Investigation of Topographical Alterations in Titanium-Zirconium-Alloy Implant Threads following Er:YAG Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Selection of the Implants
- SLA® (Sand-blasted, Large-grit, Acid-etched), length 14 mm, diameter 4.8 mm: The surface was sandblasted (grain size 250–500 μm), which produced a macro-roughness of about 20–40 μm between the peaks (peak-to-peak). Due to acid etching, a micro-roughness of about 2–4 μm was achieved. The implant was made purely of titanium. Figure 1 shows an SLA-Standard implant.
- Roxolid®, length 14 mm, diameter 4.8 mm: Roxolid is a titanium-zirconium alloy (85% titanium, 15% zirconium). The zirconium percentage makes the implant appear lighter. Figure 2 shows a Roxolid implant.
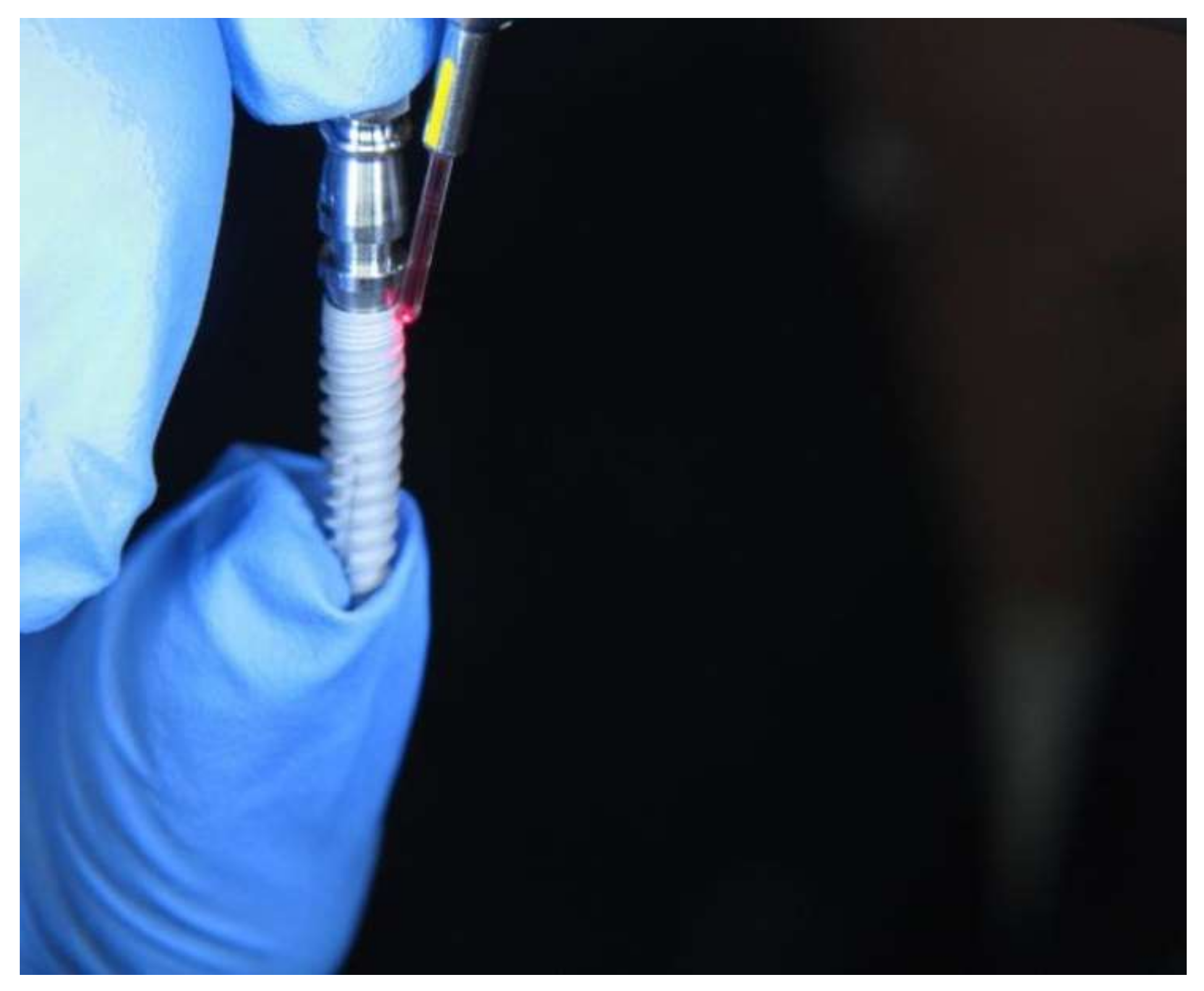
2.2. Laser Application
2.3. Roughness Analysis
- The Profile depth (Pt) (Figure 5)
- Arithmetic average roughness (Ra) (Figure 6), which was calculated according to
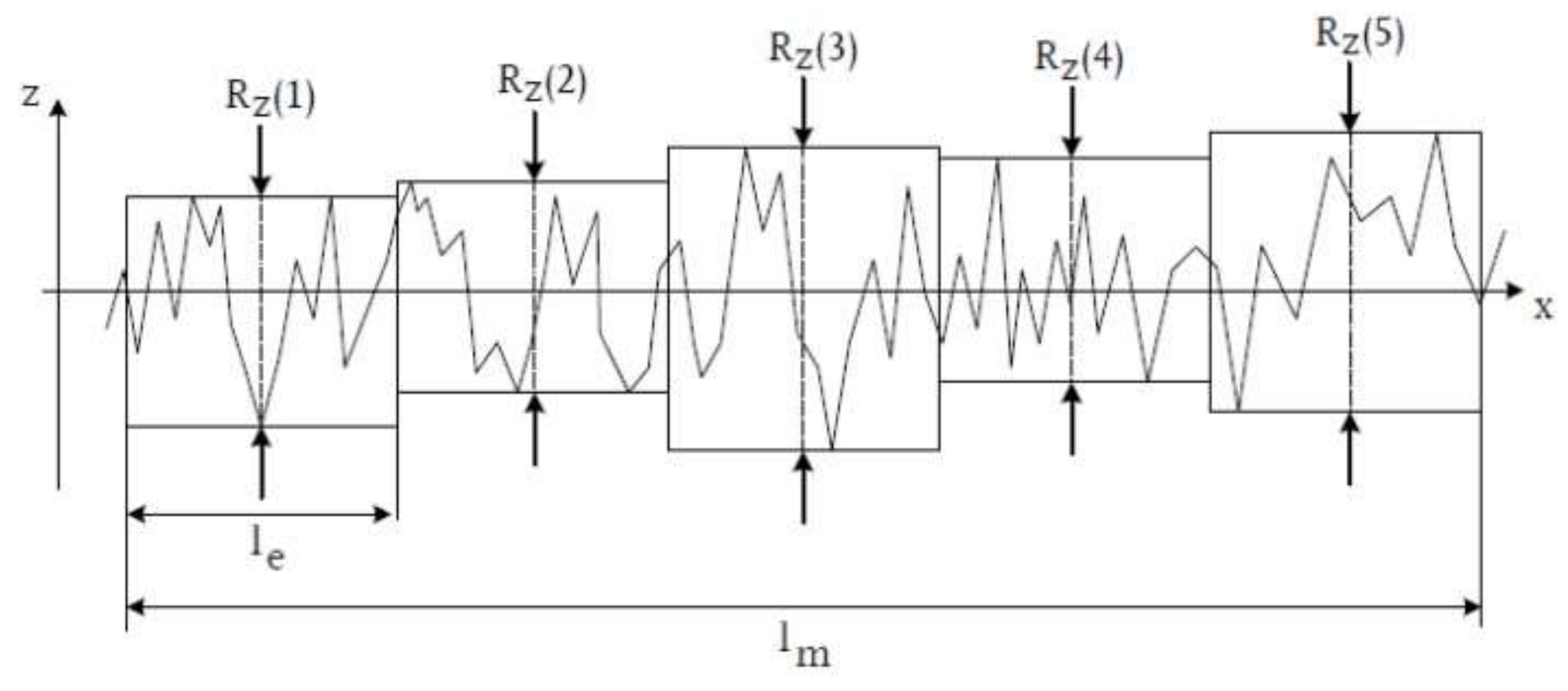
- Mean surface roughness Rz, determined from the arithmetic mean of the individual roughness depths Z1–Z5 of five adjacent individual measuring sections in the roughness profile.


