Thermal Diffusivity Characteristics of the IN718 Alloy Tested with the Modified Pulse Method
Abstract
1. Introduction
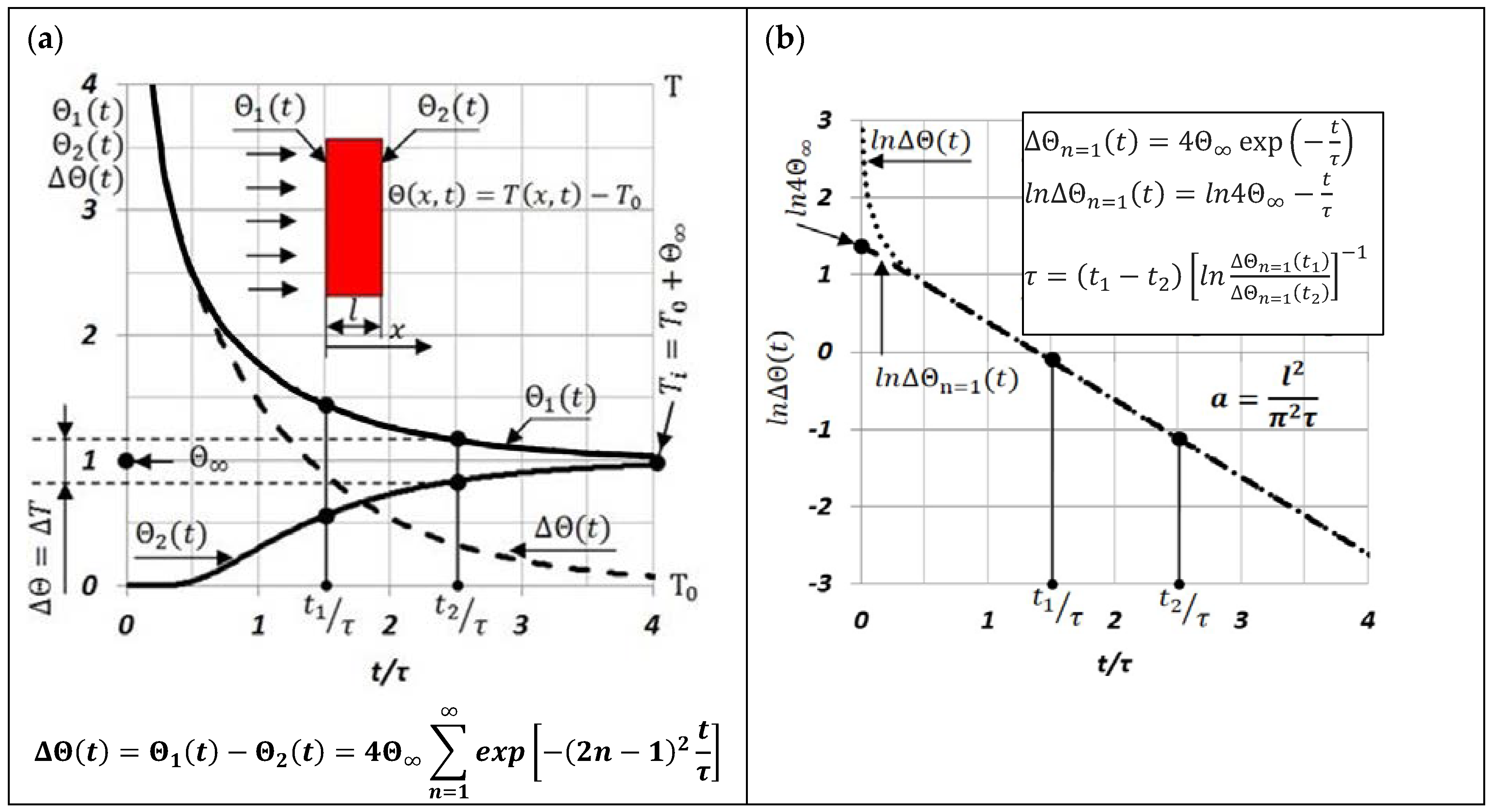
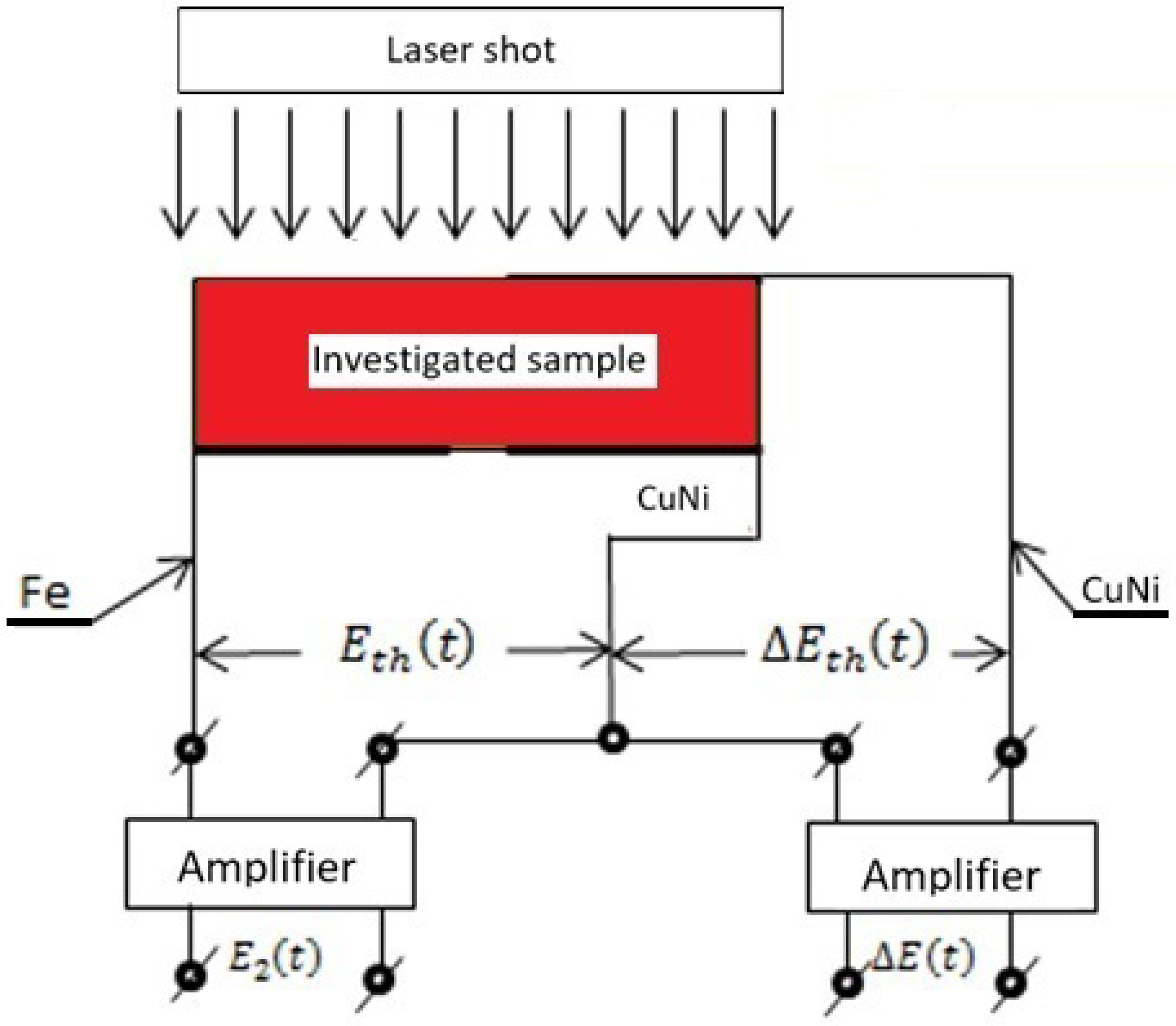
2. Experiments
- The Seebeck coefficient of the differential thermocouple “CuNi–sample–CuNi” is determined from the dependence ;
