Effect of Lanthanum Addition on Formation Behaviors of Inclusions in Q355B Weathering Steel
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
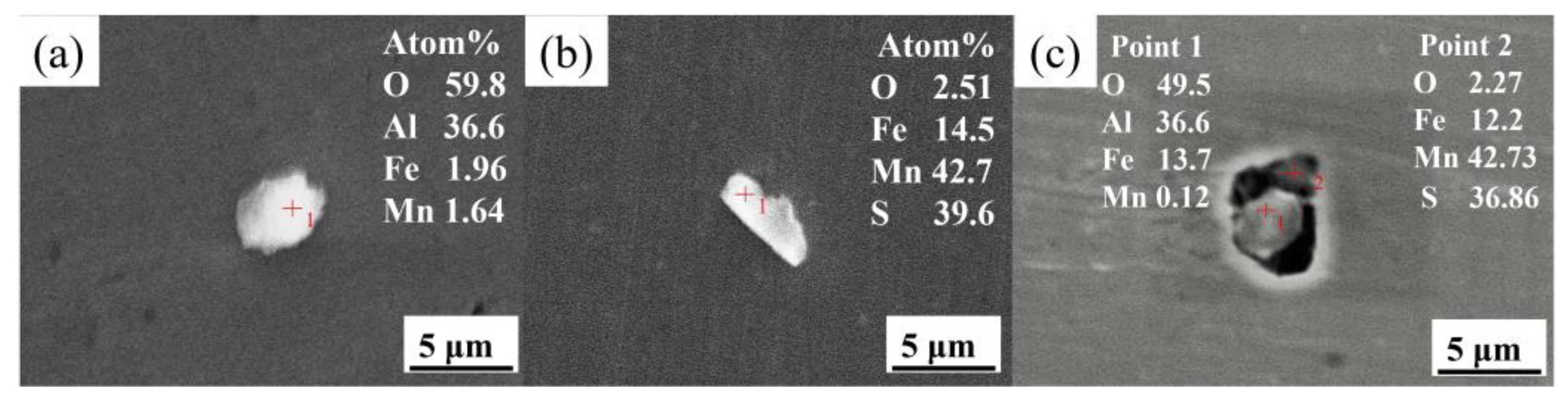
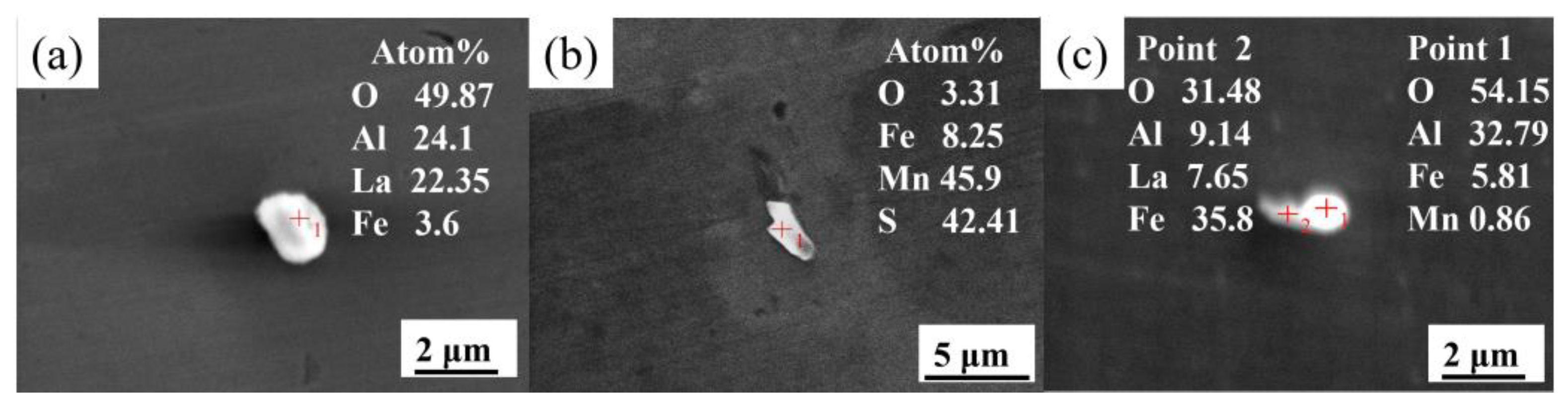
