Microstructure and Properties of Densified Gd2O3 Bulk
Abstract
1. Introduction
2. Experimental Materials and Procedures
2.1. Preparation of Gd2O3 Bulks
2.2. Microstructures and Phases
2.3. Properties
3. Results
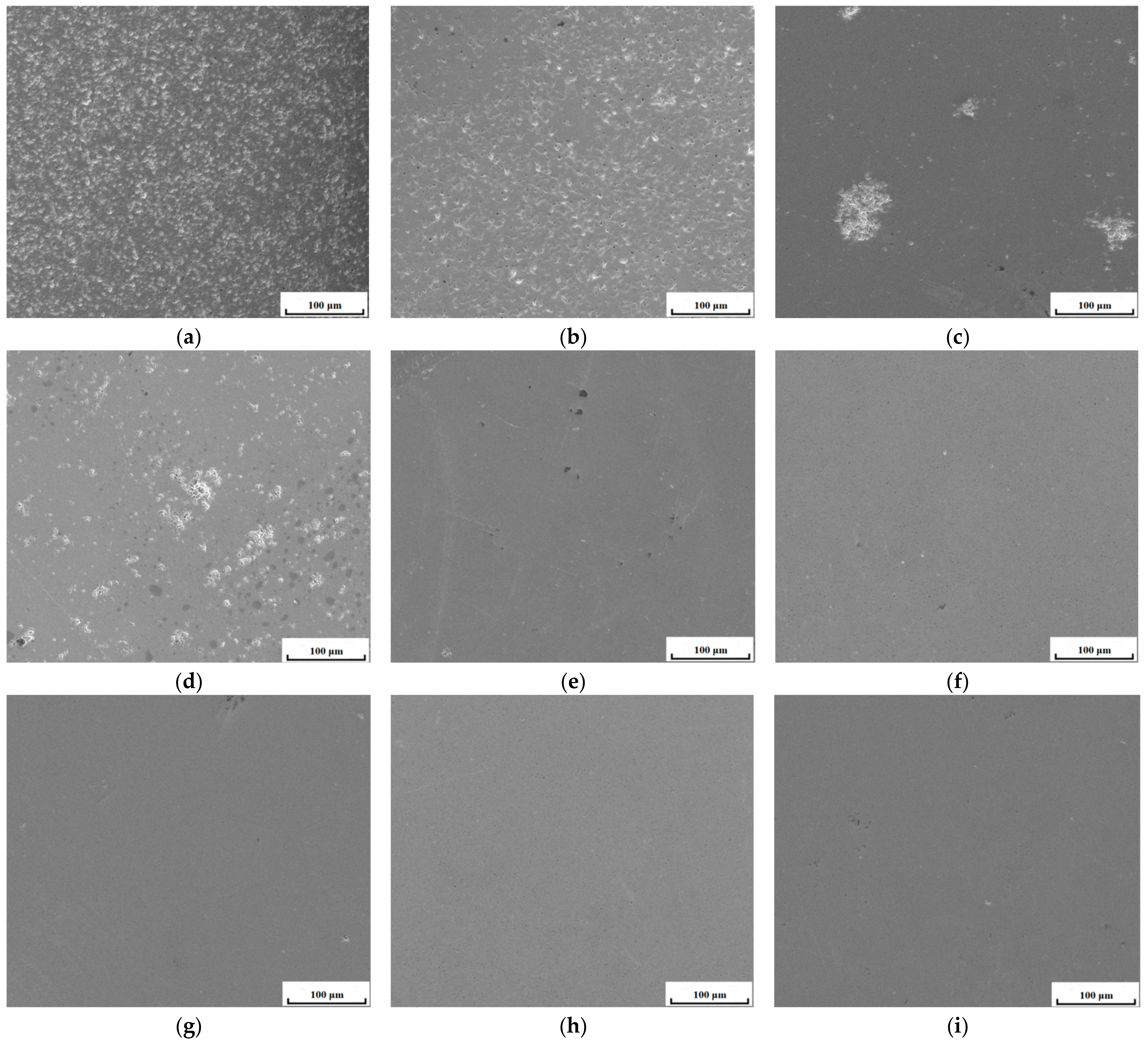
3.1. Microstructure
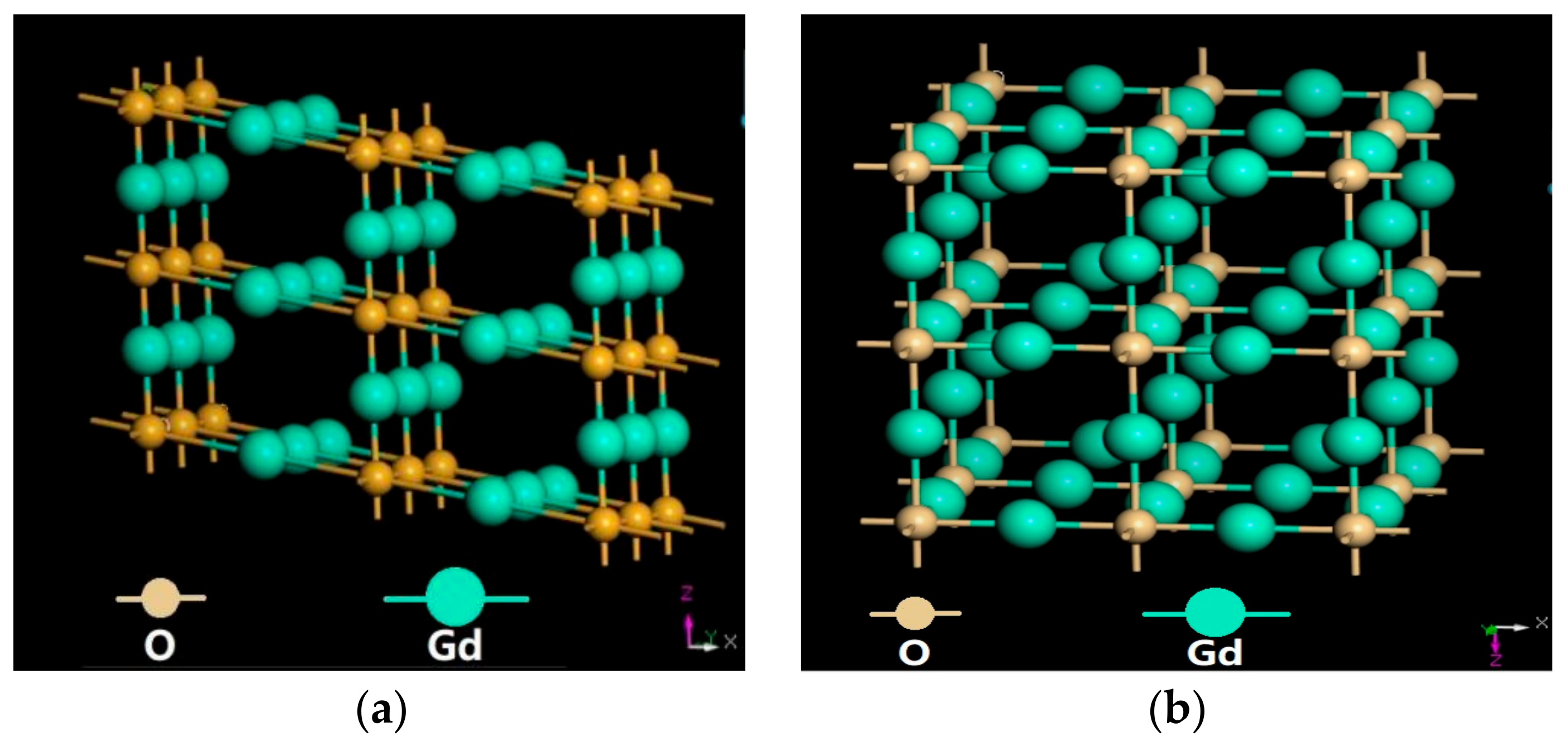
3.2. Phases
3.3. Densification
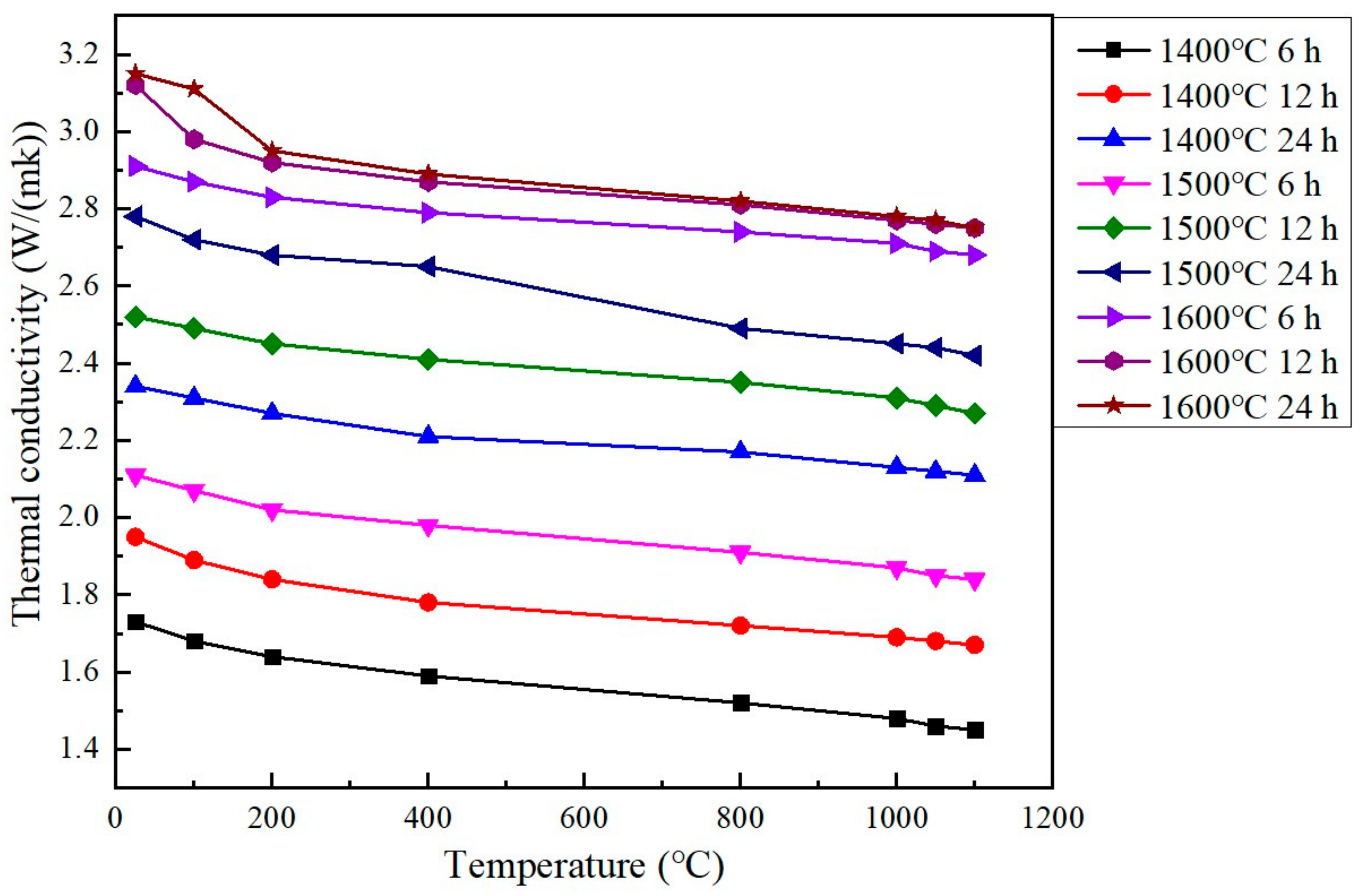
