Design and Fabrication of Mature Engineered Pre-Cardiac Tissue Utilizing 3D Bioprinting Technology and Enzymatically Crosslinking Hydrogel
Abstract
1. Introduction
2. Materials and Methods
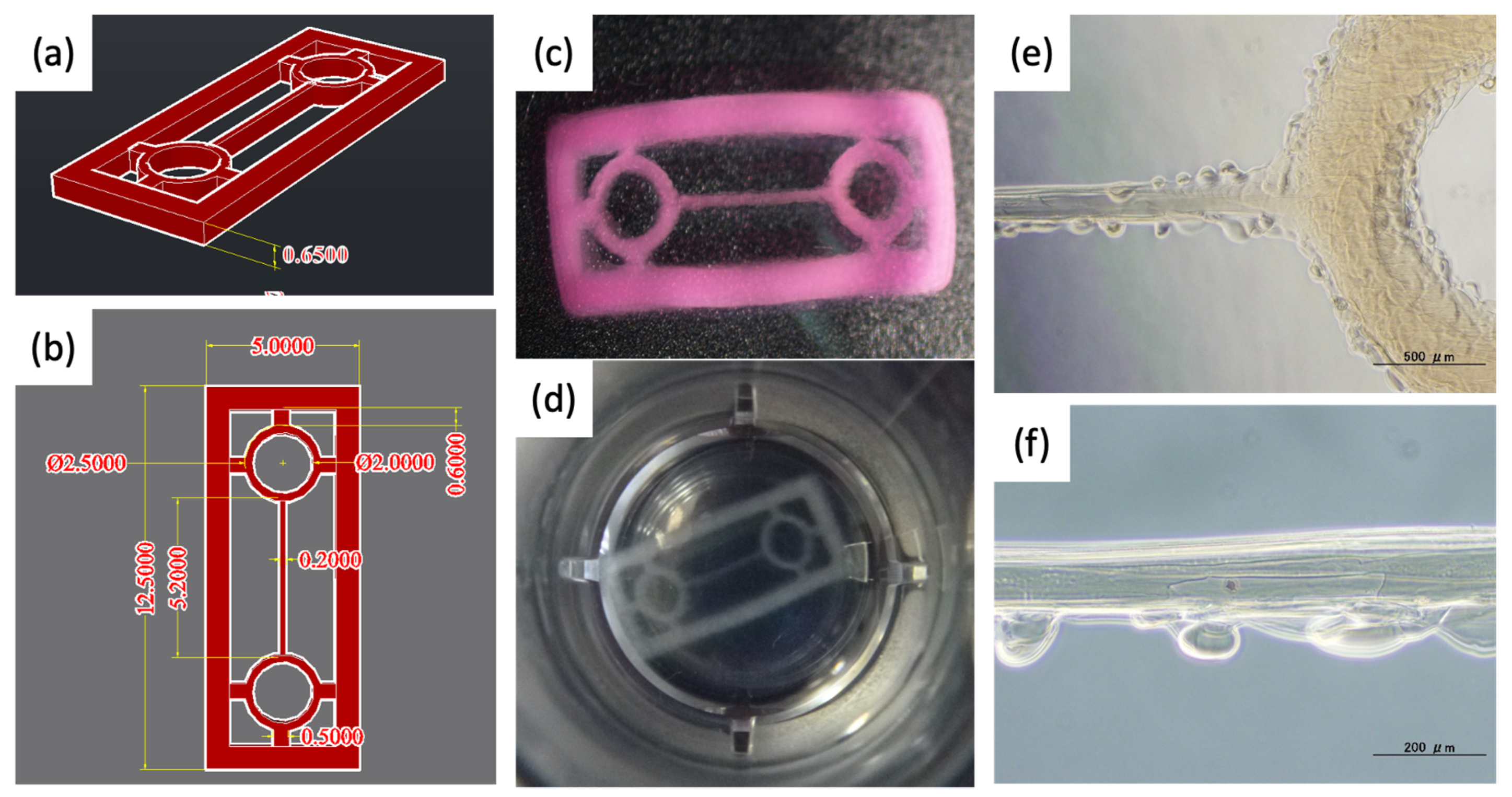
2.1. Designing a Scaffold for a Component of Cardiac Tissues
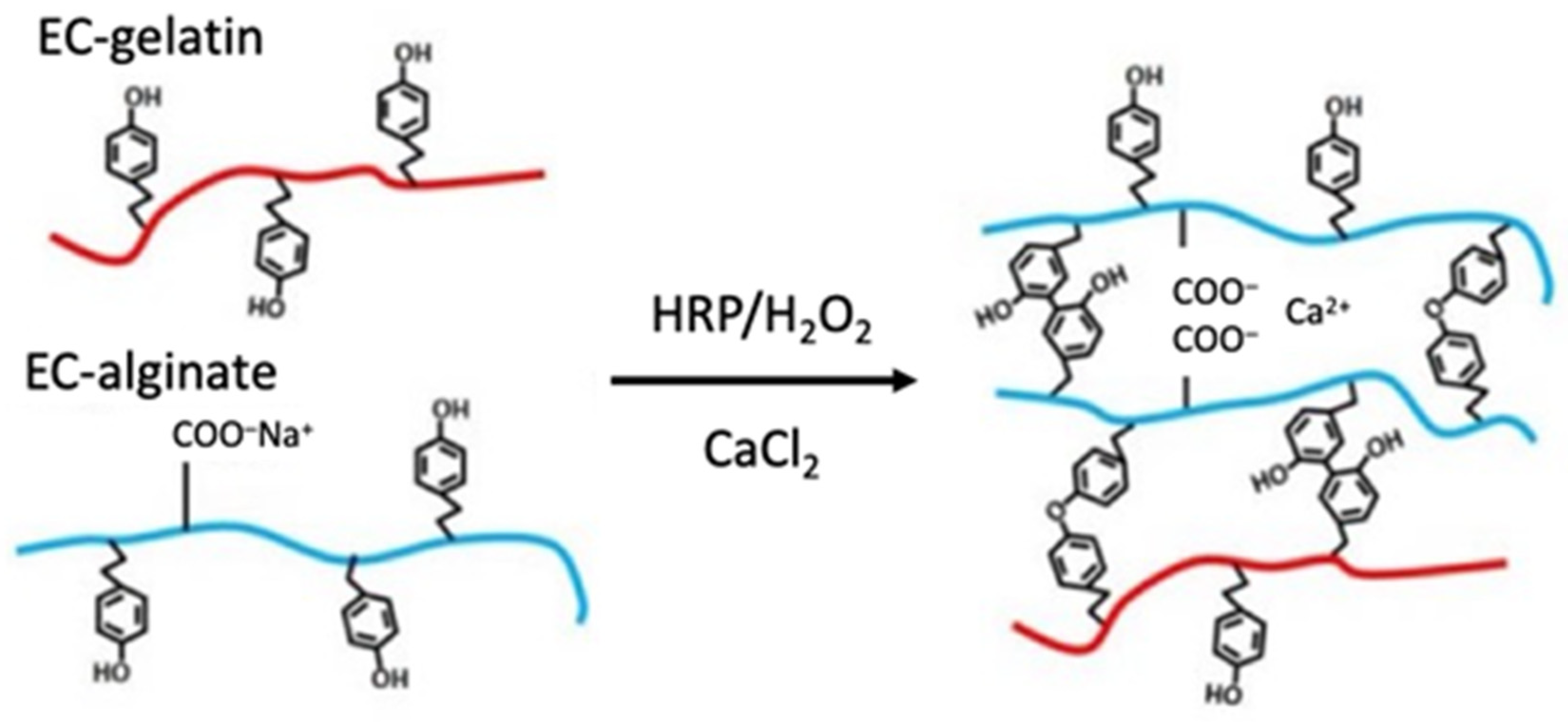
2.2. Synthesis of Enzymatically Crosslinking Alginate and Gelatin
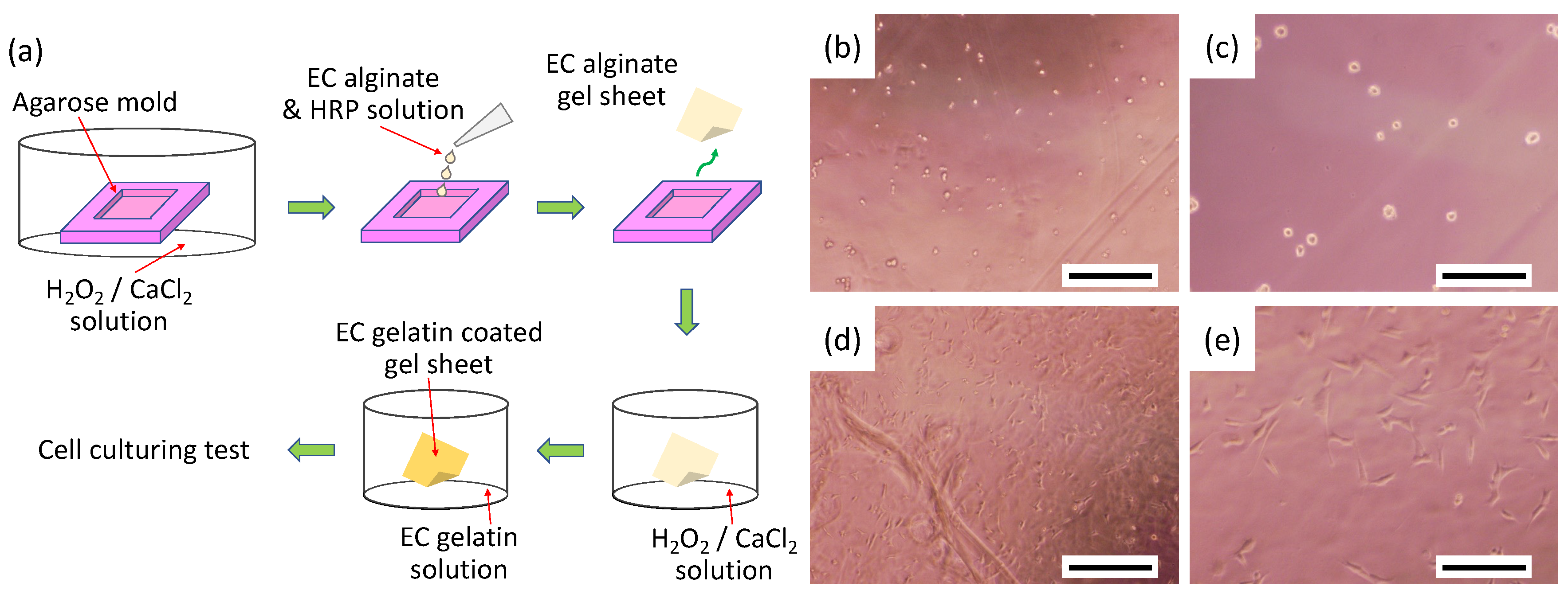
2.3. Cell Adhesion Property onto EC Alginate Hydrogel Sheets Covered with EC Gelatin
2.4. Fabrication and Pre-Treatment of Scaffolds Using a 3D Bioprinter
2.5. Primary Culture of Neonatal Rat Cardiomyocytes
2.6. Fabrication and Observation of Engineered Cardiac Tissues
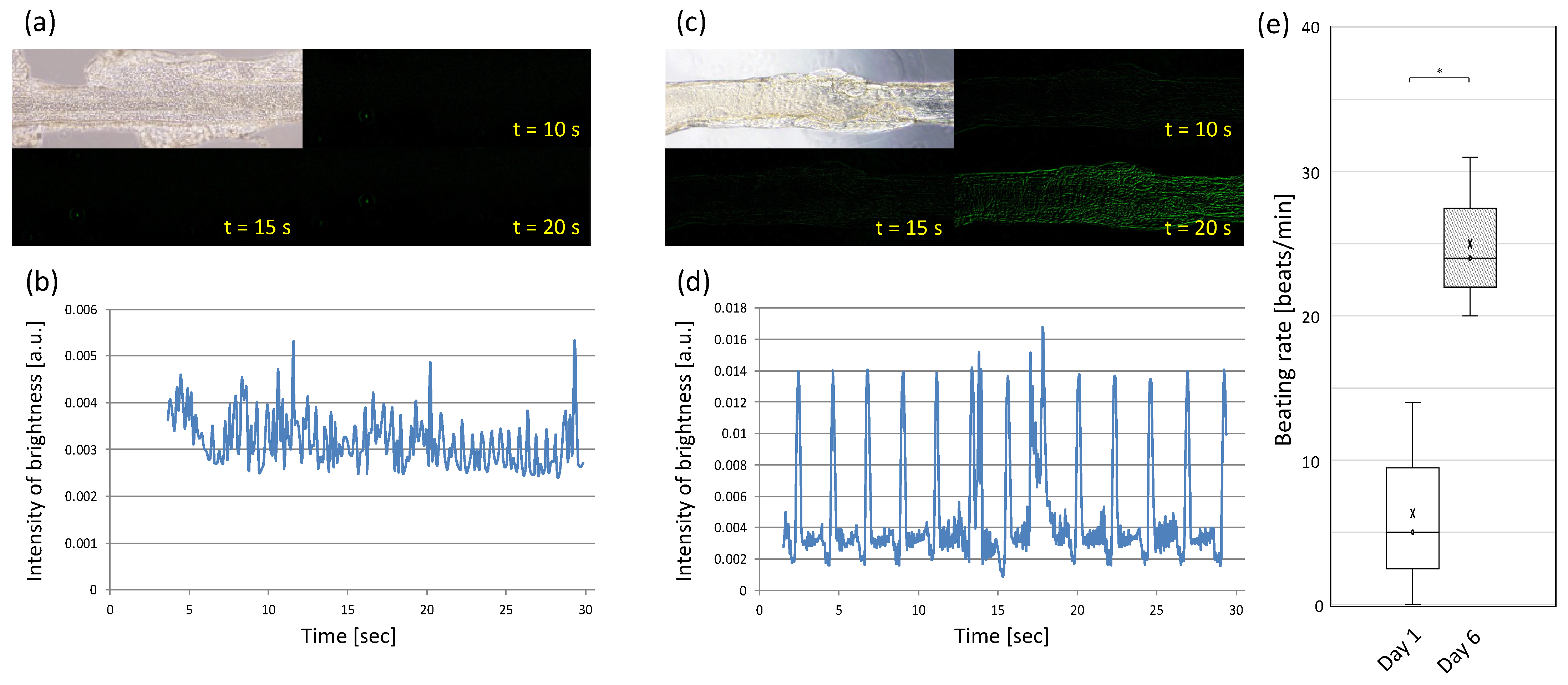
2.7. Drug Testing and Ca Imaging of Pre-Cardiac Tissues
3. Results and Discussion
3.1. Preparation of Biomaterial Inks
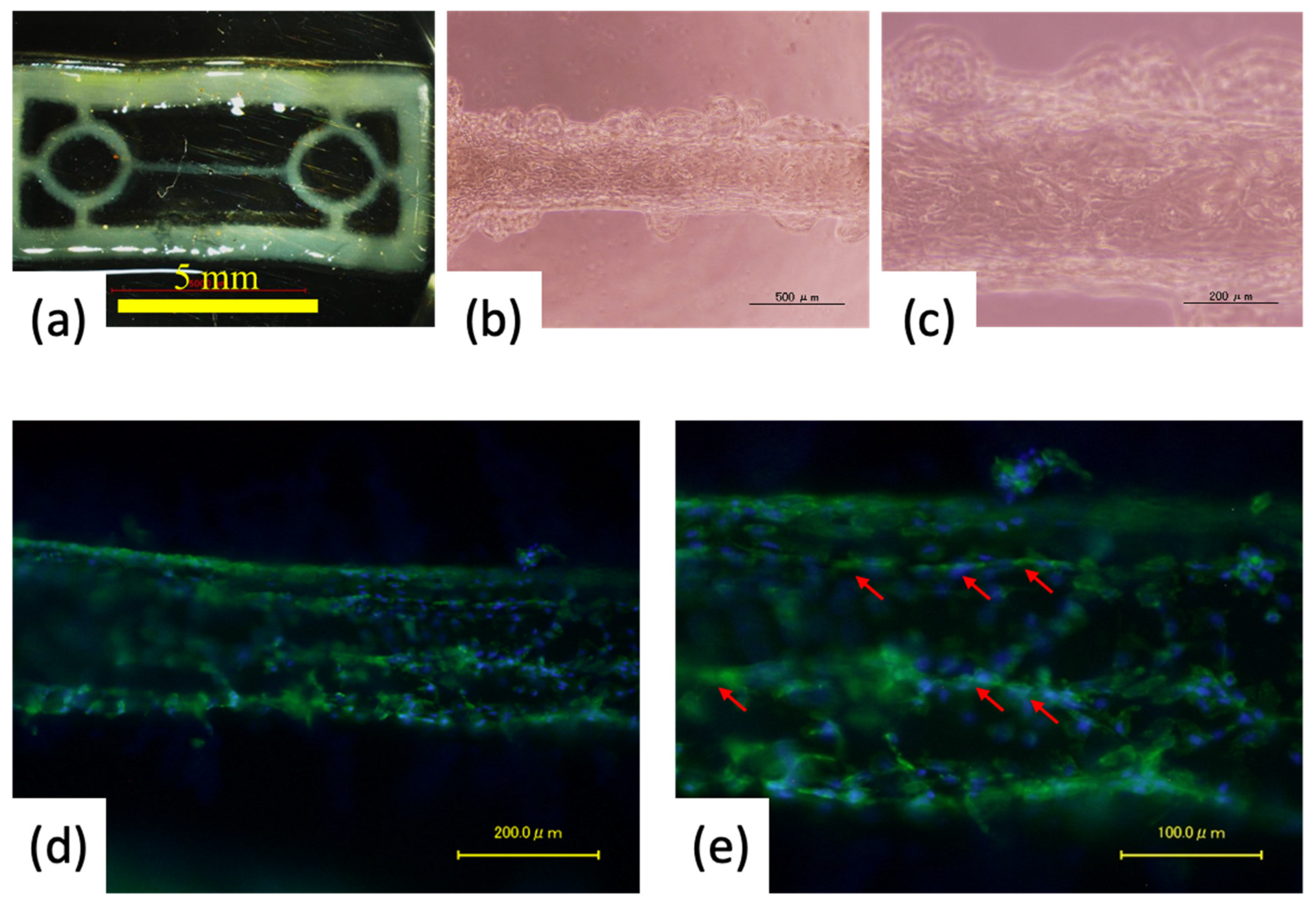
3.2. Fabrication of Pre-Cardiac Tissues Utilizing Acellular Scaffolds
3.3. Cell Orientation onto the Fiber-Shaped Scaffold