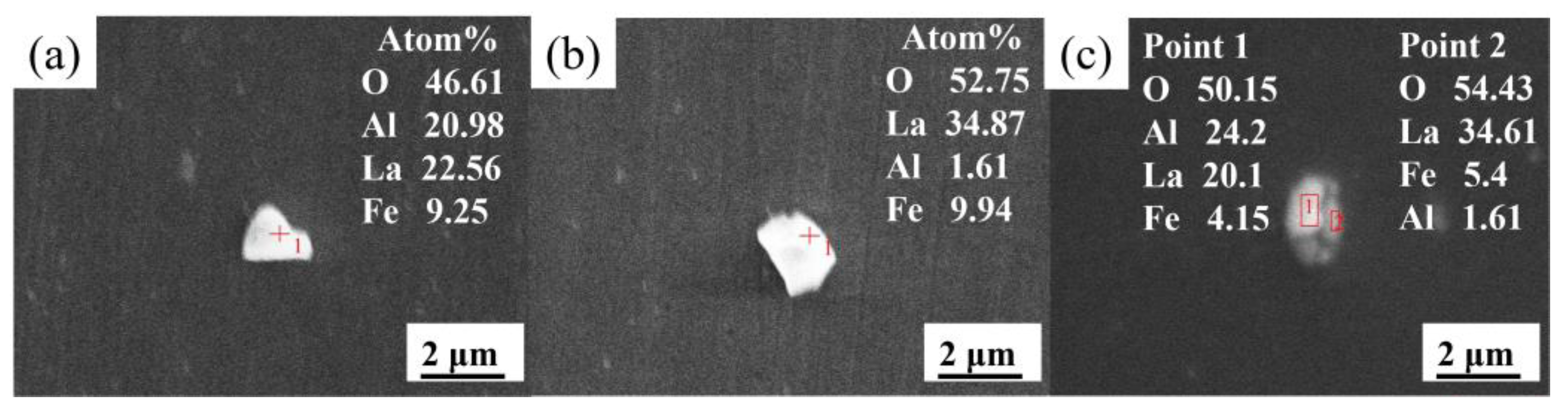
3.1. Effect of La Treatment on Characteristics of Inclusions
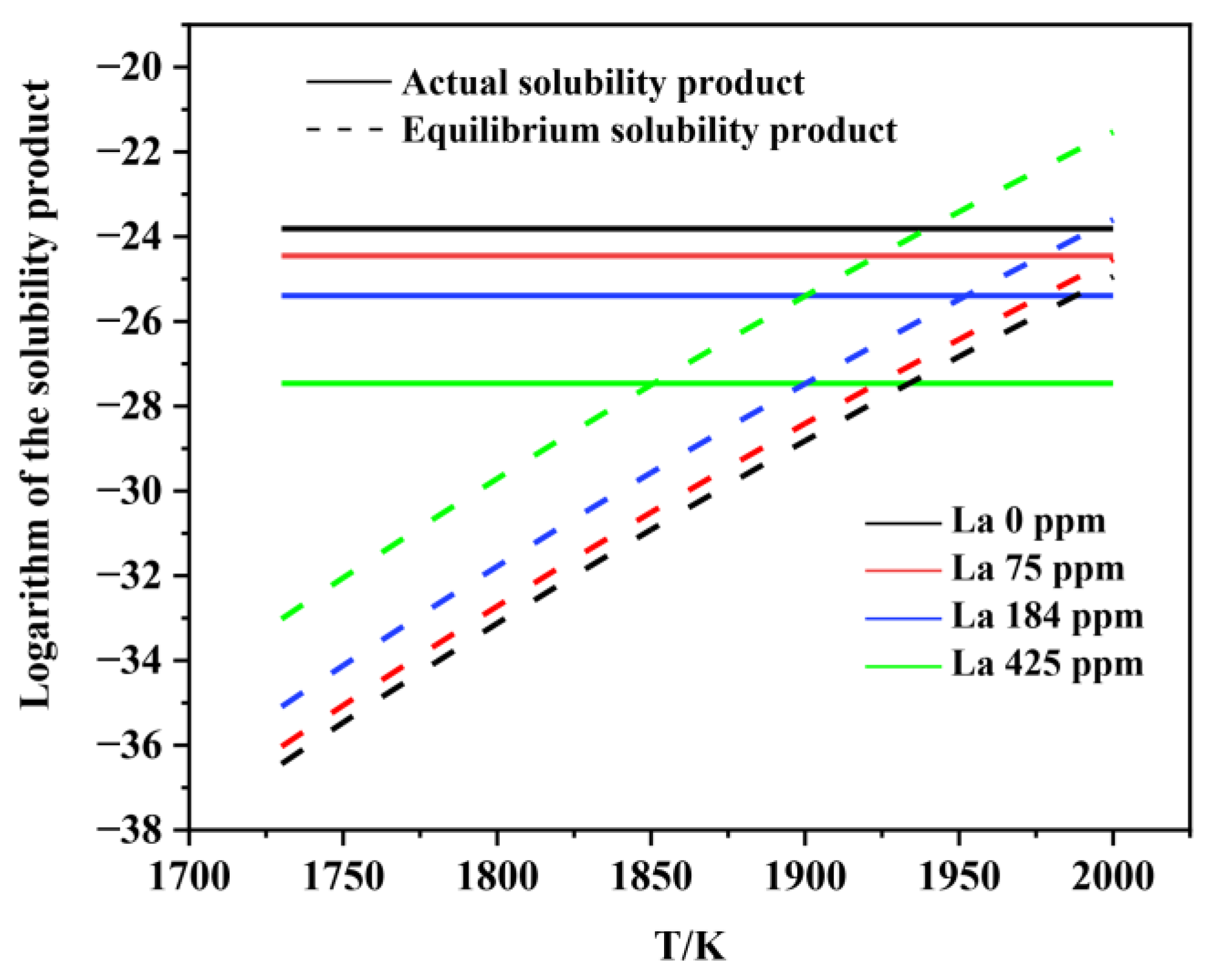
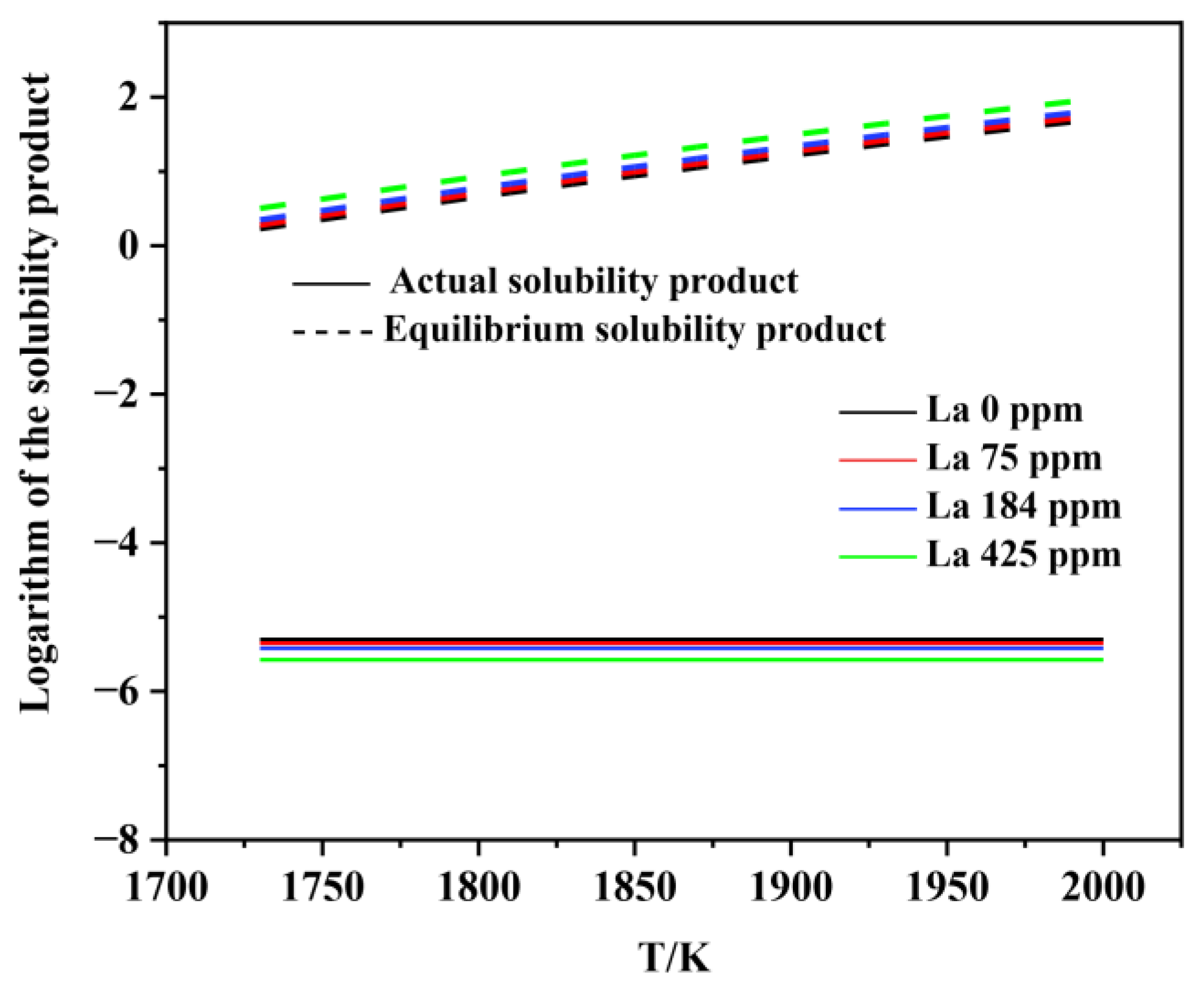
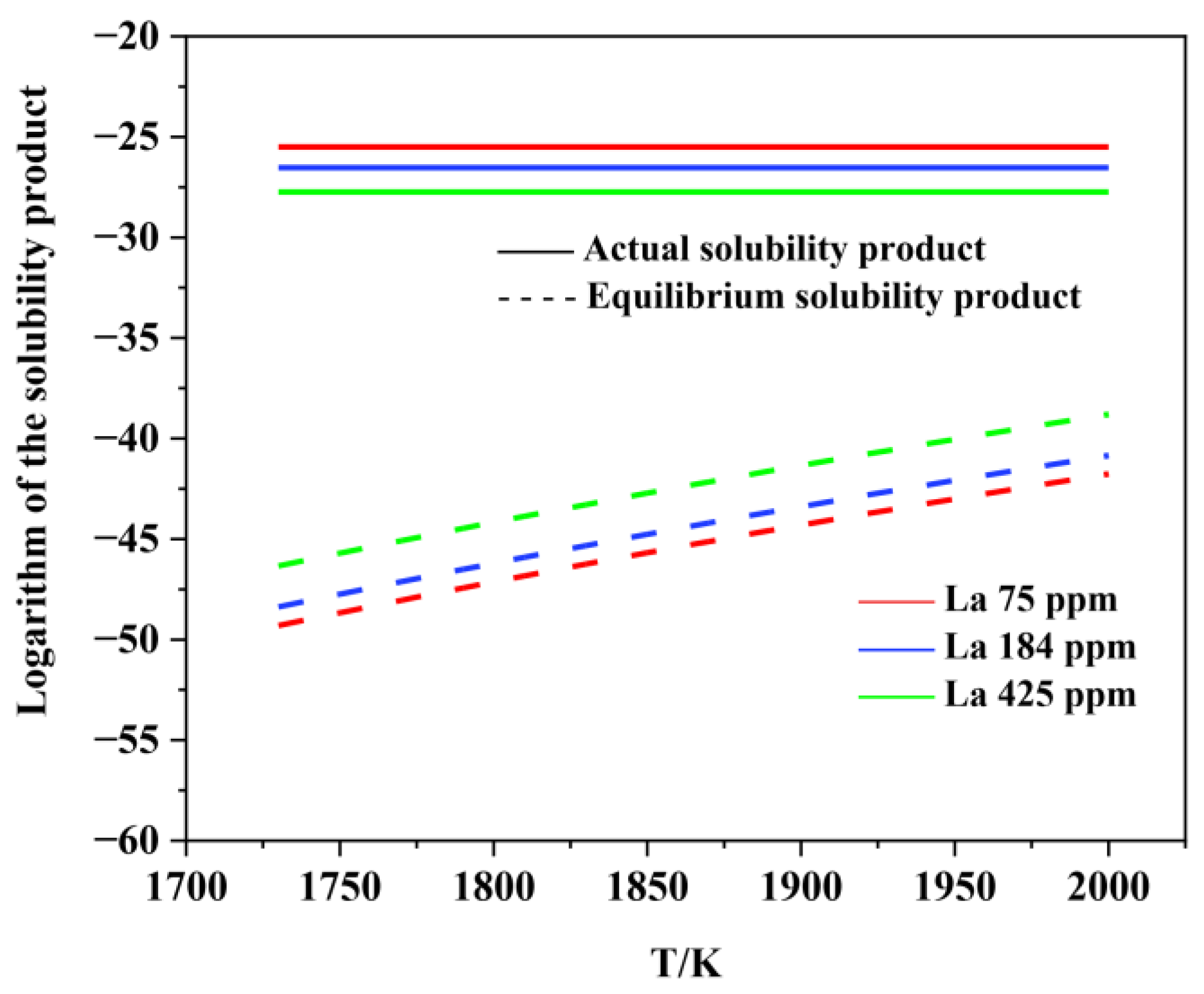
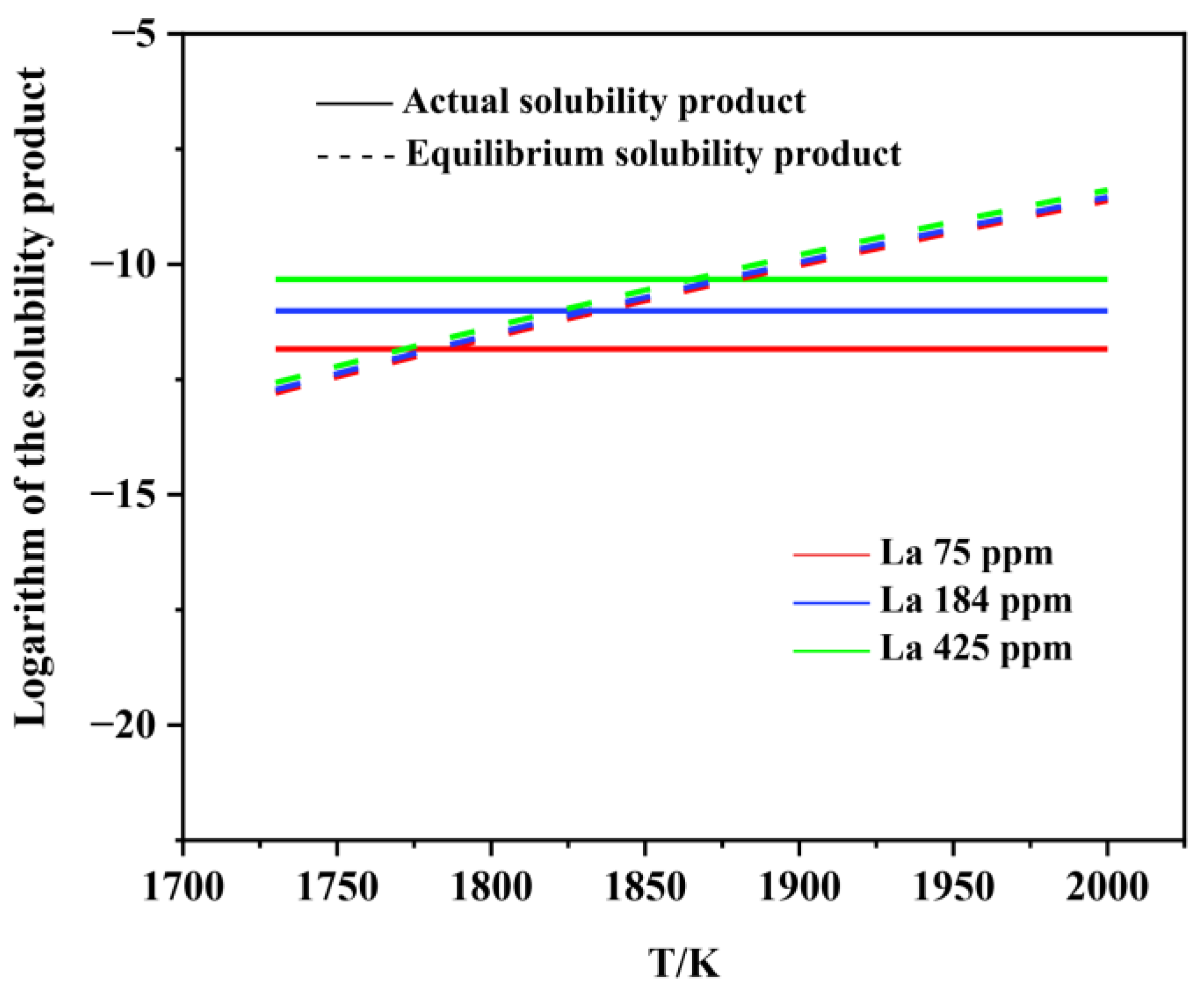