2.4. Statistical Analysis
3. Results
3.1. SLA®
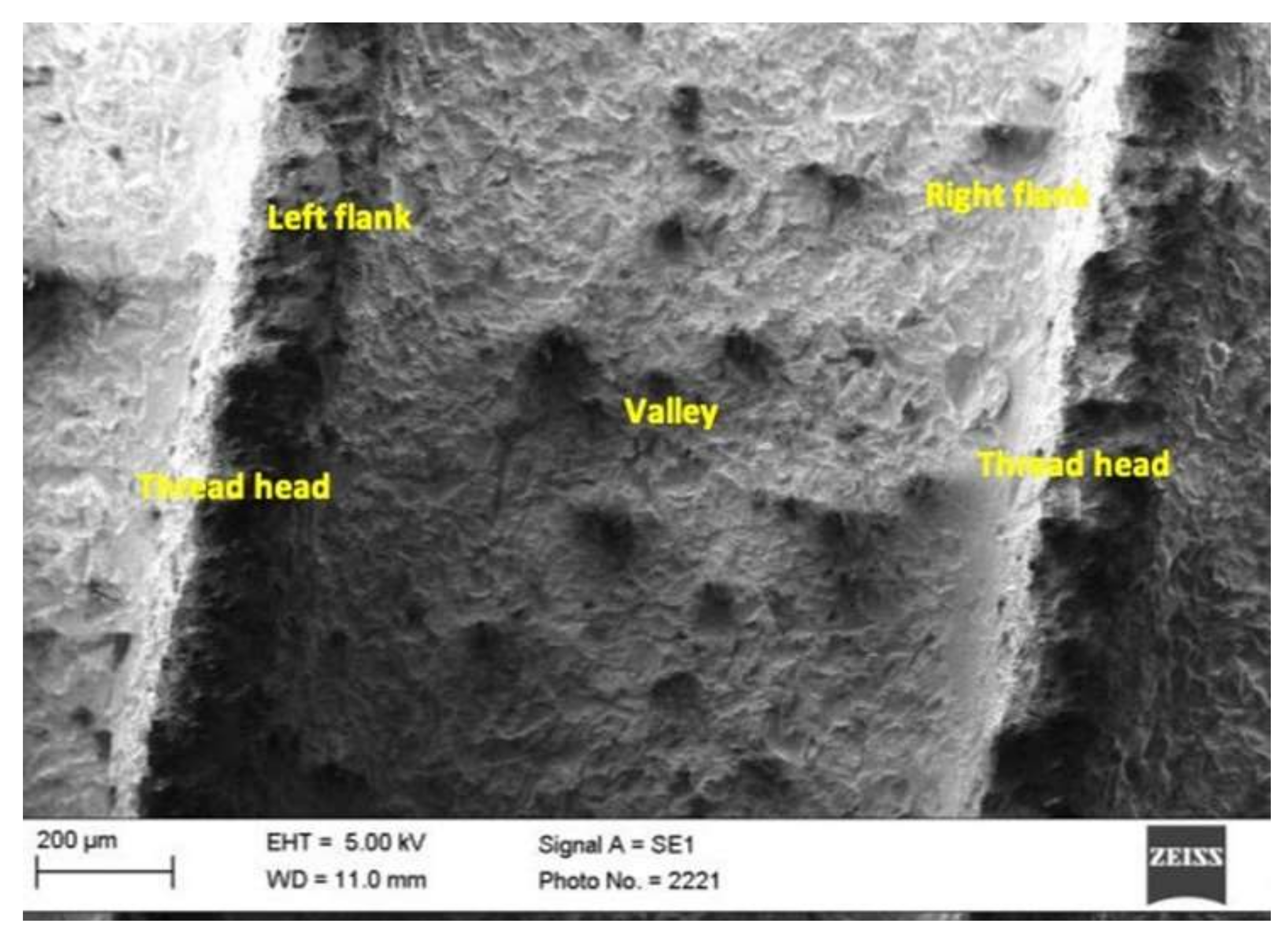
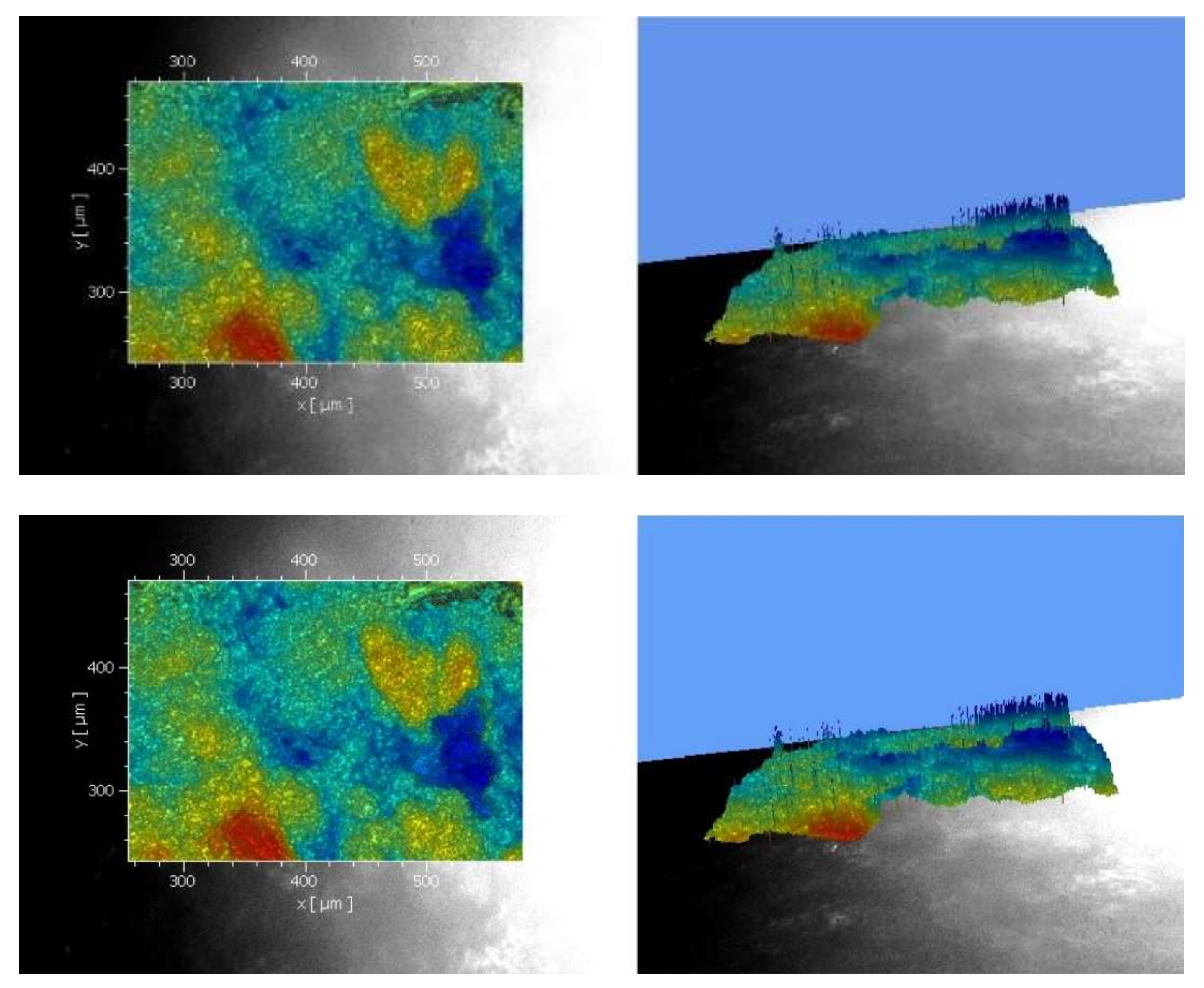
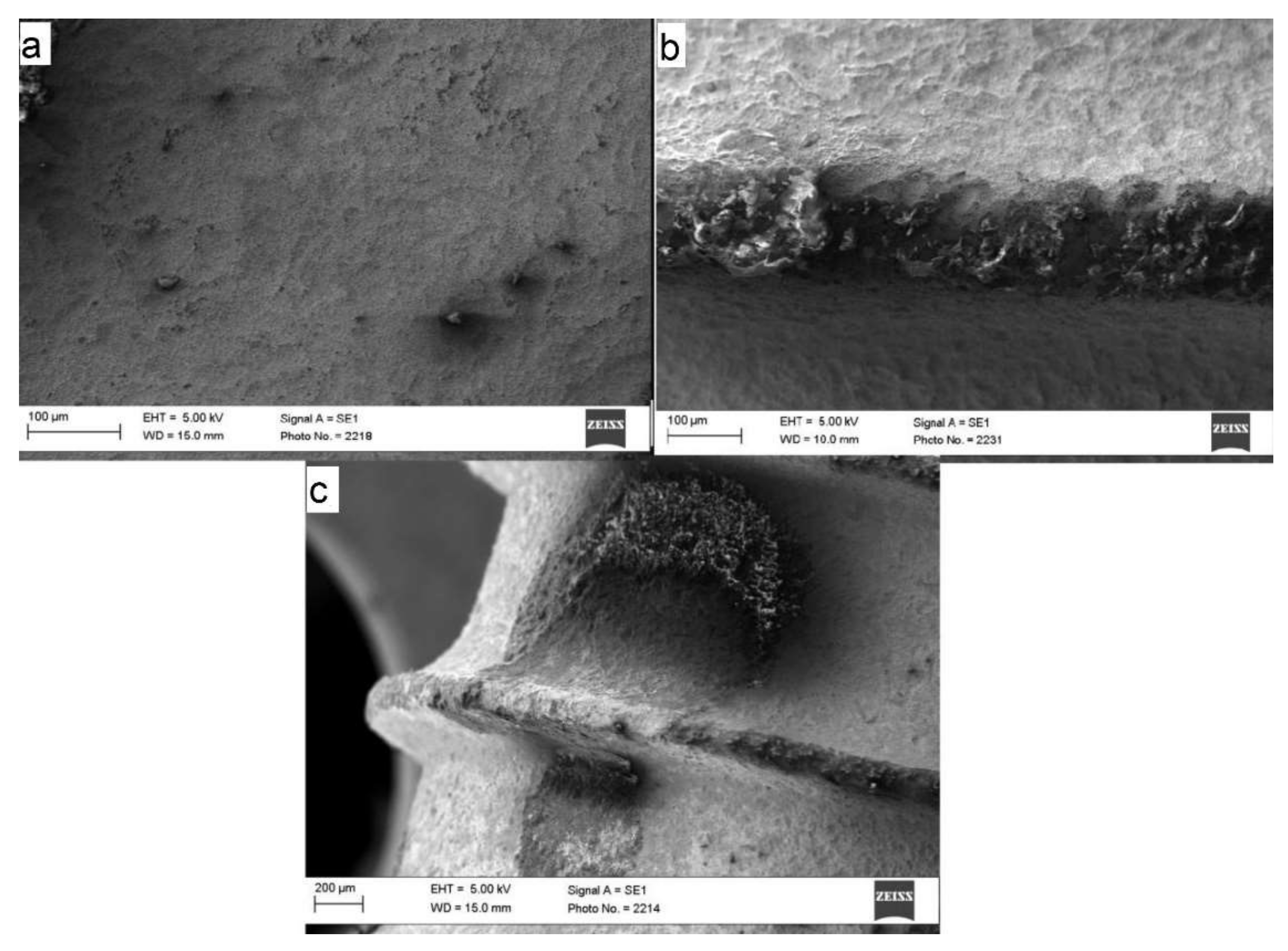
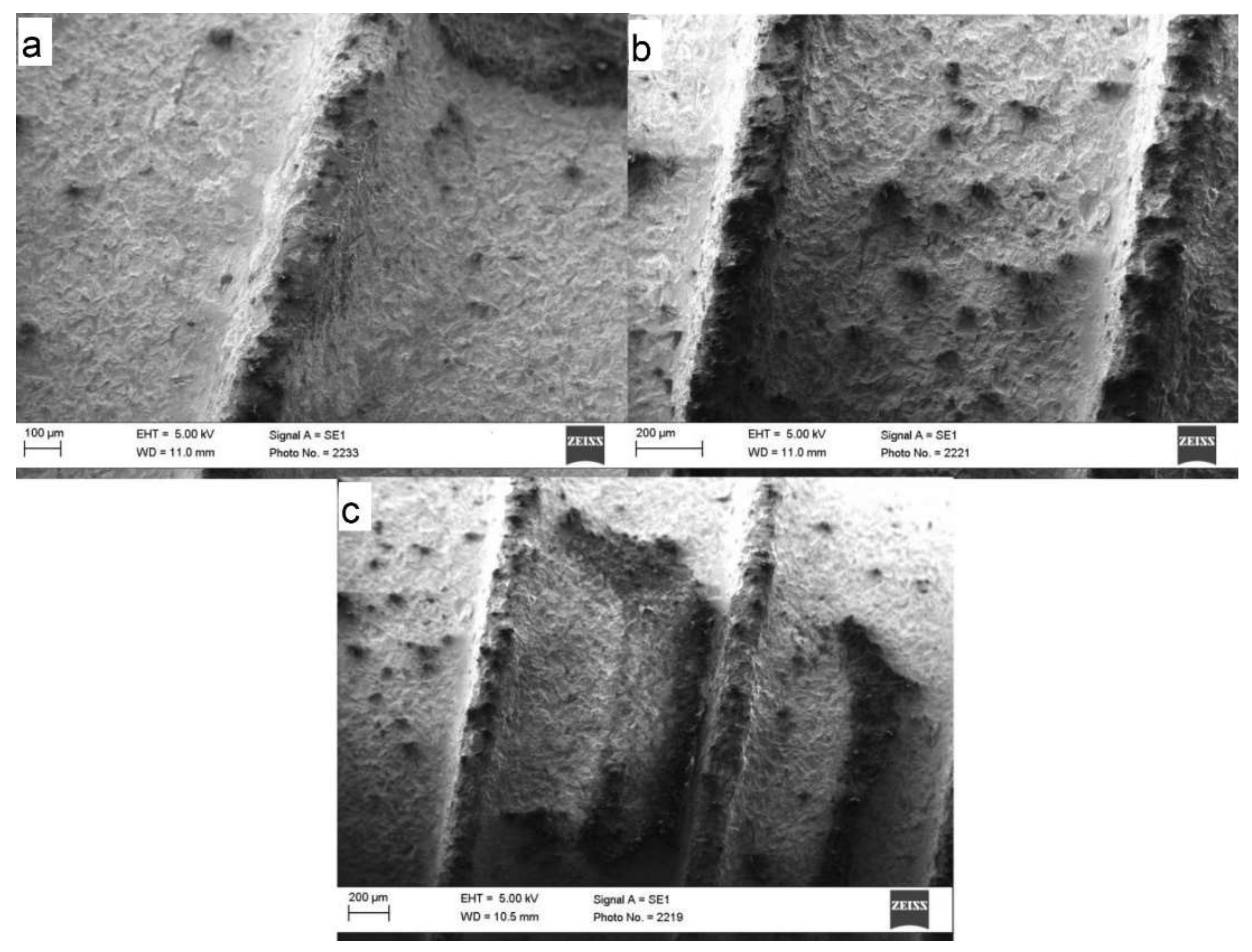
3.2. Scanning Electron Microscope (SEM)
3.3. Roxolid®
3.4. SEM Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Peiodontol. 2015, 42 (Suppl. S16), S152–S157. [Google Scholar] [CrossRef] [PubMed]
- Florke, C.; Janning, J.; Hinrichs, C.; Behrens, E.; Liedtke, K.R.; Sen, S.; Christofzik, D.; Wiltfang, J.; Gulses, A. In-vitro assessment of the efficiency of cold atmospheric plasma on decontamination of titanium dental implants. Int. J. Implant Dent. 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.H.; Suarez Lopez Del Amo, F.; Wang, H.L. Laser therapy for treatment of peri-implant mucositis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 766–782. [Google Scholar] [CrossRef]
- Natto, Z.S.; Aladmawy, M.; Levi, P.A., Jr.; Wang, H.L. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2015, 30, 338–345. [Google Scholar] [CrossRef]
- Fornaini, C.; Merigo, E.; Vescovi, P.; Bonanini, M.; Antonietti, W.; Leoci, L.; Lagori, G.; Meleti, M. Different laser wavelengths comparison in the second-stage implant surgery: An ex vivo study. Lasers Med. Sci. 2015, 30, 1631–1639. [Google Scholar] [CrossRef]
- Nicholson, J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Gülses, A.; Behrens, E.; Aktas, O.C.; Acil, Y.; Wiltfang, J. Provisorische Implantate: Chirurgische Prinzipien und materialwissenschaftliche Aspekte. Implantologie 2022, 3, 291–300. [Google Scholar]
