Flexible Organic Electrochemical Transistors. Materials 2020, 13, 2577. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Li, W.; Liu, X.; Li, F. Label-Free and Enzyme-Free Homogeneous Electrochemical Biosensing Strategy Based on Hybridization Chain Reaction: A Facile, Sensitive, and Highly Specific MicroRNA Assay. Anal. Chem. 2015, 87, 11368–11374. [Google Scholar] [CrossRef] [PubMed]
- Svigelj, R.; Zuliani, I.; Grazioli, C.; Dossi, N.; Toniolo, R. An Effective Label-Free Electrochemical Aptasensor Based on Gold Nanoparticles for Gluten Detection. Nanomater 2022, 12, 987. [Google Scholar] [CrossRef]
- Xu, H.; Song, Y.; Zhu, P.; Zhao, W.; Liu, T.; Wang, Q.; Zhao, T. Alcohol Sensor Based on Surface Plasmon Resonance of ZnO Nanoflowers/Au Structure. Materials 2022, 15, 189. [Google Scholar] [CrossRef]
- Sarcina, L.; Macchia, E.; Loconsole, G.; D’Attoma, G.; Saldarelli, P.; Elicio, V.; Palazzo, G.; Torsi, L. Surface Plasmon Resonance Assay for Label-Free and Selective Detection of Xylella Fastidiosa. Adv. NanoBiomed Res. 2021, 1, 2100043. [Google Scholar] [CrossRef]
- Mahmoodi, S.R.; Xie, P.; Zachs, D.P.; Peterson, E.J.; Graham, R.S.; Kaiser, C.R.W.; Lim, H.H.; Allen, M.G.; Javanmard, M. Single-step label-free nanowell immunoassay accurately quantifies serum stress hormones within minutes. Sci. Adv. 2021, 7, eabf4401. [Google Scholar] [CrossRef]
- Seo, Y.; Jeong, S.; Lee, J.; Choi, H.S.; Kim, J.; Lee, H. Innovations in biomedical nanoengineering: Nanowell array biosensor. Nano Converg. 2018, 5, 9. [Google Scholar] [CrossRef]
- Mahmoodi, S.R.; Xie, P.; Allen, M.; Javanmard, M. Multiwell Plate Impedance Analysis of a Nanowell Array Sensor for Label-Free Detection of Cytokines in Mouse Serum. IEEE Sens. Lett. 2020, 4, 4500104. [Google Scholar] [CrossRef]
- Guo, Y.; Pan, X.; Zhang, W.; Hu, Z.; Wong, K.-W.; He, Z.; Li, H.-W. Label-free probes using DNA-templated silver nanoclusters as versatile reporters. Biosens. Bioelectron. 2020, 150, 111926. [Google Scholar] [CrossRef] [PubMed]
- Peltomaa, R.; Glahn-Martínez, B.; Benito-Peña, E.; Moreno-Bondi, M.C. Optical Biosensors for Label-Free Detection of Small Molecules. Sensors 2018, 18, 4126. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pinkse, P.W.H.; Segerink, L.I.; Eijkel, J.C.T. Bottom-Up Assembled Photonic Crystals for Structure-Enabled Label-Free Sensing. ACS Nano 2021, 15, 9299–9327. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.; Spitzer, D. 3D Core–Shell TiO2@MnO2 Nanorod Arrays on Microcantilevers for Enhancing the Detection Sensitivity of Chemical Warfare Agents. ACS Appl. Mater. Interfaces 2021, 13, 47185–47197. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Walt, D.R. Highly Sensitive and Multiplexed Protein Measurements. Chem. Rev. 2019, 119, 293–321. [Google Scholar] [CrossRef]
- Li, C.; Zhang, G.; Wu, S.; Zhang, Q. Aptamer-based microcantilever-array biosensor for profenofos detection. Anal. Chim. Acta 2018, 1020, 116–122. [Google Scholar] [CrossRef]
- Cooper, O.; Phan, H.-P.; Fitzpatrick, T.; Dinh, T.; Huang, H.; Nguyen, N.-T.; Tiralongo, J. Picomolar detection of carbohydrate-lectin interactions on piezoelectrically printed microcantilever array. Biosens. Bioelectron. 2022, 205, 114088. [Google Scholar] [CrossRef]
- Gupta, A.; Akin, D.; Bashir, R. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 2004, 84, 1976–1978. [Google Scholar] [CrossRef]
- Longo, G.; Alonso-Sarduy, L.; Rio, L.M.; Bizzini, A.; Trampuz, A.; Notz, J.; Dietler, G.; Kasas, S. Rapid detection of bacterial resistance to antibiotics using AFM cantilevers as nanomechanical sensors. Nat. Nanotechnol. 2013, 8, 522–526. [Google Scholar] [CrossRef]