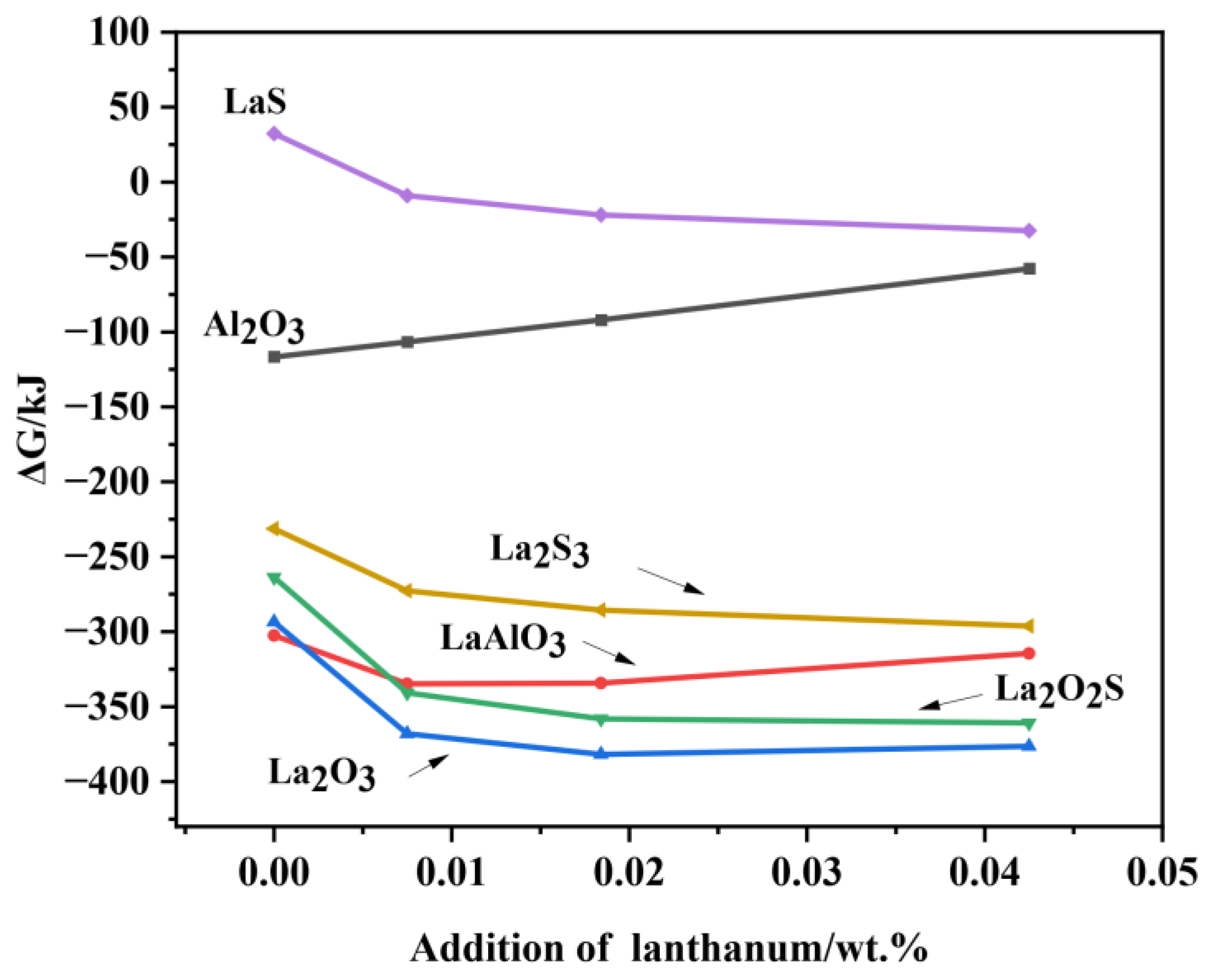
3.2. Thermodynamic Analysis on the Formation and Modification of Inclusions
4. Conclusions
- The original harmful inclusions in Q355B weathering steel could be modified to fine and dispersive rare earth inclusions by adding the appropriate amount of La. The main inclusions in RE-free steel are large-sized irregular Al2O3 and MnS, with an average size of about 5.35 μm. As the La content increases from 0.0075 to 0.0184 wt.%, the main inclusions transform from MnS, LaAlO3, and Al2O3-LaAlO3 complex inclusion into LaAlO3, La2O3, MnS, and LaAlO3-La2O3 complex inclusion. Meanwhile, the average size of inclusions significantly decreases from 3.4 to 2.48 μm and the distribution is more dispersive. When the La content increases to 0.0425 wt.%, the original MnS and Al2O3 inclusions are completely modified into La2O2S and La2O3 but the inclusions demonstrate serious agglomeration and growth.