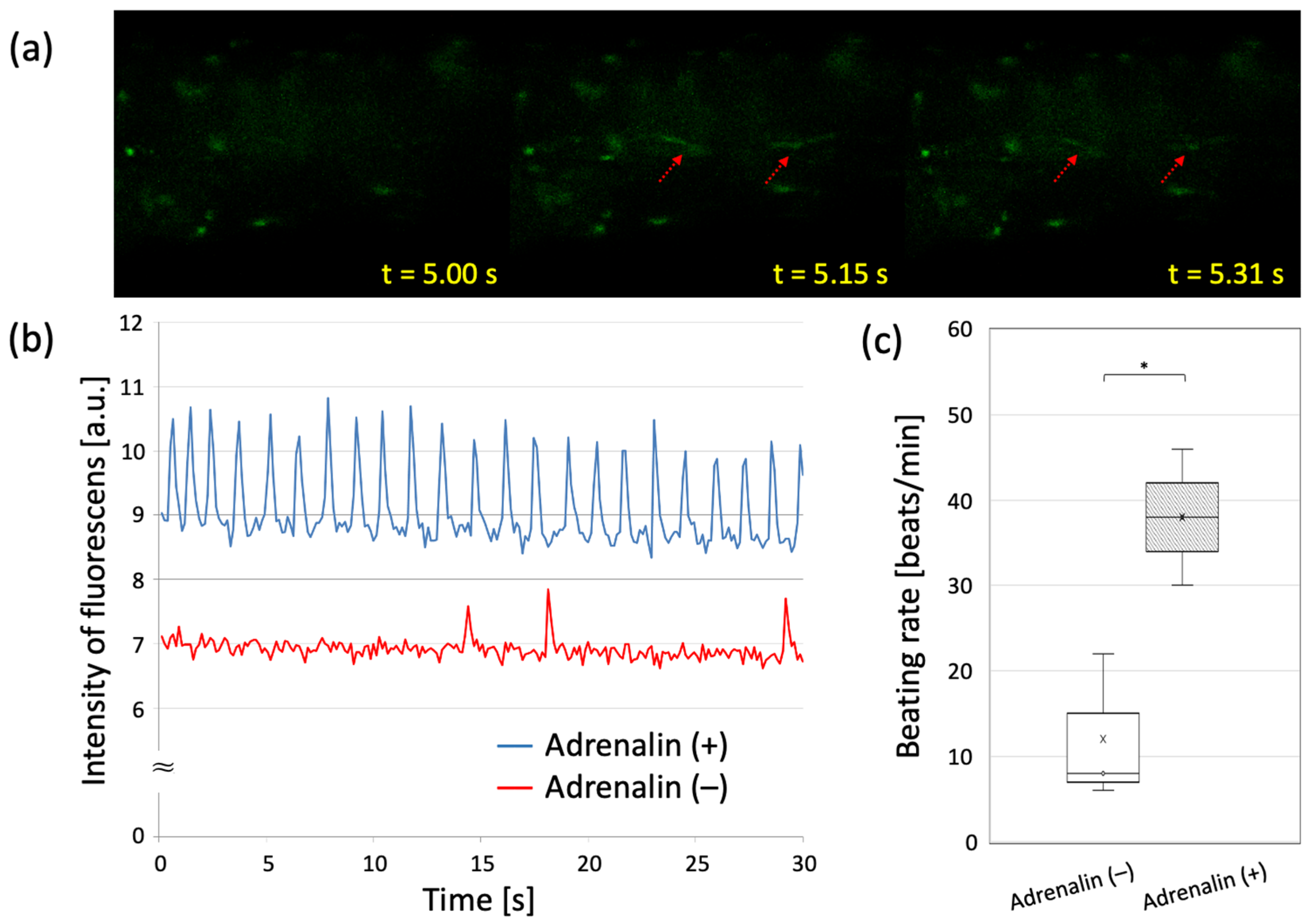
3.4. Evaluating Drug-Response of Cardiac Building Units
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashammakhi, N.; GhavamiNejad, A.; Tutar, R.; Fricker, A.; Roy, I.; Chatzistavrou, X.; Apu, E.H.; Nguyen, K.-L.; Ahsan, T.; Pountos, I.; et al. Highlights on Advancing Frontiers in Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 633–664. [Google Scholar] [CrossRef] [PubMed]
- Zamani, M.; Karaca, E.; Huang, N.F. Multicellular Interactions in 3D Engineered Myocardial Tissue. Front. Cardiovasc. Med. 2018, 5, 147. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, Y.; Nishimura, K.; Tambara, K.; Yamamoto, M.; Lu, F.; Tabata, Y.; Komeda, M. Prevascularization with gelatin microspheres containing basic fibroblast growth factor enhances the benefits of cardiomyocyte transplantation. J. Thorac. Cardiovasc. Surg. 2002, 124, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Oved, H.; Silberman, E.; Dvir, T. Bioengineering approaches to treat the failing heart: From cell biology to 3D printing. Nat. Rev. Cardiol. 