3.4. Thermal Conductivity
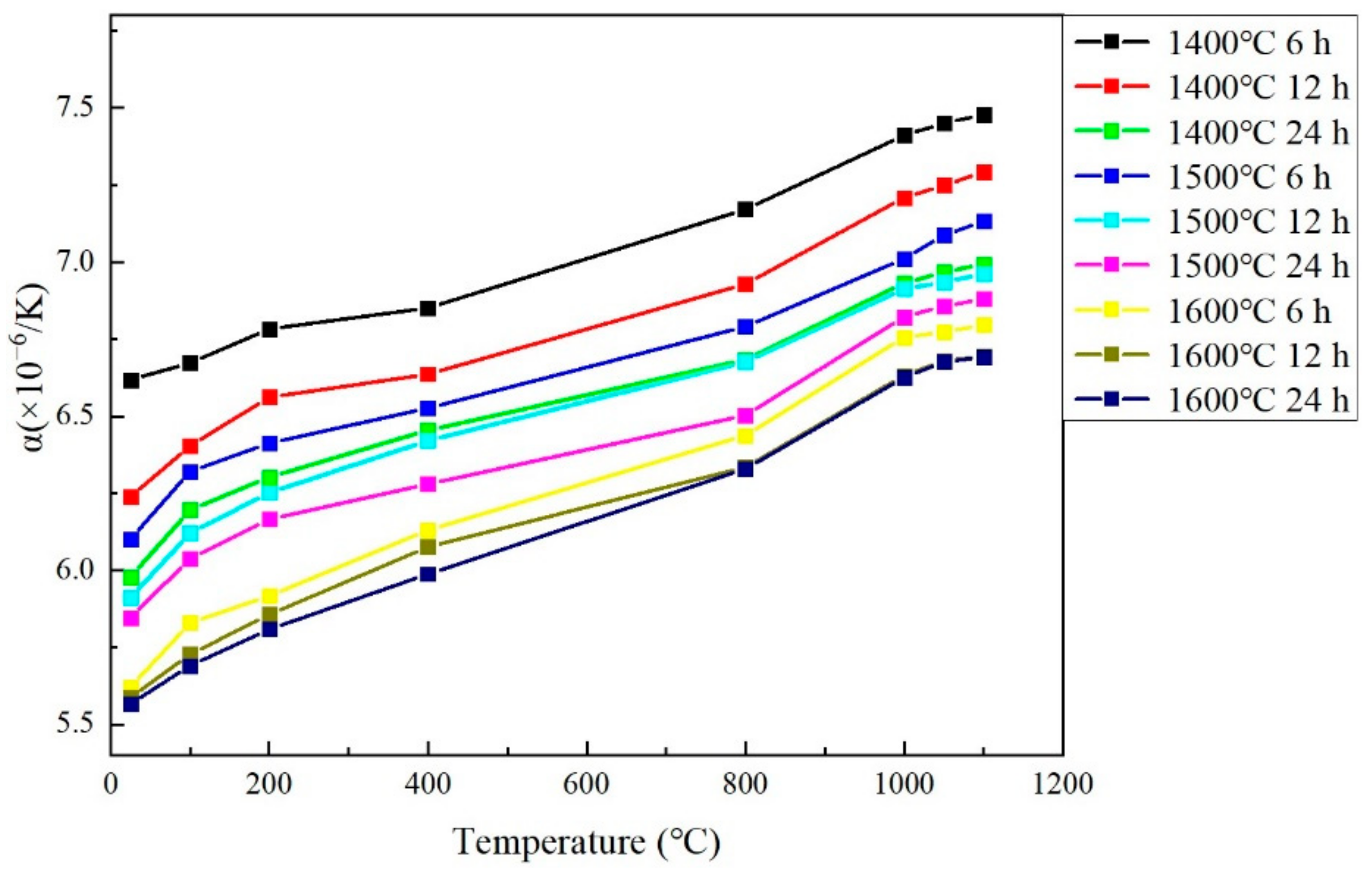
3.5. Thermal Expansion Coefficient
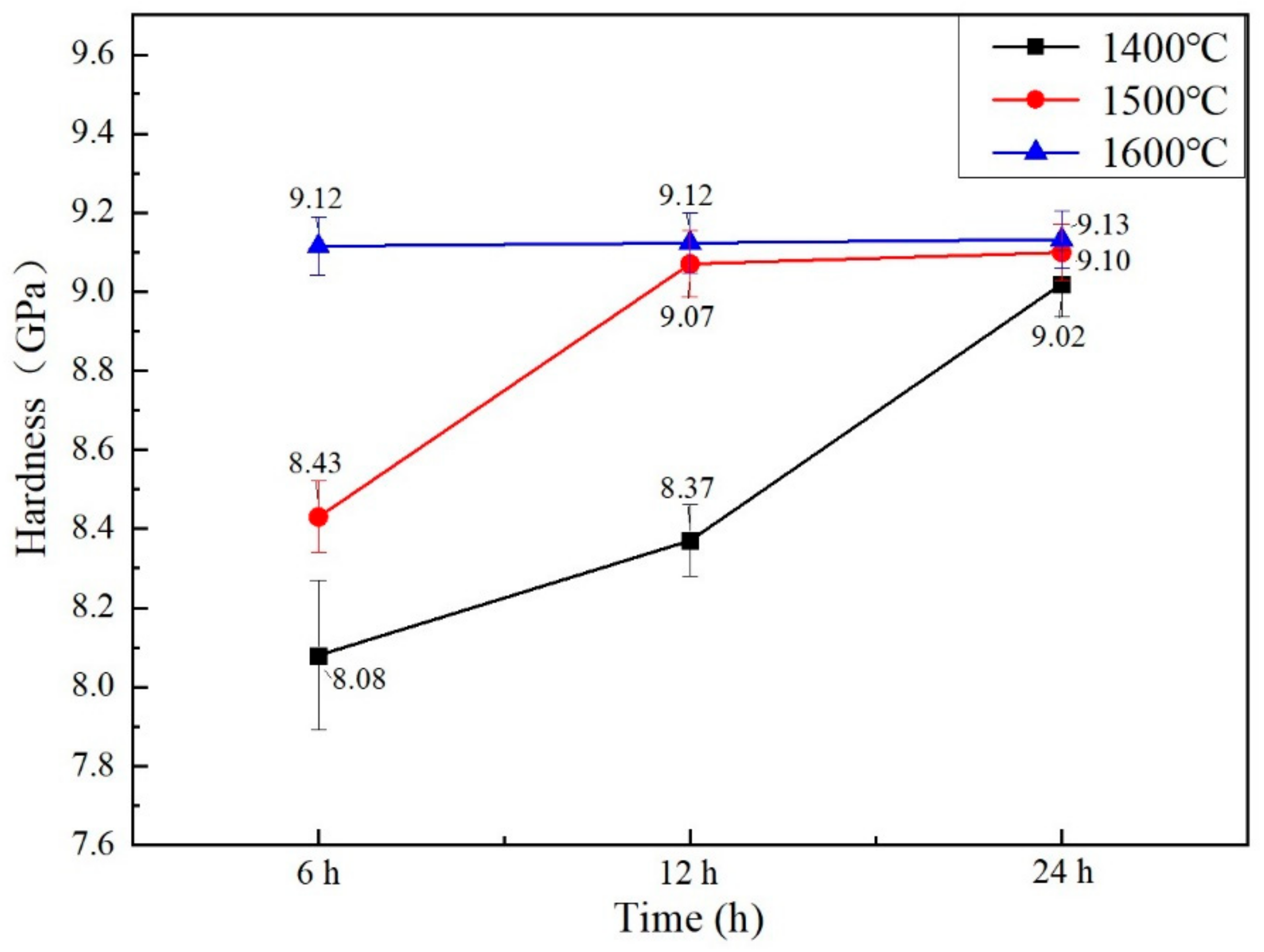
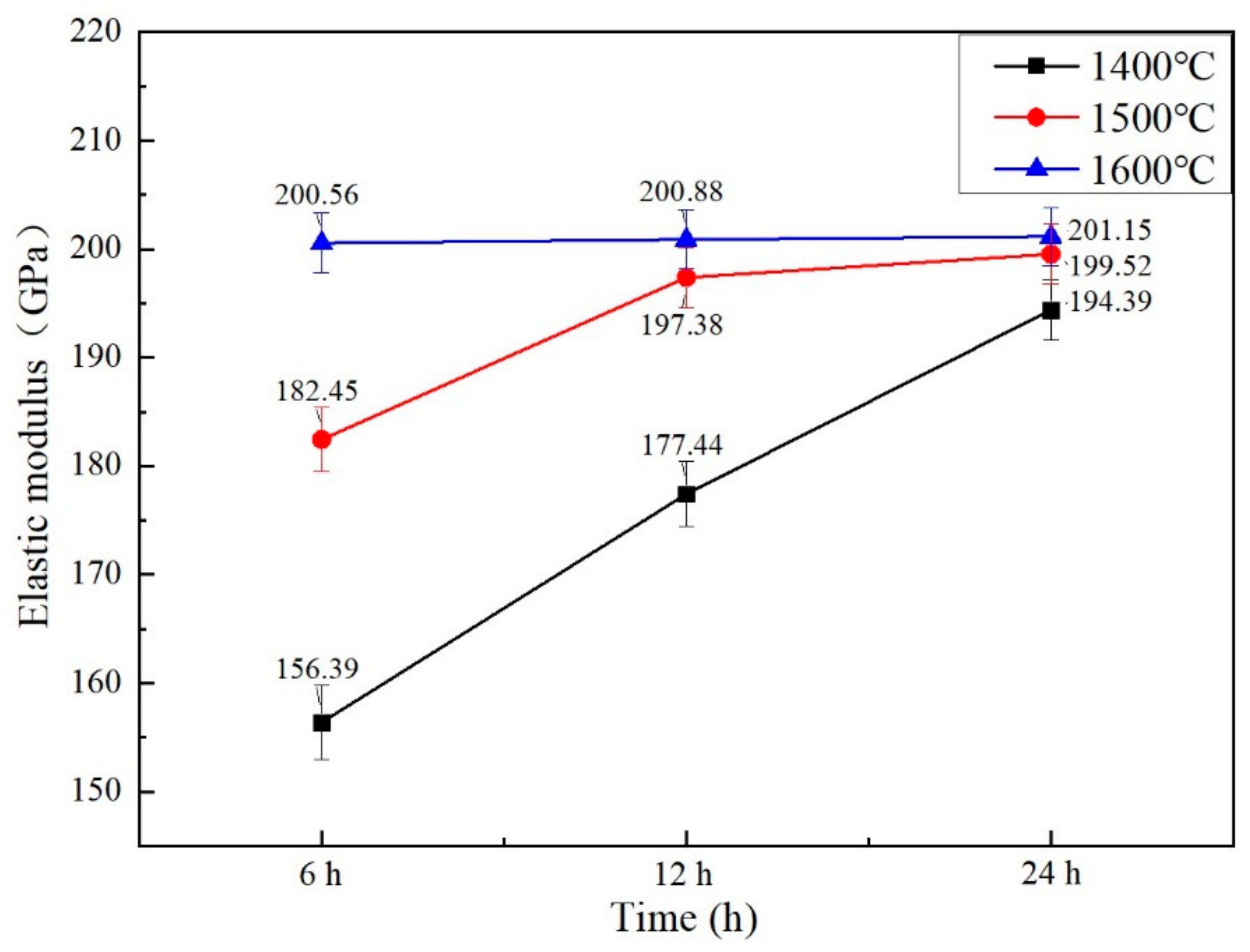