- The Seebeck coefficient of the thermocouple “Fe–CuNi” is determined from the dependence ;
- If the variations of and are minor, then and are constant values;
- The gain factor of both amplifiers was constant and amounted to .
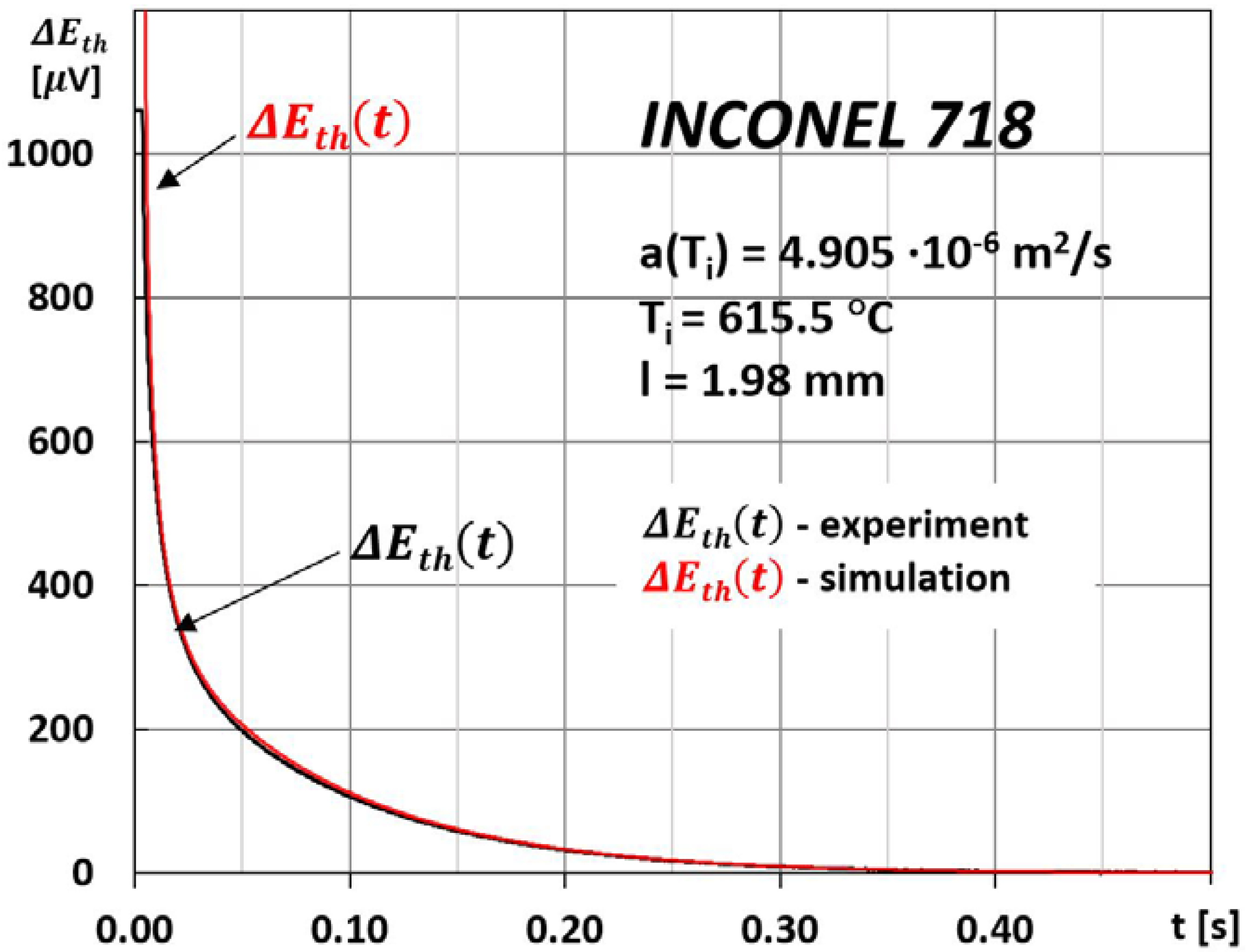
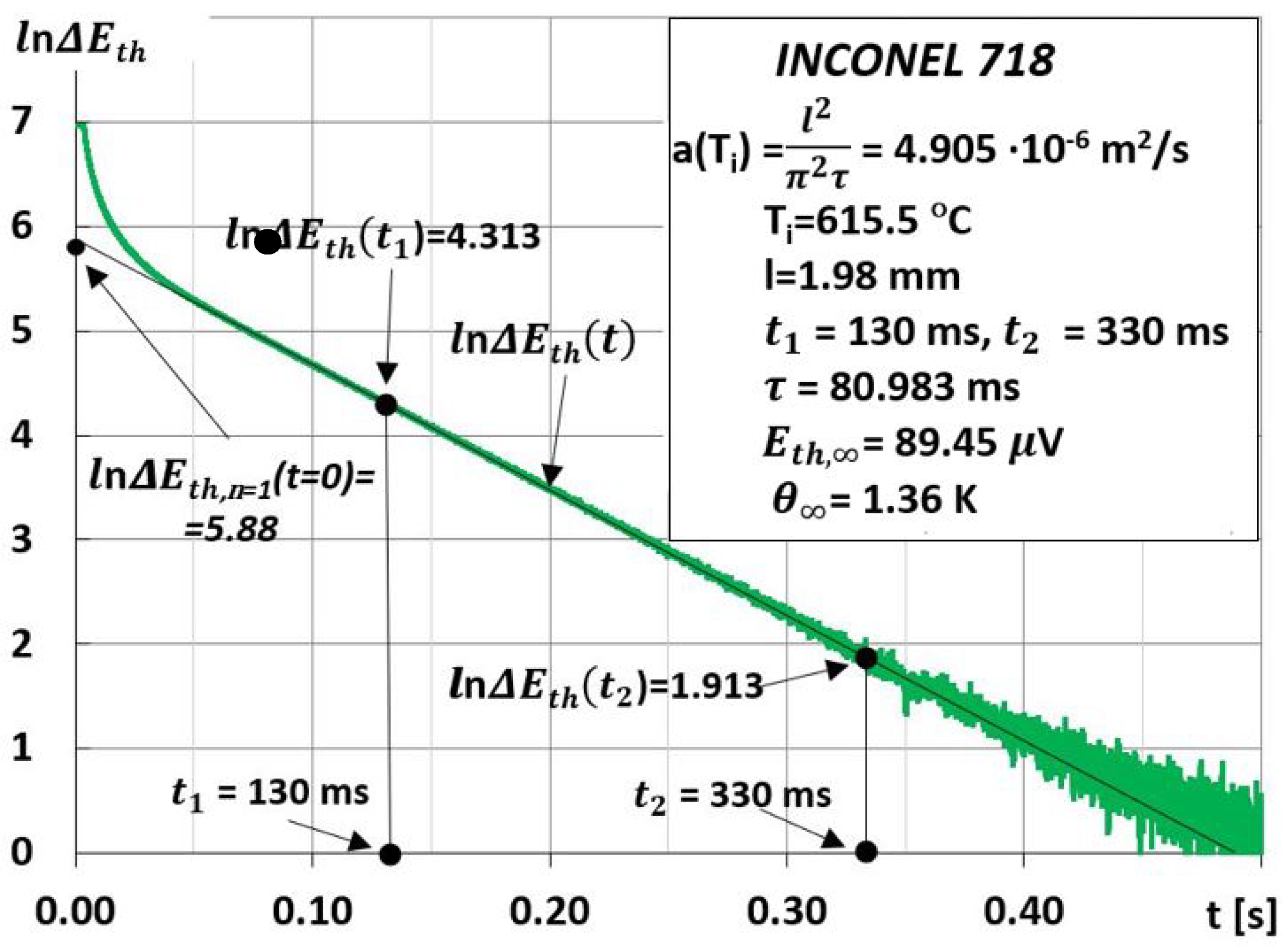
3. Measurements
4. Discussion
5. Conclusions
- A significant advantage of the MPM method is the use of the results of measuring the temperature difference between the extreme surfaces of the test sample, after a laser shot in its face, to determine the characteristic time , and thus the thermal diffusivity .
- The use of this method of measuring , and thus and , allows one to eliminate even components from these measurement signals (n = 2, 4, 6,...).