2022, 19, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Alonzo, M.; El Khoury, R.; Nagiah, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. 3D Biofabrication of a Cardiac Tissue Construct for Sustained Longevity and Function. Appl. Mater. Interfaces 2022, 14, 21800–21813. [Google Scholar] [CrossRef]
- Bliley, J.; Tashman, J.; Stang, M.; Coffin, B.; Shiwarski, D.; Lee, A.; Hinton, T.; Feinberg, A. FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication 2022, 14, 024106. [Google Scholar] [CrossRef]
- Kalhori, D.; Zakeri, N.; Zafar-Jafarzadeh, M.; Moroni, L.; Solati-Hashjin, M. Cardiovascular 3D bioprinting: A review on cardiac tissue development. Bioprinting 2022, 28, e00221. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.-H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Annu. Rev. Biomed. 2017, 45, 148–163. [Google Scholar] [CrossRef]
- Hong, N.; Yang, G.-H.; Lee, J.H.; Kim, G.H. 3D bioprinting and its in vivo applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 444–459. [Google Scholar] [CrossRef]
- Noor, N.; Shapira, A.; Edri, R.; Gal, I.; Wertheim, L.; Dvir, T. 3D Printing of Personalized Thick and Perfusable Cardiac Patches and Hearts. Adv. Sci. 2019, 6, 1900344. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D bioprinting of collagen to rebuild components of the human heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Groll, J.; Boland, T.; Blunk, T.; Burdick, J.A.; Cho, D.-W.; Dalton, P.D.; Derby, B.; Forgacs, G.; Li, Q.; Mironov, V.A.; et al. Biofabrication: Reappraising the definition of an evolving field. Biofabrication 2016, 8, 013001. [Google Scholar] [CrossRef]
- Sakai, S.; Kawakami, K. Both ionically and enzymatically crosslinkable alginate-tyramine conjugate as materials for cell encapsulation. J. Biomed. Mater. Res. A. 2008, 85, 345–351. [Google Scholar] [CrossRef]
- Nakamura, M.; Mir, T.A.; Arai, K.; Ito, S.; Yoshida, T.; Iwanaga, S.; Kitano, H.; Obara, C.; Nikaido, T. Bioprinting with pre-cultured cellular constructs towards tissue engineering of hierarchical tissues. Int. J. Bioprint. 2015, 1, 39–48. [Google Scholar] [CrossRef]