- The thermodynamic calculations indicate that Al2O3 and various lanthanum-containing inclusions are formed in the liquid phase. As the La content in molten steel increases from 0 to 0.0425wt.%, the Al2O3 inclusion is inclined to be modified into lanthanum oxide and lanthanum oxysulfide. The modification process is Al2O3 → LaAlO3 → La2O3 → La2O2S, which is very consistent with the experimental observations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yamashita, M.; Miyuki, H.; Matsuda, Y.; Nagano, H.; Misawa, T. The long term growth of the protective rust layer formed on weathering steel by atmospheric corrosion during a quarter of a century. Corros. Sci. 1994, 36, 283–299. [Google Scholar] [CrossRef]
- Morel, J.M.; Creus, J.; Gaillet, L.; Chatel, V.; Astic, J.Y. Evaluation of the Durability of Weathering Steel—Effects of environmentals changes on corrosion behavior of low alloyed steels. CE/Papers 2021, 4, 1644–1649. [Google Scholar] [CrossRef]
- Keiser, J.T.; Brown, C.W.; Heidersbach, R.H. Characterization of the passive film formed on weathering steels. Corros. Sci. 1983, 23, 251–259. [Google Scholar] [CrossRef]
- Larrabee, C.P. Corrosion Resistance of High-Strength Low-Alloy Steels as Influenced by Composition and Environment. Corros. Eng. 2014, 3, 259–271. [Google Scholar] [CrossRef]
- Królikowska, A.; Komorowski, L.; Kunce, I.; Wojda, D.; Zacharuk, K.; Paszek, U.; Wierzbicki, T.; Bilewska, K. Corrosion Assessment of a Weathering Steel Bridge Structure after 30 Years of Service. Materials 2021, 14, 3788. [Google Scholar] [CrossRef]
- Torkkeli, J.; Saukkonen, T.; Hanninen, H. Effect of MnS inclusion dissolution on carbon steel stress corrosion cracking in fuel-grade ethanol. Corros. Sci. 2015, 96, 14–22. [Google Scholar] [CrossRef]
- Wei, J.; Dong, J.H.; Ke, W.; He, X.Y. Influence of Inclusions on Early Corrosion Development of Ultra-Low Carbon Bainitic Steel in NaCl Solution. Corros. J. Sci. Eng. 2015, 71, 1476–1480. [Google Scholar] [CrossRef]
- Shibaeva, T.V.; Laurinavichyute, V.K.; Tsirlina, G.A.; Arsenkin, A.M.; Grigorovich, K.V. The effect of microstructure and non-metallic inclusions on corrosion behavior of low carbon steel in chloride containing solutions. Corros. Sci. 2014, 80, 299–308. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Qi, Y.; Chen, L.; Chen, C. Mechanism of (Mg,Al,Ca)-oxide inclusion-induced pitting corrosion in 316l stainless steel exposed to sulphur environments containing chloride ion. Corros. Sci. 2013, 67, 20–31. [Google Scholar] [CrossRef]
- Moghaddam, S.M.; Sadeghi, F.; Paulson, K.; Weinzapfel, N.; Correns, M.; Bakolas, V.; Dinkel, M. Effect of non-metallic inclusions on butterfly wing initiation, crack formation, and spall geometry in bearing steels. Int. J. Fatigue 2015, 80, 203–215. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, X.R.; Yan, E.; Misra, R.; Du, C.M.; Du, F. The influence of inclusion factors on ultra-high cyclic deformation of a dual phase steel. Mater. Sci. Eng. A. 2019, 754, 275–281. [Google Scholar] [CrossRef]
- Liu, X.G.; Wang, C.; Gui, J.T.; Xiao, Q.Q.; Guo, B.F. Effect of MnS inclusions on deformation behavior of matrix based on in-situ experiment. Mater. Sci. Eng. 2019, 746, 239–247. [Google Scholar] [CrossRef]
- Wilson, W.G.; Kay, D.A.R.; Vahed, A. The use of thermodynamics and phase equilibria to predict the behavior of the rare earth elements in steel. JOM 1974, 26, 14–23. [Google Scholar] [CrossRef]
- Wang, L.M.; Lin, Q.; Yue, L.J.; Liu, L.; Guo, F.; Wang, F.M. Study of application of rare earth elements in advanced low alloy steels. J. Alloys Compd. 2008, 451, 534–553. [Google Scholar] [CrossRef]