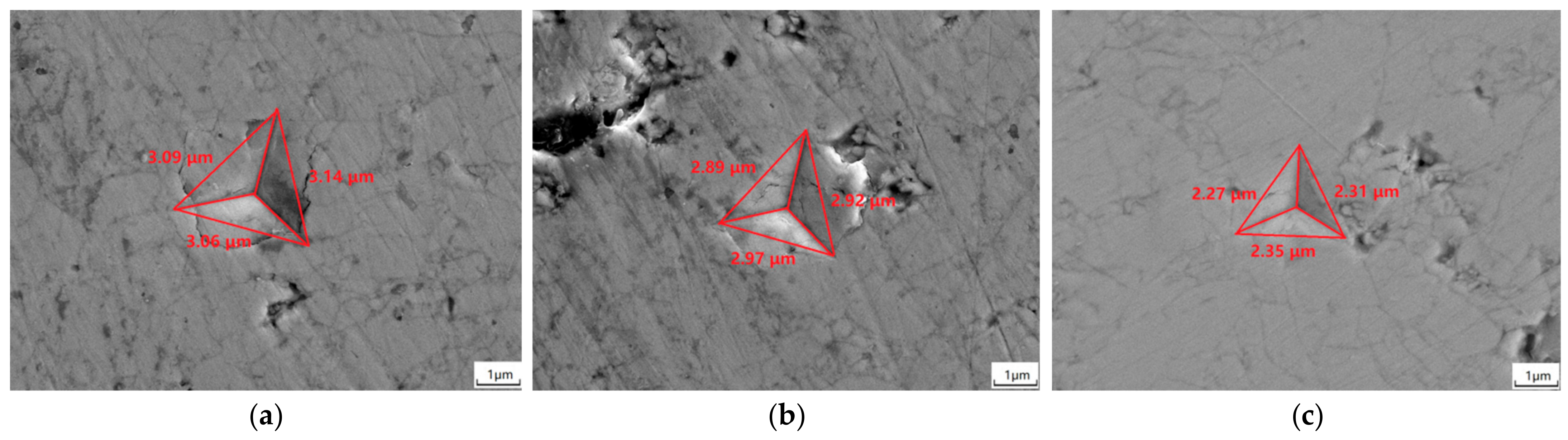
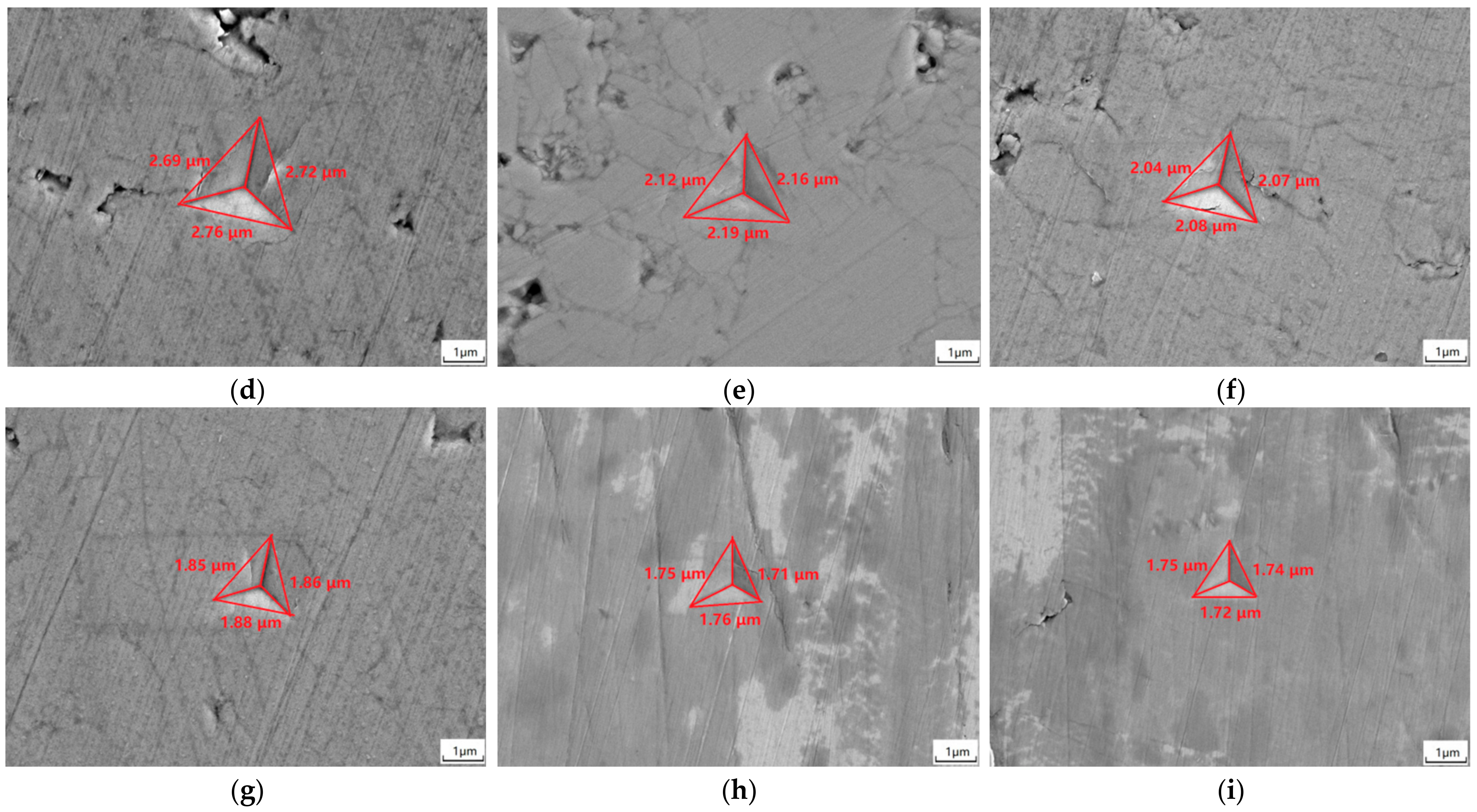
3.6. Mechanical Properties
4. Discussion
5. Conclusions
- (1)
- The Gd2O3 bulk became denser and denser when sintering temperature rose and sintering time extended. The densification of the Gd2O3 bulk reached 96.16% after sintering at 1600 °C for 24 h, which reached a near-fully dense state.