- Arai, K.; Tsukamoto, Y.; Yoshida, H.; Sanae, H.; Mir, T.A.; Sakai, S.; Yoshida, T.; Okabe, M.; Nikaido, T.; Taya, M.; et al. The development of cell-adhesive hydrogel for 3D printing. Int. J. Bioprint. 2016, 2, 153–162. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Ohta, S.; Nakamura, M.; Ito, T. 3D inkjet printing of star block copolymer hydrogels cross-linked using various metallic ions. RSC Adv. 2017, 7, 55571–55576. [Google Scholar] [CrossRef]
- Draget, K.I.; Bræk, G.S.; Smidsrød, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Yang, J.-S.; Xie, Y.-J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Cao, L.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Egg-box model-based gelation of alginate and pectin: A review. Carbohydr. Polym. 2020, 242, 116389. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Shimizu, T.; Yamato, M.; Kikuchi, A.; Okano, T. Electrical coupling of cardiomyocyte sheets occurs rapidly via functional gap junction formation. Biomaterials 2006, 27, 4765–4774. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, Y.; Shimizu, T.; Matsuura, K.; Sekine, H.; Tanaka, N.; Tadakuma, K.; Yamato, M.; Kaneko, M.; Okano, T. Cell Sheet Technology for Cardiac Tissue Engineering. Card. Tissue Eng. 2014, 1181, 139–155. [Google Scholar]
- Goodenough, D.A.; Goliger, J.A.; Paul, D.L. Connexins, connexons, and intercellular communication. Annu. Rev. Biochem. 1996, 65, 475–502. [Google Scholar] [CrossRef]
- Severs, N.J. Pathophysiology of gap junctions in heart disease. J. Cardiovasc. Electrophysiol. 1994, 5, 462–475. [Google Scholar] [CrossRef]
- Gros, D.B.; Jongsma, H.J. Connexins in mammalian heart function. BioEssays 1996, 18, 719–730. [Google Scholar] [CrossRef]
- Oyamada, M.; Kimura, H.; Oyamada, Y.; Miyamoto, A.; Ohshika, H.; Mori, M. The Expression, Phosphorylation, and Localization of Connexin 43 and Gap-Junctional Intercellular Communication during the Establishment of a Synchronized Contraction of Cultured Neonatal Rat Cardiac Myocytes. Exp. Cell Res. 1994, 212, 351–358. [Google Scholar] [CrossRef]