- Ayna, M.; Gülses, A. Adapting a simple surgical manual tool to a 3D printed implantology protocol: The use of a universal screwdriver for fixation of custom-made laser sintered titanium subperiosteal implants. 3D Print. Med. 2022, 8, 31. [Google Scholar] [CrossRef]
- Baltatu, I.; Sandu, A.V.; Vlad, M.D.; Spataru, M.C.; Vizureanu, P.; Baltatu, M.S. Mechanical Characterization and In Vitro Assay of Biocompatible Titanium Alloys. Micromachines 2022, 13, 430. [Google Scholar] [CrossRef]
- Madalina Simona, B.; Vizureanu, P.; Bălan, T.; Lohan, N.; Ţugui, C. Preliminary Tests for Ti-Mo-Zr-Ta Alloys as Potential Biomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012023. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17 (Suppl. S2), 68–81. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Valero, A.; Buitrago-Vera, P.; Sola-Ruiz, M.F.; Ferrer-Garcia, J.C. Decontamination of dental implant surface in peri-implantitis treatment: A literature review. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, e869–e876. [Google Scholar] [CrossRef]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: Radiographic outcome. Clin. Oral Implant. Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Schmit, J.; Pakuła, A. White Light Interferometry. In Handbook of Advanced Nondestructive Evaluation; Ida, N., Meyendorf, N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 421–467. [Google Scholar]