- (2)
- There was no phase transformation during sintering of the preformed Gd2O3 bulks. All of the sintered Gd2O3 bulks were composed of about 90% cubic and 10% monoclinic structures.
- (3)
- When sintering temperature rose and sintering time extended, the hardness, elastic modulus, fracture toughness and thermal conductivity of the sintered Gd2O3 bulk increased and the CTE decreased gradually. Gd2O3 bulk sintered at 1600 °C for 24 h possessed the maximum elastic modulus, hardness, fracture toughness and thermal conductivity of 201.15 GPa, 9.13 GPa, 15.03 MPa·m0.5 and 2.75 W/(m·k) (at 1100 °C), and the minimum CTE of 6.69 × 10−6/°C (at 1100 °C).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, T. The journey of gas turbine localization. Energy 2019, 12, 16–22. [Google Scholar]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Cai, L.X.; He, Y.; Wang, S.S.; Li, Y.; Li, F. Thermal-Fluid-Solid coupling analysis on the temperature and thermal stress field of a Nickel-Base superalloy turbine blade. Materials 2021, 14, 3315. [Google Scholar] [CrossRef] [PubMed]
- Vassen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Mishra, R.K.; Nandi, V.; Bhat, R.R. Failure Analysis of High-Pressure compressor blade in an aero gas turbine engine. J. Fail. Anal. Prev. 2018, 18, 465–470. [Google Scholar] [CrossRef]
- Bakan, E.; Vaßen, R. Ceramic top coats of plasma-sprayed thermal barrier coatings: Materials, processes, and properties. J. Therm. Spray. Technol. 2017, 26, 992–1010. [Google Scholar] [CrossRef]
- Wu, S.; Zhao, Y.T.; Li, W.G.; Liu, W.L.; Wu, Y.P.; Liu, F.K. Research progresses on ceramic materials of thermal barrier coatings on gas turbine. Coatings 2021, 11, 79. [Google Scholar] [CrossRef]
- Huang, J.; Wang, W.; Li, Y.; Fang, H.; Tu, S. A novel strategy to control the microstructure of plasma-sprayed ysz thermal barrier coatings. Surf. Coat. Technol. 2020, 402, 126304. [Google Scholar] [CrossRef]
- Raghavan, S.; Wang, H.; Porter, W.D. Thermal properties of zirconia co-doped with trivalent and pentanvalent oxides. Acta Mater. 2001, 49, 169–179. [Google Scholar] [CrossRef]
- Chen, X.G.; Zhang, H.S.; Liu, Y.X.; Zhao, Y.D.; Ren, B.; Tang, A.; Lu, K. Thermal properties of RE2AlTaO7 (RE = Gd and Yb) oxides. Ceram. Int. 2018, 44, 10762–10765. [Google Scholar] [CrossRef]
- Li, Y.J.; Huang, J.B.; Wang, W.Z.; Ye, D.D.; Fang, H.J.; Gao, D.; Tu, S.T.; Guo, X.P.; Yu, Z.X. Control of the Pore Structure of Plasma-Sprayed Thermal Barrier Coatings through the Addition of Unmelted Porous YSZ Particles. Coatings 2021, 11, 360. [Google Scholar] [CrossRef]
- Allen, A.; Ilavsky, J.; Long, G. Microstructural characterization of yttria-stabilized zirconia plasma-sprayed deposits using multiple small-angle neutron scattering. Acta Mater. 2001, 49, 1661–1675. [Google Scholar] [CrossRef]
- Boissonnet, G.; Chalk, C.; Nicholls, J.R. Thermal insulation of YSZ and Erbia-Doped Yttria-Stabilised Zirconia EB-PVD thermal barrier coating systems after CMAS attack. Materials 2020, 13, 4382. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, Z.Y.; Liu, G.X.; He, L.M. Sm-doped Gd2Zr2O7 thermal barrier coatings: Thermal expansion coefficient, structure and failure. Vacuum 2021, 190, 110314. [Google Scholar] [CrossRef]
- Gao, P.H.; Yang, G.J.; Sao, S.T.; Li, J.P.; Yang, Z.; Guo, Y.C. Heredity and variation of hollow structure from powders to coatings through atmospheric plasma spraying. Surf. Coat. Technol. 2016, 305, 76–82. [Google Scholar] [CrossRef]
- Nicholls, J.R.; Lawson, K.J.; Johnstone, A.; Rickerby, D.S. Methods to reduce the thermal conductivity of EB-PVD TBCs. Surf. Coat. Technol. 2002, 151, 383–391. [Google Scholar] [CrossRef]