- Kasas, S.; Ruggeri, F.S.; Benadiba, C.; Maillard, C.; Stupar, P.; Tournu, H.; Dietler, G.; Longo, G. Detecting nanoscale vibrations as signature of life. Proc. Natl. Acad. Sci. USA 2015, 112, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Bennett, I.; Pyne, A.L.B.; McKendry, R.A. Cantilever Sensors for Rapid Optical Antimicrobial Sensitivity Testing. ACS Sens. 2020, 5, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ma, X.; Guan, Y.; Tang, J.; Zhang, B. Microcantilever Array Biosensor for Simultaneous Detection of Carcinoembryonic Antigens and α-Fetoprotein Based on Real-Time Monitoring of the Profile of Cantilever. ACS Sens. 2019, 4, 3034–3041. [Google Scholar] [CrossRef]
- Lee, G.; Eom, K.; Park, J.; Yang, J.; Haam, S.; Huh, Y.-M.; Ryu, J.K.; Kim, N.H.; Yook, J.I.; Lee, S.W.; et al. Real-Time Quantitative Monitoring of Specific Peptide Cleavage by a Proteinase for Cancer Diagnosis. Angew. Chem. Int. Ed. 2012, 51, 5837–5841. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, P.M.; Tamayo, J.; Ruz, J.J.; Puertas, S.; Polo, E.; Grazu, V.; de la Fuente, J.M.; Calleja, M. Tackling reproducibility in microcantilever biosensors: A statistical approach for sensitive and specific end-point detection of immunoreactions. Analyst 2013, 138, 863–872. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Bang, D.; Jang, K.; Kim, E.; Haam, S.; Na, S. Multimodal label-free detection and discrimination for small molecules using a nanoporous resonator. Nat. Commun. 2014, 5, 3456. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Rao, D.; Wu, X.; Zhang, Q.; Wu, S. Aptamer-based microcantilever-array biosensor for ultra-sensitive and rapid detection of okadaic acid. Microchem. J. 2021, 160, 105644. [Google Scholar] [CrossRef]
- Kosaka, P.M.; Pini, V.; Ruz, J.J.; da Silva, R.A.; González, M.U.; Ramos, D.; Calleja, M.; Tamayo, J. Detection of cancer biomarkers in serum using a hybrid mechanical and optoplasmonic nanosensor. Nat. Nanotechnol. 2014, 9, 1047–1053. [Google Scholar] [CrossRef]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Garratty, G. Immune Hemolytic Anemia Associated with Negative Routine Serology. Semin. Hematol. 2005, 42, 156–164. [Google Scholar] [CrossRef]
- Semple, J.W.; Freedman, J. Autoimmune Pathogenesis and Autoimmune Hemolytic Anemia. Semin. Hematol. 2005, 42, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Risinger, M.; Kalfa, T.A. Red cell membrane disorders: Structure meets function. Blood 2020, 136, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Lee, D.; Lee, S.W.; Lee, J.H.; Lee, G.; Yoon, D.S. Coagulation-Inspired Direct Fibrinogen Assay Using Plasmonic Nanoparticles Functionalized with Red Blood Cell Membranes. ACS Nano 2021, 15, 6386–6394. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, I.; Lee, W.; Kim, M.; Park, J.; Lee, G.; Yoon, D.S.; Park, J. Highly sensitive and wide-range nanoplasmonic detection of fibrinogen using erythrocyte membrane-blanketed nanoparticles. Biosens. Bioelectron. 2019, 135, 216–223. [Google Scholar] [CrossRef]
- Kim, I.; Kim, C.; Lee, D.; Lee, S.W.; Lee, G.; Yoon, D.S. A bio-inspired highly selective enzymatic glucose sensor using a red blood cell membrane. Analyst 2020, 145, 2125–2132. [Google Scholar] [CrossRef]
- Kim, I.; Kwon, D.; Lee, D.; Lee, G.; Yoon, D.S. Permselective glucose sensing with GLUT1-rich cancer cell membranes. Biosens. Bioelectron. 2019, 135, 82–87. [Google Scholar] [CrossRef]
- Kim, I.; Kim, Y.I.; Lee, S.W.; Jung, H.G.; Lee, G.; Yoon, D.S. Highly permselective uric acid detection using kidney cell membrane–functionalized enzymatic biosensors. Biosens. Bioelectron. 2021, 190, 113411. [Google Scholar] [CrossRef]
- Kim, I.; Kwon, D.; Lee, D.; Lee, T.H.; Lee, J.H.; Lee, G.; Yoon, D.S. A highly permselective electrochemical glucose sensor using red blood cell membrane. Biosens. Bioelectron. 2018, 102, 617–623. [Google Scholar] [CrossRef]