- Park, J.B.; Yang, S.M.; Ko, Y. Evaluation of the Surface Characteristics of Various Implant Abutment Materials Using Confocal Microscopy and White Light Interferometry. Implant Dent. 2015, 24, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Chala, M.; Anagnostaki, E.; Mylona, V.; Chalas, A.; Parker, S.; Lynch, E. Adjunctive Use of Lasers in Peri-Implant Mucositis and Peri-Implantitis Treatment: A Systematic Review. Dent. J. 2020, 8, 68. [Google Scholar] [CrossRef]
- Keller, U.; Hibst, R. Experimental studies of the application of the Er:YAG laser on dental hard substances: II. Light microscopic and SEM investigations. Lasers Surg. Med. 1989, 9, 345–351. [Google Scholar] [CrossRef]
- Yan, M.; Liu, M.; Wang, M.; Yin, F.; Xia, H. The effects of Er:YAG on the treatment of peri-implantitis: A meta-analysis of randomized controlled trials. Lasers Med. Sci. 2015, 30, 1843–1853. [Google Scholar] [CrossRef]
- Li, L.; Deng, J.; Ren, S. The clinical efficacy of Er:YAG lasers in the treatment of peri-implantitis: A systematic review and meta-analysis. Ann. Palliat. Med. 2021, 10, 9002–9014. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Konstantinidis, I.; Karoussis, I.K.; Ma, X.; Chu, H. Systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J. Periodontol. 2014, 85, 1203–1213. [Google Scholar] [CrossRef]
- Grandin, H.M.; Berner, S.; Dard, M. A Review of Titanium Zirconium (TiZr) Alloys for Use in Endosseous Dental Implants. Materials 2012, 5, 1348–1360. [Google Scholar] [CrossRef]
- Schwarz, F.; Bieling, K.; Nuesry, E.; Sculean, A.; Becker, J. Clinical and histological healing pattern of peri-implantitis lesions following non-surgical treatment with an Er:YAG laser. Lasers Surg. Med. 2006, 38, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Rothamel, D.; Sculean, A.; Georg, T.; Scherbaum, W.; Becker, J. Effects of an Er:YAG laser and the Vector ultrasonic system on the biocompatibility of titanium implants in cultures of human osteoblast-like cells. Clin. Oral Implant. Res. 2003, 14, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Aoki, A.; Oda, S.; Yoneyama, T.; Ishikawa, I. Effects of the Er:YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J. Clin. Laser Med. Surg. 2003, 21, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.I.; Min, H.K.; Park, B.H.; Kwon, Y.H.; Park, J.B.; Herr, Y.; Heo, S.J.; Chung, J.H. The effect of Er:YAG laser irradiation on the scanning electron microscopic structure and surface roughness of various implant surfaces: An in vitro study. Lasers Med. Sci. 2011, 26, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.R. Titanium and Zirconia Particle Release from Implant Surfaces during Er: YAG Laser Decontamination. Master’s Thesis, Temple University, Philadelphia, PA, USA, 2021. [Google Scholar]
- Zhang, Y.; Yu, W.; Jiang, X.; Lv, K.; Sun, S.; Zhang, F. Analysis of the cytotoxicity of differentially sized titanium dioxide nanoparticles in murine MC3T3-E1 preosteoblasts. J. Mater. Sci. Mater. Med. 2011, 22, 1933–1945. [Google Scholar] [CrossRef]
- Wang, C.W.; Ashnagar, S.; Gianfilippo, R.D.; Arnett, M.; Kinney, J.; Wang, H.L. Laser-assisted regenerative surgical therapy for peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef]
- Kreisler, M.; Gotz, H.; Duschner, H. Effect of Nd:YAG, Ho:YAG, Er:YAG, CO2, and GaAIAs laser irradiation on surface properties of endosseous dental implants. Int. J. Oral Maxillofac. Implant. 2002, 17, 202–211. [Google Scholar]