- Therefore, there is a significant expansion of the time interval in which we can treat the logarithm of the function , , and as linearly dependent on;
- A relatively small error in determining the thermal diffusivity by the MPM method, which depends mainly on the accuracy of the measurements of the thickness of the tested sample, and the characteristic time τ. In this case, the error was estimated to be less than 3%;
- Precise determination of the discrete temperature value in which is measured as well as averaging the temperature range of this value ; where (see Figure 1a);
- A simple way to determine the ratio of temperature differences between the extreme surfaces of the tested sample by replacing it with . At the same time, the values of and are taken from the experimentally recorded changes of , as shown in Figure 4. Additionally, for small, several-degree changes in temperature in the time interval between and depending on , it can be assumed that ;
- Due to the differential measurement of the thermal Emf in the “CuNi–sample–CuNi” system, the harmful influence of external electromagnetic fields on the measurement signal was eliminated.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meola, C.; Carlomagno, G.M. Recent advances in the use of infrared thermography. Meas. Sci. Technol. 2004, 15, 27–58. [Google Scholar] [CrossRef]
- Erdoğdu, F. A review on simultaneous determination of thermal diffusivity and heat transfer coefficient. J. Food Eng. 2006, 86, 453–459. [Google Scholar] [CrossRef]
- Huang, L.; Liu, L.-S. Simultaneous determination of thermal conductivity and thermal diffusivity of food and agricultural materials using a transient plane-source method. J. Food Eng. 2009, 95, 179–185. [Google Scholar] [CrossRef]
- Hadi, S.; Nishitani, M.; Wijayanta, A.T.; Kurata, K.; Takamatsu, H. Measurement of Thermal Conductivity and Thermal Diffusivity of Solid Materials Using a Novel Stamp Sensor: A Feasibility Study with Numerical Analysis. J. Therm. Sci. Technol. 2012, 7, 536–548. [Google Scholar] [CrossRef][Green Version]
- San Martin, C.; Torres, C.; Esparza, D.; Bonilla, D. Thermal diffusivity measurements of spherical samples using active infrared thermography. Infrared Phys. Technol. 2012, 55, 469–474. [Google Scholar] [CrossRef]
- Cernuschi, F.; Bison, P.G.; Marinetti, S.; Figari, A.; Lorenzoni, L.; Grinzato, E. Comparison of thermal diffusivity measurement techniques. In Proceedings of the 6th International Conference on Quantitative InfraRed Thermography, Dubrovnik, Croatia, 24–27 September 2002; pp. 211–221. [Google Scholar] [CrossRef]
- Cape, J.A.; Lehman, G.W. Temperature and Finite Pulse-Time Effects in the Flash Method for Measuring Thermal Diffusivity. J. Appl. Phys. 1963, 34, 1909. [Google Scholar] [CrossRef]
- Glavina, M.Y.; Scala, K.D.; Ansorena, R.; Valle, C.E. Estimation of thermal diffusivity of foods using transfer functions. LWT-Food Sci. Technol. 2006, 39, 455–459. [Google Scholar] [CrossRef]
- Baba, T.; Ono, A. Improvement of the laser flash method to reduce uncertainty in thermal diffusivity measurements. Meas. Sci. Technol. 2001, 12, 2046. [Google Scholar] [CrossRef]
- Maglic, K.D.; Cezairliyan, A.; Peletsky, V.E. Compendium of Thermophysical Property Measurement Methods—Volume 2: Recommended Measurement Techniques and Practices; Springer Science and Business Media: New York, NY, USA, 1992. [Google Scholar]
- Vălu, O.S.; Staicu, D.; Beneš, O.; Konings, R.J.M.; Lajarge, P. Heat capacity, thermal conductivity and thermal diffusivity of uranium–americium mixed oxides. J. Alloys Compd. 2014, 614, 144–150. [Google Scholar] [CrossRef]
- Reza, A.; Zayachuk, Y.; Yu, H.; Hofmann, F. Transient grating spectroscopy of thermal diffusivity degradation in deuterium implanted tungsten. Scr. Mater. 2020, 174, 6–10. [Google Scholar] [CrossRef]
- Bellucci, S.; Bovesecchi, G.; Cataldo, A.; Coppa, P.; Corasaniti, S.; Potenza, M. Transmittance and Reflectance Effects during Thermal Diffusivity Measurements of GNP Samples with the Flash Method. Materials 2019, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Magliĉ, K.D. Compendium of Thermophysical Property Measurement Method; Magliĉ, K.D., Ed.; Plenum Press: New York, NY, USA, 1984; pp. 299–454. [Google Scholar]
- Vozár, L.; Hohenauer, W. Flash method of measuring the thermal diffusivity, a review. In Proceedings of the 16th European Conference on Thermophysical Properties, Imperial College, London, UK, 1–4 September 2002; pp. 1–25. [Google Scholar]
- Hemberger, F.; Ebert, H.P.; Fricke, J. Determination of the Local Thermal Diffusivity of Inhomogeneous Samples by a Modified Laser-Flash Method. Int. J. Thermophys. 2007, 28, 1509–1521. [Google Scholar] [CrossRef]
- Laskar, J.M.; Bagavathiappan, S.; Sardar, M.; Jayakumar, T.; Philip, J.; Raj, B. Measurement of thermal diffusivity of solids using infrared thermography. Mater. Lett. 2008, 62, 2740–2742. [Google Scholar] [CrossRef]
- Martínez, K.; Marín, E.; Glorieux, C.; Lara-Bernal, A.; Calderón, A.; Peña Rodríguez, G.; Ivanov, R. Thermal diffusivity measurements in solids by photothermal infrared radiometry: Influence of convection–radiation heat losses. Int. J. Therm. Sci. 2015, 98, 202–207. [Google Scholar] [CrossRef]
- Massard, H.; Pinto, C.S.C.; Couto, P.; Orlande, H.R.B.; Cotta, R.M. Analysis of flash method physical models for the measurement of thermal diffusivity of solid materials. In Proceedings of the 10th Brazilian Congress of Thermal Sciences and Engineering—ENCIT 2004, Rio de Janeiro, Brazil, 29 November–3 December 2004. Paper CIT04-0537. [Google Scholar]
- Woodfield, P.L.; Monde, M.; Mitsutake, Y. On estimating thermal diffusivity using analytical inverse solution for unsteady one-dimensional heat conduction. Int. J. Heat Mass Trans. 2007, 50, 1202–1205. [Google Scholar] [CrossRef]