- An, G.S.; Li, W.S.; Feng, L.; Cheng, B.; Wang, Z.P.; Li, Z.Y.; Zhang, Y. Isothermal oxidation and TGO growth behaviors of YAG/YSZ double-ceramic-layer thermal barrier coatings. Ceram. Int. 2021, 47, 24320–24330. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Han, Y.X.; Lin, C.C.; Zheng, W. Effect of spraying power on the morphology of YSZ splat and micro-structure of thermal barrier coating. Ceram. Int. 2021, 47, 18956–18963. [Google Scholar] [CrossRef]
- Cong, S.; Ran, G.; Li, Y.P.; Chen, Y. Ball-milling properties and sintering behavior of Al-based Gd2O3–W shielding materials used in spent-fuel storage. Powder Technol. 2020, 369, 127–136. [Google Scholar] [CrossRef]
- Bahamirian, M.; Hadavi, S.M.M.; Farvizi, M.; Rahimipour, M.R.; Keyvani, A. Phase stability of ZrO29.5Y2O35.6Yb2O35.2Gd2O3 compound at 1100 °C and 1300 °C for advanced TBC applications. Ceram. Int. 2019, 45, 7344–7350. [Google Scholar] [CrossRef]
- Yan, Z.; Peng, H.R.; Yuan, K.; Zhang, X. Optimization of Yb2O3-Gd2O3-Y2O3 Co-Doped ZrO2 Agglomerated and Calcined Powders for Air Plasma Spraying. Coatings 2021, 11, 373. [Google Scholar] [CrossRef]
- Eranezhuth, W.A.; Soumya, S.; Rajashekhar, S.; Ravi, K. Structural, functional and mechanical properties of spark plasma sintered gadolinia (Gd2O3). Ceram. Int. 2016, 42, 1384–1391. [Google Scholar] [CrossRef]
- Doleker, K.M.; Karaoglanli, A.C. Comparison of oxidation behavior of YSZ and Gd2Zr2O7 thermal barrier coatings (TBCs). Surf. Coat. Technol. 2017, 318, 198–207. [Google Scholar] [CrossRef]
- Shen, Z.Y.; Liu, Z.; Liu, G.X.; Mu, R.D.; He, L.M.; Dai, J.W. GdYbZrO thermal barrier coatings by EB-PVD: Phase, microstructure, thermal properties and failure. Surf. Interfaces 2021, 24, 101123. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Q.S.; Liu, Y.B.; Ning, X.J. Microstructure, thermal characteristics, and thermal cycling behavior of the ternary rare earth oxides (La2O3, Gd2O3, and Yb2O3) co-doped YSZ coatings. Surf. Coat. Technol. 2020, 403, 126387. [Google Scholar] [CrossRef]
- Gao, P.H.; Zeng, S.C.; Jin, C.; Zhang, B.; Chen, B.Y.; Yang, Z.; Guo, Y.C.; Liang, M.X.; Li, J.P.; Li, Q.P.; et al. Effect of Gd2O3 Addition on the Microstructure and Properties of Gd2O3-Yb2O3-Y2O3-ZrO2 (GYYZO) Ceramics. Materials 2021, 14, 7470. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.D.; Öte, M.; Königstein, T. A highly porous thermal barrier coating based on Gd2O3-Yb2O3 co-doped YSZ. Surf. Coat. Technol. 2019, 366, 349–354. [Google Scholar] [CrossRef]
- Zhang, J.X.; Bai, Y.; Li, E.B.; Dong, H.Y.; Ma, W. Yb2O3-Gd2O3 codoped strontium zirconate composite ceramics for potential thermal barrier coating applications. Int. J. Appl. Ceram. Technol. 2020, 17, 1608–1618. [Google Scholar] [CrossRef]
- Shang, F.L.; Zhang, X.; Guo, X.C.; Zhao, P.F.; Chang, Y. Determination of high temperature mechanical properties of thermal barrier coatings by nanoindentation. Surf. Eng. 2014, 30, 283–289. [Google Scholar] [CrossRef]
- Kyongjun, A.; Kakkaveri, S.; Ravichandran, R.E.; Dutton, S.L. Semiatin. Microstructure, Texture, and Thermal Conductivity of Single-Layer and Multilayer Thermal Barrier Coatings of Y2O3-Stabilized ZrO2 and Al2O3 Made by Physical Vapor Deposition. J. Am. Ceram. Soc. 1999, 82, 399–406. [Google Scholar] [CrossRef]
- Rice, R.W.; Wu, C.C.; Borchelt, F. Hardness-Grain-Size relations in ceramics. J. Am. Ceram. Soc. 1994, 77, 2539–2553. [Google Scholar] [CrossRef]
- Jan, F.J.; Virkar, A.V. Fabrication, Microstructural characterization, and mechanical properties of polycrystalline t′-Zirconia. J. Am. Ceram. Soc. 1990, 73, 3650–3657. [Google Scholar] [CrossRef]
- Schaedler, T.A.; Leckie, R.M.; Krmer, S.; Evans, A.G.; Levi, C.G. Toughening of nontransformable T′-YSZ by addition of titania. J. Am. Ceram. Soc. 2007, 90, 3896–3901. [Google Scholar] [CrossRef]