- Deppe, H.; Ahrens, M.; Behr, A.V.; Marr, C.; Sculean, A.; Mela, P.; Ritschl, L.M. Thermal effect of a 445 nm diode laser on five dental implant systems: An in vitro study. Sci. Rep. 2021, 11, 20174. [Google Scholar] [CrossRef]
- Sikora, C.; Alfaro, M.; Yuan, J.; Barao, V.; Sukotjo, C.; Mathew, M. Wear and Corrosion Interactions at the Titanium/Zirconia Interface: Dental Implant Application: Tribocorrosion of Implant and Abutment Materials. J. Prosthodont. 2018, 27, 842–852. [Google Scholar] [CrossRef]
- Fahlstedt, P.; Bunaes, D.F.; Lie, S.A.; Leknes, K.N. Dental implant surface temperatures following double wavelength (2780/940 nm) laser irradiation in vitro. Clin. Exp. Dent. Res. 2021, 7, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 2015, 68, 217–269. [Google Scholar] [CrossRef] [PubMed]




| Measuring Range | Head | Valley | Flank (R) | Flank (L) |
|---|---|---|---|---|
| Thread “3” untreated | 2.38 | 1.52 | 1.25 | 1.73 |
| Thread “3” treated with 160 mJ | 1.87 * | 1.53 | 1.18 | 1.42 * |
| Thread “4” untreated | 2.01 | 1.88 | 1.52 | 1.4 |
| Thread “4” treated with 180 mJ | 1.78 * | 1.65 * | 1.32 * | 1.35 |
| Thread “5” untreated | 1.71 | 1.78 | 1.59 | 1.64 |
| Thread “5” treated with 200 mJ | 1.33 * | 1.27 * | 1.23 * | 1.71 |
| Thread “6” untreated | 1.66 | 1.74 | 1.5 | 1.82 |
| Thread “6” treated with 250 mJ | 1.07 * | 1.44 * | 1.52 | 1.86 |
| Measuring Range | Head | Valley | Flank (R) | Flank (L) |
|---|---|---|---|---|
| Thread “3” untreated | 2.14 | 1.65 | 1.69 | 1.62 |