- Parker, W.J.; Jenkins, R.J.; Butler, C.P.; Abbot, G.L. Flash Method of Determining Thermal Diffusivity, Heat Capacity, and Thermal Conductivity. J. Appl. Phys. 1961, 32, 1679–1684. [Google Scholar] [CrossRef]
- Ukrainczyk, N. Thermal diffusivity estimation using numerical inverse solution for 1D heat conduction. Int. J. Heat Mass Trans. 2009, 52, 5674–5681. [Google Scholar] [CrossRef]
- Terpiłowski, J.; Rudzki, R.; Szczepaniak, R.; Woroniak, G. Thermal diffusivity investigation of Fe61Ni39, Fe52Ni48 and Fe40Ni60 binary iron–nickel alloys using the modified pulse method. J. Alloys Compd. 2016, 657, 748–754. [Google Scholar] [CrossRef]
- Terpiłowski, J.; Rudzki, R.; Szczepaniak, R.; Woroniak, G. Investigation of thermal diffusivity of Fe80Ni20 alloy by means of modified pulse method. J. Alloys Compd. 2018, 735, 560–568. [Google Scholar] [CrossRef]
- Hu, W.; Huang, Z.; Yu, Q.; Wang, Y.; Jiao, Y.; Lei, C.; Cai, L.; Zhai, H.; Zhou, Y. Ti2AlC triggered in-situ ultrafine TiC/Inconel 718 composites: Microstructure and enhanced properties. J. Mater. Sci. Technol. 2020, 51, 70–78. [Google Scholar] [CrossRef]
- Chamanfar, A.; Sarrat, L.; Jahazi, M.; Asadi, M.; Weck, A.; Koul, A.K. Microstructural characteristics of forged and heat treated Inconel-718 disks. Mater. Des. (1980–2015) 2013, 52, 791–800. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Huang, W.; Jing, G.; Wang, Z.; Zeng, X. Controllable in-situ aging during selective laser melting: Stepwise precipitation of multiple strengthening phases in Inconel 718 alloy. J. Mater. Sci. Technol. 2019, 35, 1925–1930. [Google Scholar] [CrossRef]
- Kang, D.S.; Koizumi, Y.; Yamanaka, K.; Aoyagi, K.; Bian, H.; Chiba, A. Significant impact of yttrium microaddition on high temperature tensile properties of Inconel 713C superalloy. Mater. Lett. 2018, 227, 40–43. [Google Scholar] [CrossRef]
- Anbarasan, N.; Gupta, B.K.; Prakash, S.; Muthukumar, P.; Oyyaravelu, P.; Kumar, R.J.F.; Jerome, S. Effect of Heat Treatment on the Microstructure and Mechanical Properties of Inconel 718. Mater. Today Proc. 2018, 5, 7716–7724. [Google Scholar] [CrossRef]
- Karthik, G.M.; Asghari-Rad, P.; Sathiyamoorthi, P.; Zargaran, A.; Kim, E.S.; Kim, T.S.; Kim, H.S. Architectured multi-metal CoCrFeMnNi-Inconel 718 lamellar composite by high-pressure torsion. Scr. Mater. 2021, 195, 113722. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Guan, Y. Thermodynamics analysis and rapid solidification of laser polished Inconel 718 by selective laser melting. Appl. Surf. Sci. 2020, 511, 145423. [Google Scholar] [CrossRef]
- Rifat, M.; DeMeter, E.C.; Basu, S. Microstructure evolution during indentation of Inconel-718 created by additive manufacturing. Mater. Sci. Eng. A 2020, 781, 139204. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Guo, Q.; Ma, Z.; Li, H.; Liu, Y. Improving creep resistance of nickel-based superalloy Inconel 718 by tailoring gamma double prime variants. Scr. Mater. 2019, 164, 66–70. [Google Scholar] [CrossRef]
- Wan, H.Y.; Luo, Y.W.; Zhang, B.; Song, Z.M.; Wang, L.Y.; Zhou, Z.J.; Li, C.P.; Chen, G.F.; Zhang, G.P. Effects of surface roughness and build thickness on fatigue properties of selective laser melted Inconel 718 at 650 °C. Int. J. Fatigue 2020, 137, 105654. [Google Scholar] [CrossRef]
- Parida, R.P.; Senthilkumar, V. Experimental studies of defect generation in selective laser melted Inconel 718 alloy. Mater. Today Proc. 2021, 39, 1372. [Google Scholar] [CrossRef]
- Ruan, J.J.; Ueshima, N.; Oikawa, K. Growth behavior of the δ-Ni3Nb phase in superalloy 718 and modified KJMA modeling for the transformation-time-temperature diagram. J. Alloys Compd. 2020, 814, 152289. [Google Scholar] [CrossRef]
- Zhao, Y.; Aoyagi, K.; Daino, Y.; Yamanaka, K.; Chiba, A. Significance of powder feedstock characteristics in defect suppression of additively manufactured Inconel 718. Addit. Manuf. 2020, 34, 101277. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Sun, Z.; Lin, J.; Lv, Y.; Xu, B. Microstructural evolution and mechanical properties of Inconel 718 superalloy thin wall fabricated by pulsed plasma arc additive manufacturing. J. Alloys Compd. 2020, 819, 152936. [Google Scholar] [CrossRef]
- Jambor, M.; Bokuvka, O.; Novy, F.; Trsko, L.; Belan, J. Phase Transformations in Nickel base Superalloy Inconel 718 during Cyclic Loading at High Temperature. Prod. Eng. Arch. 2017, 15, 15–18. [Google Scholar] [CrossRef]
- Zhao, Y.; Hao, L.; Zhang, Q.; Xiong, W. Phase transformations during continuous cooling in Inconel 718 alloys manufactured by laser powder bed fusion and suction casting. Mater. Charact. 2022, 185, 111764. [Google Scholar] [CrossRef]
- Terpiłowski, J.; Szczepaniak, R.; Woroniak, G.; Rudzki, R. Adaptation of the modified pulse method for determination of thermal diffusivity of solids in the vicinity of the second-order phase transition point. Arch. Thermodyn. 2013, 34, 73–92. [Google Scholar] [CrossRef]
- Terpiłowski, J.; Jóźwiak, S.; Rudzki, R.; Szczepaniak, R.; Woroniak, G. Investigation of Phase Transformation of Fe65Ni35 Alloy by the Modified Pulse Method. Materials 2020, 13, 3425. [Google Scholar] [CrossRef]
- Terpiłowski, J. A modified flash method for determination of thermal diffusivity in solids. Arch. Thermodyn. 2003, 24, 59–80. [Google Scholar]
- Mills Kenneth, C. Recommended Values of Thermophysical Properties for Selected Commercial Alloys; Woodhead Publishing Ltd.: Cambridge, UK; ASM International: Materials Park, OH, USA, 2002. [Google Scholar]
- Material Property Database. MPDB; v. 7.49; JAHM Software 1 nc, JAHM Software: North Reading, UK, 2012. Available online: www.jahm.com (accessed on 29 April 2022).