- Cao, X.Q.; Vassen, R.; Stoever, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Schelling, P.K.; Phillpot, S.R.; Grimes, R.W. Optimum pyrochlore compositions for low thermal conductivity. Philos. Mag. Lett. 2004, 84, 127–137. [Google Scholar] [CrossRef]
- Lehmann, H.; Pitzer, D.; Pracht, G.; Vassen, R.; Stover, D. Thermal conductivity and thermal expansion coefficients of the Lanthanum Rare-Earth-Element zirconate system. J. Am. Ceram. Soc. 2010, 86, 1338–1344. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Guo, L.; Yang, Y.P.; Guo, H.B.; Zhang, H.J.; Gong, S.K. Influence of Gd2O3 and Yb2O3 co-doping on phase stability, thermo-physical properties and sintering of 8YSZ. Chin. J. Aeronaut. 2012, 25, 948–953. [Google Scholar] [CrossRef]


| Processing | Parameters |
|---|---|
| Powders | Gd2O3 |
| Ball-to-powder ratio in weight | 10:1 |
| Grinding ball | Agate ball |
| Wetting agent | sodium stearate (1 wt.%) |
| Rotation rate | 120 revolutions per minute |
| Time | 20 h |
| Processing | Parameters |
|---|---|
| Pressure | 200 MPa |
| Keeping time | 5 min |
| Sinter temperature | 1600 °C, 1500 °C, 1400 °C |
| Sinter times | 6, 12, 24 h |
| Bulks | 1400 °C | 1500 °C | 1600 °C |
|---|---|---|---|
| 6 h | 17.9% | 7.3% | 0.9% |
| 12 h | 8.6% | 3.8% | 0.4% |
| 24 h | 4.5% | 2.5% | 0.2% |
| Bulks | 1400 °C (g/cm3) | 1500 °C (g/cm3) | 1600 °C (g/cm3) |
|---|---|---|---|
| 6 h | 6.086 | 6.867 | 7.334 |
| 12 h | 6.765 | 7.128 | 7.377 |
| 24 h | 7.071 | 7.228 | 7.394 |
| Bulks | 1400 °C | 1500 °C | 1600 °C |
|---|---|---|---|
| 6 h | 79.15% | 89.21% | 95.38% |
| 12 h | 87.98% | 92.71% | 95.94% |
| 24 h | 91.96% | 94.01% | 96.16% |
| Bulks | 1400 °C | 1500 °C | 1600 °C |
|---|---|---|---|
| 6 h | 10.91 ± 1.21 | 12.75 ± 1.54 | 14.97 ± 1.32 |
| 12 h | 12.34 ± 1.33 | 13.84 ± 1.42 | 15.02 ± 1.43 |
| 24 h | 13.50 ± 1.28 | 14.03 ± 1.37 | 15.03 ± 1.56 |
| Crystal Phase | Lattice (Å) | Space Group | Wykoff Coordinates | Angle |
|---|---|---|---|---|
| M-Gd2O3 | a = 14.095 b = 3.5765 c = 8.7692 | C2/M | Gd (0.25,0.25,0) O (0,0,0) O (0.5,0,0) | α = γ = 90° β = 100.08° |
| C-Gd2O3 | a = b = c = 10.813 | IA-3 | Gd (0.25,0,0) O (0.50,0.50,0.50) | α = β = γ = 90° |
| Bulks | Hardness (GPa) | Elastic Modulus (GPa) | Fracture Toughness (MPa·m0.5) | Thermal Conductivity W/(m·k) | Thermal Expansion Coefficient (at 1100 °C) |
|---|---|---|---|---|---|
| Gd2O3 | 9.13 | 201.15 | 15.03 | 2.75 (at 1100 °C) | 6.69 × 10−6/°C |
| ZrO2 | 12.0 [31] | 210 [31] | 6.0 [31] | 3.0 (at RT) [30] | 9 × 10−6/K [30] |
| 8YSZ | 13 [31] | 230 [32] | 5.1 [33] | 1.85 (at 1000 °C) [16] | 10 × 10−6/K [34] |
| 6GdSZ | 10 [35] | 200 [35] | —— | 1.5 (at 1100 °C) [36] | 11.5 × 10−6/K [36] |
| 15 wt%Gd2O3-GYYZO | 15.61 [26] | 306.88 [26] | 7.822 [26] | 1.04 (at 1100 °C) [26] | 7.89 × 10−6/K [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, P.-H.; Jin, C.; Zeng, S.-C.; Xie, R.-G.; Zhang, B.; Chen, B.-Y.; Yang, Z.; Guo, Y.-C.; Liang, M.-X.; Li, J.-P.; et al. Microstructure and Properties of Densified Gd2O3 Bulk. Materials 2022, 15, 7793. https://doi.org/10.3390/ma15217793
Gao P-H, Jin C, Zeng S-C, Xie R-G, Zhang B, Chen B-Y, Yang Z, Guo Y-C, Liang M-X, Li J-P, et al. Microstructure and Properties of Densified Gd2O3 Bulk. Materials. 2022; 15(21):7793. https://doi.org/10.3390/ma15217793
Chicago/Turabian StyleGao, Pei-Hu, Can Jin, Sheng-Cong Zeng, Rui-Guang Xie, Bo Zhang, Bai-Yang Chen, Zhong Yang, Yong-Chun Guo, Min-Xian Liang, Jian-Ping Li, and et al. 2022. "Microstructure and Properties of Densified Gd2O3 Bulk" Materials 15, no. 21: 7793. https://doi.org/10.3390/ma15217793
APA StyleGao, P.-H., Jin, C., Zeng, S.-C., Xie, R.-G., Zhang, B., Chen, B.-Y., Yang, Z., Guo, Y.-C., Liang, M.-X., Li, J.-P., Zhang, L.-N., Yan, Z.-Y., Jia, L., & Zhao, D. (2022). Microstructure and Properties of Densified Gd2O3 Bulk. Materials, 15(21), 7793. https://doi.org/10.3390/ma15217793