- Mir, T.A.; Iwanaga, S.; Kurooka, T.; Toda, H.; Sakai, S.; Nakamura, M. Biofabrication offers future hope for tackling various obstacles and challenges in tissue engineering and regenerative medicine: A Perspective. Int. J. Bioprint. 2019, 5, 153. [Google Scholar] [CrossRef]

| XY-Axis Pitch Size [μm/pixles] | Z-Axis Pitch Width [μm] | Nozzle Head Speed [μm/s] | Number of Laminations |
|---|---|---|---|
| 50 | 95 | 32,000 | 7 |
| Chemicals | A275 [-] (at 0.1 w/v%) | Degree of Tyramine | |
|---|---|---|---|
| [g/g-polymer] | [mmol/g-polymer] | ||
| Tyramine | 8.63 * | — | — |
| Sodium alginate | 0.002 (a) | — | — |
| EC alginate | 0.735 ± 0.001 (b) | 0.093 | 0.535 |
| Gelatin | 0.060 ± 0.005 (b) | — | — |
| EC gelatin | 0.374 ± 0.004 (b) | 0.038 | 0.219 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanaga, S.; Hamada, Y.; Tsukamoto, Y.; Arai, K.; Kurooka, T.; Sakai, S.; Nakamura, M. Design and Fabrication of Mature Engineered Pre-Cardiac Tissue Utilizing 3D Bioprinting Technology and Enzymatically Crosslinking Hydrogel. Materials 2022, 15, 7928. https://doi.org/10.3390/ma15227928
Iwanaga S, Hamada Y, Tsukamoto Y, Arai K, Kurooka T, Sakai S, Nakamura M. Design and Fabrication of Mature Engineered Pre-Cardiac Tissue Utilizing 3D Bioprinting Technology and Enzymatically Crosslinking Hydrogel. Materials. 2022; 15(22):7928. https://doi.org/10.3390/ma15227928
Chicago/Turabian StyleIwanaga, Shintaroh, Yuta Hamada, Yoshinari Tsukamoto, Kenichi Arai, Taketoshi Kurooka, Shinji Sakai, and Makoto Nakamura. 2022. "Design and Fabrication of Mature Engineered Pre-Cardiac Tissue Utilizing 3D Bioprinting Technology and Enzymatically Crosslinking Hydrogel" Materials 15, no. 22: 7928. https://doi.org/10.3390/ma15227928
APA StyleIwanaga, S., Hamada, Y., Tsukamoto, Y., Arai, K., Kurooka, T., Sakai, S., & Nakamura, M. (2022). Design and Fabrication of Mature Engineered Pre-Cardiac Tissue Utilizing 3D Bioprinting Technology and Enzymatically Crosslinking Hydrogel. Materials, 15(22), 7928. https://doi.org/10.3390/ma15227928