| Thread “3” treated with 160 mJ | 1.75 * | 1.65 | 1.59 | 1.50 |
| Thread “4” untreated | 2.32 | 1.79 | 1.32 | 1.55 |
| Thread “4” treated with 180 mJ | 1.78 * | 1.68 | 1.23 | 1.48 |
| Thread “5” untreated | 2.1 | 2.08 | 1.54 | 1.22 |
| Thread “5” treated with 200 mJ | 1.45 * | 1.99 | 1.22 * | 1.01 * |
| Thread “6” untreated | 2.08 | 1.88 | 1.72 | 1.21 |
| Thread “6” treated with 250 mJ | 1.84 * | 1.58 * | 1.58 * | 0.7 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayna, M.; Spille, J.; Açil, Y.; Weitkamp, J.-T.; Wiltfang, J.; Esen, C.; Gülses, A. Investigation of Topographical Alterations in Titanium-Zirconium-Alloy Implant Threads following Er:YAG Irradiation. Materials 2022, 15, 7889. https://doi.org/10.3390/ma15227889
Ayna M, Spille J, Açil Y, Weitkamp J-T, Wiltfang J, Esen C, Gülses A. Investigation of Topographical Alterations in Titanium-Zirconium-Alloy Implant Threads following Er:YAG Irradiation. Materials. 2022; 15(22):7889. https://doi.org/10.3390/ma15227889
Chicago/Turabian StyleAyna, Mustafa, Johannes Spille, Yahya Açil, Jan-Tobias Weitkamp, Jörg Wiltfang, Cemal Esen, and Aydin Gülses. 2022. "Investigation of Topographical Alterations in Titanium-Zirconium-Alloy Implant Threads following Er:YAG Irradiation" Materials 15, no. 22: 7889. https://doi.org/10.3390/ma15227889
APA StyleAyna, M., Spille, J., Açil, Y., Weitkamp, J.-T., Wiltfang, J., Esen, C., & Gülses, A. (2022). Investigation of Topographical Alterations in Titanium-Zirconium-Alloy Implant Threads following Er:YAG Irradiation. Materials, 15(22), 7889. https://doi.org/10.3390/ma15227889