- Park, J.; Lee, W.; Kim, I.; Kim, M.; Jo, S.; Kim, W.; Park, H.; Lee, G.; Choi, W.; Yoon, D.S.; et al. Ultrasensitive detection of fibrinogen using erythrocyte membrane-draped electrochemical impedance biosensor. Sens. Actuators B Chem. 2019, 293, 296–303. [Google Scholar] [CrossRef]
- Lee, T.; Kim, I.; Cheong, D.Y.; Roh, S.; Jung, H.G.; Lee, S.W.; Kim, H.S.; Yoon, D.S.; Hong, Y.; Lee, G. Selective colorimetric urine glucose detection by paper sensor functionalized with polyaniline nanoparticles and cell membrane. Anal. Chim. Acta 2021, 1158, 338387. [Google Scholar] [CrossRef]
- Braun, T.; Barwich, V.; Ghatkesar, M.K.; Bredekamp, A.H.; Gerber, C.; Hegner, M.; Lang, H.P. Micromechanical mass sensors for biomolecular detection in a physiological environment. Phys. Rev. E 2005, 72, 031907. [Google Scholar] [CrossRef]
- Gupta, A.K.; Nair, P.R.; Akin, D.; Ladisch, M.R.; Broyles, S.; Alam, M.A.; Bashir, R. Anomalous resonance in a nanomechanical biosensor. Proc. Natl. Acad. Sci. USA 2006, 103, 13362–13367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulze, R.; Heinrich, M.; Nossol, P.; Forke, R.; Sborikas, M.; Tsapkolenko, A.; Billep, D.; Wegener, M.; Kroll, L.; Gessner, T. Piezoelectric P(VDF-TrFE) transducers assembled with micro injection molded polymers. Sens. Actuators A Phys. 2014, 208, 159–165. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Shu, W.; Huber, F.; Lang, H.P.; Gerber, C.; Dobson, C.M.; Welland, M.E. Label-free detection of amyloid growth with microcantilever sensors. Nanotechnology 2008, 19, 384007. [Google Scholar] [CrossRef]
- Ortega, G.A.; Zuaznabar-Gardona, J.C.; Reguera, E. Electrochemical immunoassay for the detection of IgM antibodies using polydopamine particles loaded. Biosens. Bioelectron. 2018, 116, 30–36. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wei, X.; Zheng, S.W.; Lenhart, B.J.; Xu, P.; Li, J.; Pan, J.; Albrecht, H.; Liu, C. Multiplex quantitative detection of SARS-CoV-2 specific IgG and IgM antibodies based on DNA-assisted. Biosens. Bioelectron. 2021, 181, 113134. [Google Scholar] [CrossRef]
- Bereli, N.; Bakhshpour, M.; Topçu, A.A.; Denizli, A. Surface Plasmon Resonance-Based Immunosensor for Igm Detection with Gold Nanoparticles. Micromachines 2021, 12, 1092. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.-Z.; Zheng, G.; Zhang, H.; Zhou, M.; Li, S.; Kong, Y. Dual-template molecularly imprinted electrochemical biosensor for IgG-IgM combined assay based on a dual-signal strategy. Bioelectrochemistry 2022, 148, 108267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, Z.; Hu, H.; Zhou, Q.; Liu, W.; Li, X.; Liu, Z.; Wang, Y.; Ma, Y. A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood. Lab Chip 2020, 20, 4255–4261. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.; Kim, W.; Park, J.; Lee, G. Hemolysis-Inspired, Highly Sensitive, Label-Free IgM Detection Using Erythrocyte Membrane-Functionalized Nanomechanical Resonators. Materials 2022, 15, 7738. https://doi.org/10.3390/ma15217738
Lee T, Kim W, Park J, Lee G. Hemolysis-Inspired, Highly Sensitive, Label-Free IgM Detection Using Erythrocyte Membrane-Functionalized Nanomechanical Resonators. Materials. 2022; 15(21):7738. https://doi.org/10.3390/ma15217738
Chicago/Turabian StyleLee, Taeha, Woong Kim, Jinsung Park, and Gyudo Lee. 2022. "Hemolysis-Inspired, Highly Sensitive, Label-Free IgM Detection Using Erythrocyte Membrane-Functionalized Nanomechanical Resonators" Materials 15, no. 21: 7738. https://doi.org/10.3390/ma15217738
APA StyleLee, T., Kim, W., Park, J., & Lee, G. (2022). Hemolysis-Inspired, Highly Sensitive, Label-Free IgM Detection Using Erythrocyte Membrane-Functionalized Nanomechanical Resonators. Materials, 15(21), 7738. https://doi.org/10.3390/ma15217738