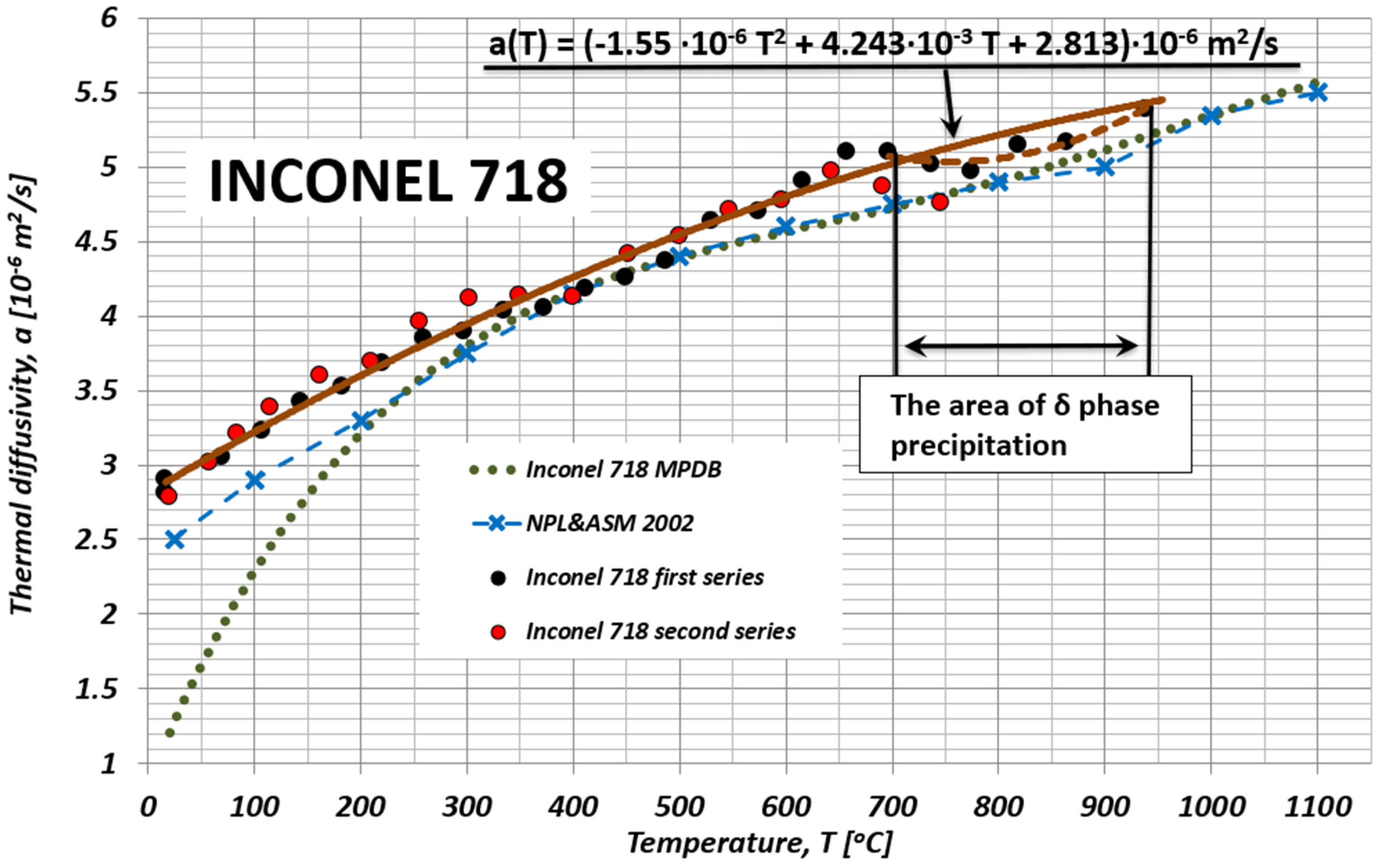
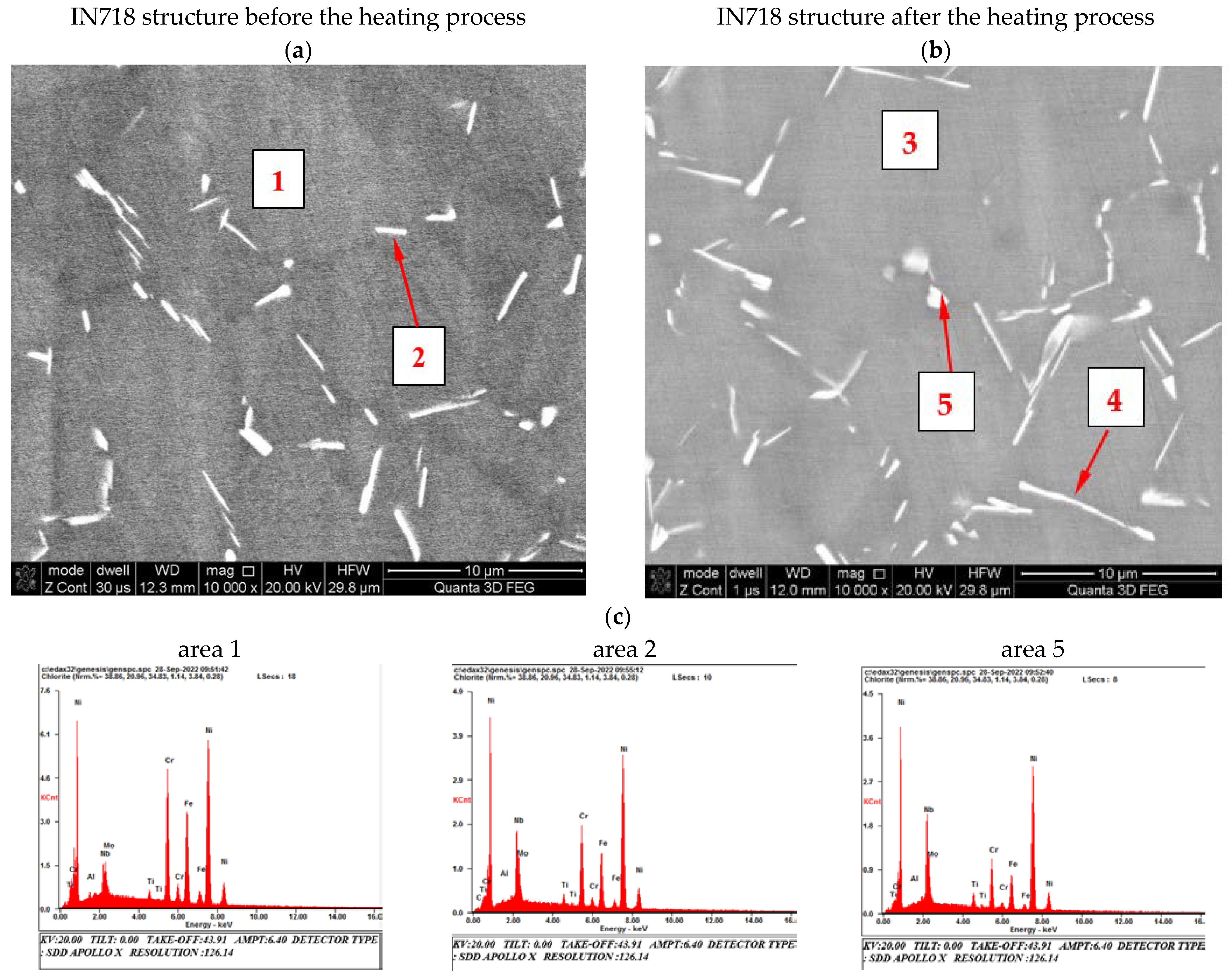
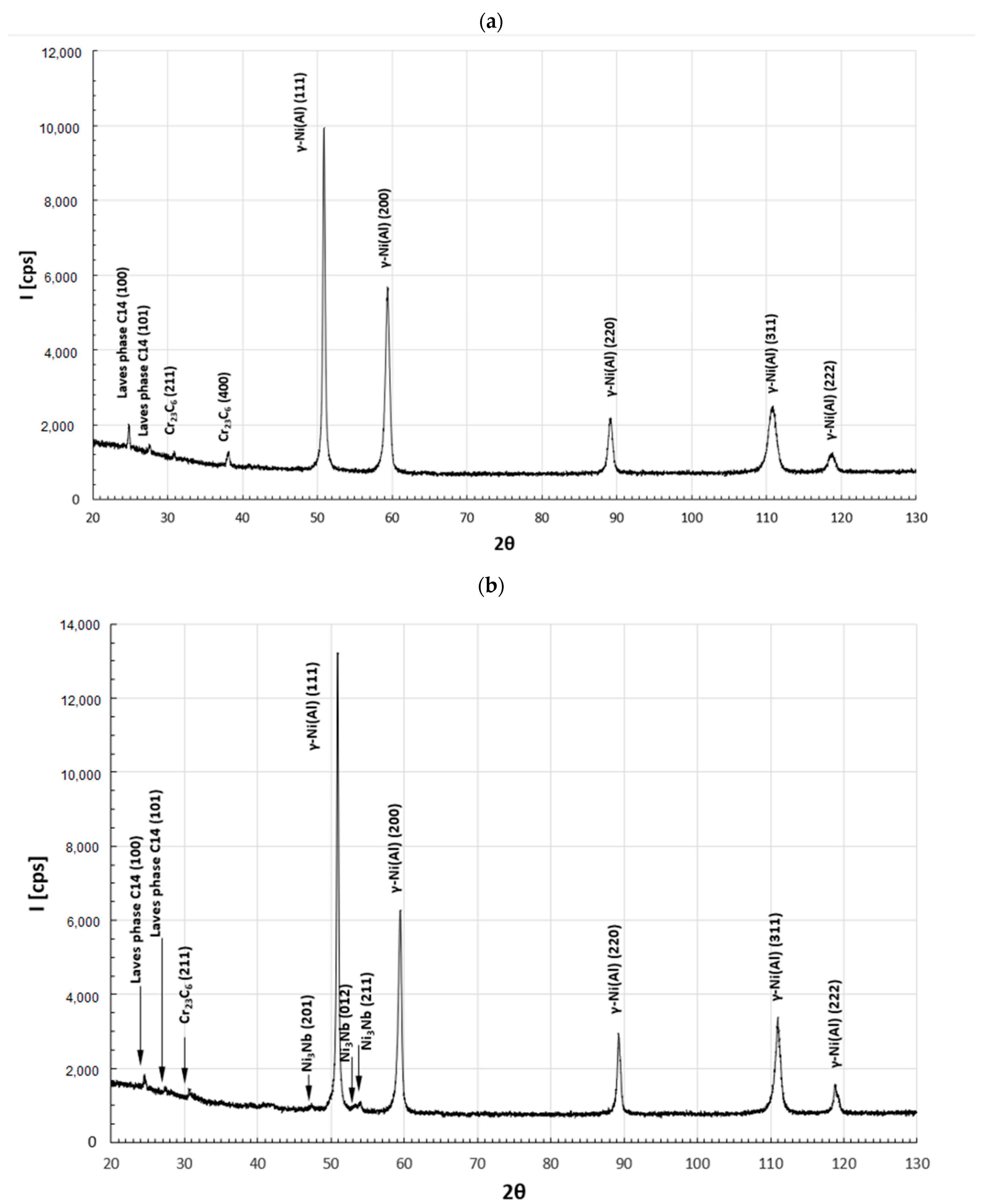
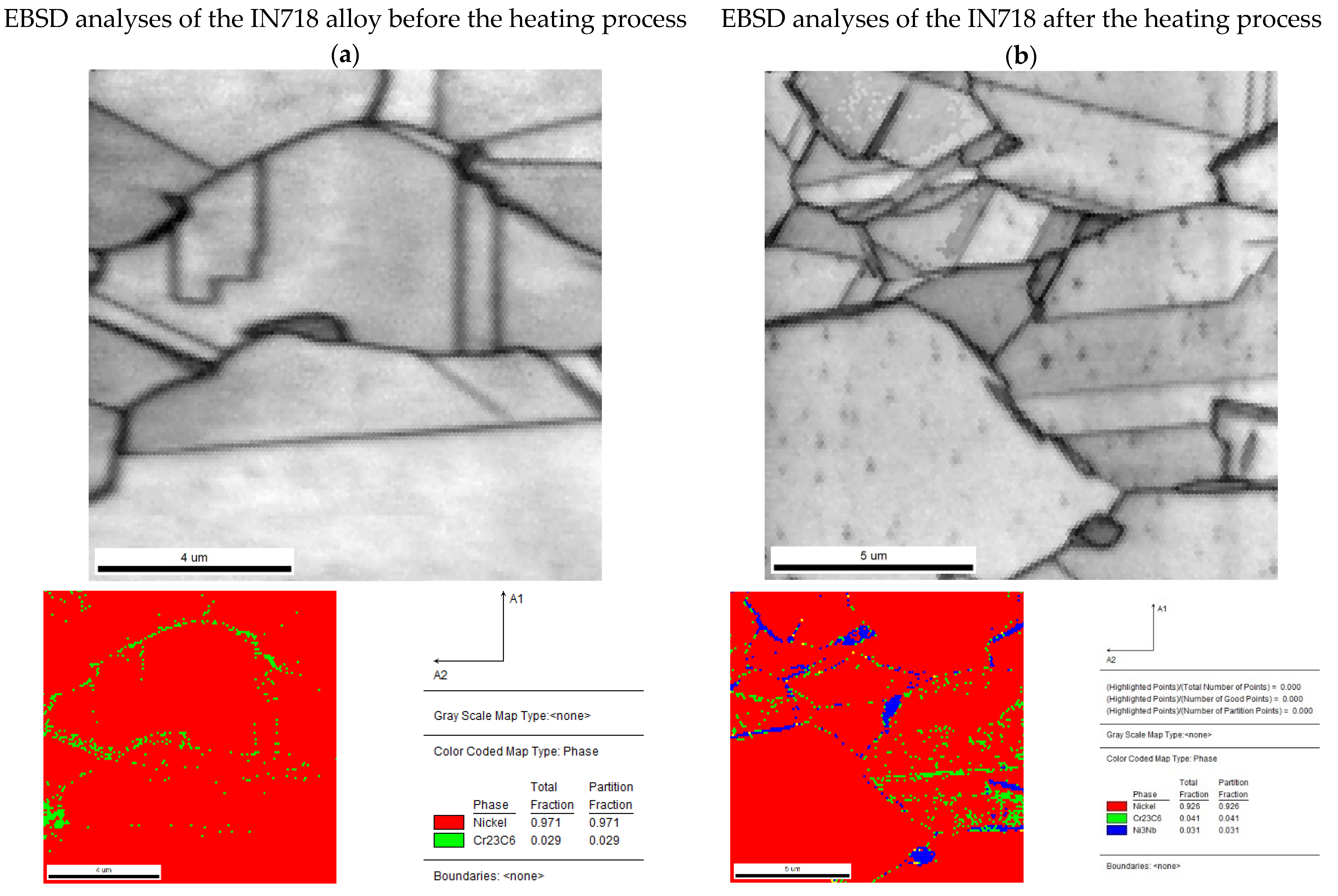
| Element [wt.%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Fe | Cr | Ti | Al | Nb | Mo | Co | Mn | Si | Cu | |
| Investigated sample | 50.77 | 18.25 | 18.28 | 1.03 | 0.83 | 5.69 | 2.83 | 1.22 | 0.37 | 0.81 | - |
| Mills [44] | 52.5 | 16.7 | 19.0 | 0.9 | 0.5 | 5.2 | 3.1 | 1.0 | 0.35 | 0.35 | 0.3 |
| MPDB [45] | 50–55 | 16–20 | 17–21 | 0.65–1.15 | 0.2–0.8 | 4.75–5.5 *) | 2.8–3.3 | max 1.0 | max 0.35 | max 0.35 | max 0.3 |
| Area 1 | Area 2 | Area 3 | Area 4 | Area 5 | |
|---|---|---|---|---|---|
| C | --- | 00.87 | --- | 01.02 | --- |
| Al | 00.52 | 00.42 | 00.47 | 00.41 | --- |
| Nb | 04.49 | 11.35 | 04.22 | 09.81 | 15.38 |
| Mo | 03.01 | 02.96 | 03.00 | 02.64 | 00.06 |
| Ti | 00.83 | 01.39 | 00.83 | 01.41 | 01.79 |
| Cr | 18.00 | 12.56 | 17.78 | 13.72 | 10.10 |
| Fe | 17.84 | 12.39 | 17.88 | 14.02 | 10.58 |
| Ni | 55.31 | 58.06 | 54.87 | 56.97 | 62.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpiłowski, J.; Jóźwiak, S.; Woroniak, G.; Szczepaniak, R. Thermal Diffusivity Characteristics of the IN718 Alloy Tested with the Modified Pulse Method. Materials 2022, 15, 7881. https://doi.org/10.3390/ma15227881
Terpiłowski J, Jóźwiak S, Woroniak G, Szczepaniak R. Thermal Diffusivity Characteristics of the IN718 Alloy Tested with the Modified Pulse Method. Materials. 2022; 15(22):7881. https://doi.org/10.3390/ma15227881
Chicago/Turabian StyleTerpiłowski, Janusz, Stanisław Jóźwiak, Grzegorz Woroniak, and Robert Szczepaniak. 2022. "Thermal Diffusivity Characteristics of the IN718 Alloy Tested with the Modified Pulse Method" Materials 15, no. 22: 7881. https://doi.org/10.3390/ma15227881
APA StyleTerpiłowski, J., Jóźwiak, S., Woroniak, G., & Szczepaniak, R. (2022). Thermal Diffusivity Characteristics of the IN718 Alloy Tested with the Modified Pulse Method. Materials, 15(22), 7881. https://doi.org/10.3390/ma15227881