- Torkamani, H.; Raygan, S.; Garcia, M.C.; Rassizadehghani, J.; Palizdar, Y.; Martin, D.S. Contributions of Rare Earth Element (La,Ce) Addition to the Impact Toughness of Low Carbon Cast Niobium Microalloyed Steels. Met. Mater. Int. 2018, 24, 773–788. [Google Scholar] [CrossRef]
- Liu, Z.; Lian, X.; Liu, T.; Yang, Y.; Dong, H. Effects of rare earth elements on corrosion behaviors of low carbon steels and weathering steels. Mater. Corros. 2019, 71, 258–266. [Google Scholar] [CrossRef]
- Lian, X.; Zhu, J.; Wang, R.; Liu, T.; Dong, H. Effects of Rare Earth (Ce and La) on Steel Corrosion Behaviors under Wet-Dry Cycle Immersion Conditions. Metals 2020, 10, 1174. [Google Scholar] [CrossRef]
- Yue, L.J.; Wang, L.L.; Wang, L.M. Influence of rare earth element on the mechanical properties of clean weathering steel. Chin. Rare Earths 2014, 35, 20–26. [Google Scholar]
- Peng, T.; Sun, J.Q.; Dong, C.C.; Yang, H.Y.; Zhang, B. Corrosion Resistance of Weathering Steel of RE Exposed in Marine Atmospheric Environment for One Year. Equip. Environ. Eng. 2017, 14, 21–24. [Google Scholar]
- Mi, F.Y.; Wang, X.D.; Liu, Z.P.; Wang, B.; Peng, Y.; Tao, D.P. Industrial Atmospheric Corrosion Resistance of P-RE Weathering Steel. J. Iron Steel Res. Int. 2011, 18, 67–73. [Google Scholar] [CrossRef]
- Yue, L.J.; Wang, L.M.; Xu, C.H. Influence of Rare Earth on Corrosion Resistance Performance in Cu-Containing Weathering Steel. Chin. Rare Earths 2009, 30, 59–64. [Google Scholar]
- Wang, R.; Chen, L.; Zhi, J.; Lian, X.; Guo, L.; Dong, H. Corrosion Behavior of Weathering Steels with Different Contents of Rare Earth Elements La and Ce. Corrosion 2022, 78, 437–448. [Google Scholar] [CrossRef]
- Yue, L.J.; Wang, L.M.; Jinsheng, H. Effects of rare earth on inclusions and corrosion resistance of 10PCuRE weathering steel. J. Rare Earths 2010, 28, 952–956. [Google Scholar] [CrossRef]
- Wang, C.; Ma, R.; Zhou, Y.; Liu, Y.; Ke, W. Effects of rare earth modifying inclusions on the pitting corrosion of 13Cr4Ni martensitic stainless steel. J. Mater. Sci. Technol. Shenyang 2021, 93, 232–243. [Google Scholar] [CrossRef]
- Li, D.; Chen, X.Q.; Fu, P.; Ma, X.; Liu, H.L.; Chen, Y.; Li, Y. Inclusion flotation-driven channel segregation in solidifying steels. Nat. Commun. 2014, 5, 5572. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Revilla, R.I.; Zhang, D.; Liu, Z.; Lutz, A.; Zhang, F.; Terryn, H. Role of Al2O3 inclusions on the localized corrosion of Q460NH weathering steel in marine environment. Corros. Sci. 2018, 138, 96–104. [Google Scholar] [CrossRef]
- Smirnov, L.A.; Rovnushkin, V.A.; Oryshchenko, A.S.; Kalinin, G.Y.; Milyuts, V.G. Modification of steel and alloys with rare-earth elements. part 2*. Metallurgist 2016, 59, 1053–1061. [Google Scholar] [CrossRef]
- Li, H.; Yu, Y.C.; Ren, X.; Zhang, S.H.; Wang, S.B. Evolution of Al2O3 inclusions by cerium treatment in low carbon high manganese steel. J. Iron Steel Res. Int. 2017, 24, 925–934. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.P.; Zhi, J.G.; Duan, C.Y.; Gao, S.; Wang, M. Effect of Rare Earth Ce on the Morphology and Distribution of Al2O3 Inclusions in High Strength IF Steel Containing Phosphorus during Continuous Casting and Rolling Process. Iron Steel Inst. Jpn. 2021, 61, 657–666. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, L.; Zhang, L.; Wang, Y.; Shu, Y.; Zhou, Y. Investigation of RE-O-S-As Inclusions in High Carbon Steels. Metall. Mater. Trans. B 2017, 48, 2849–2858. [Google Scholar] [CrossRef]
- Huang, Y.; Cheng, G.; Li, S.; Dai, W. Effect of cerium on the behavior of inclusions in H13 steel. Steel Res. Int. 2018, 89, 1800371. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, L. Effect of Cerium Content on Inclusions in an Ultra-Low-Carbon Aluminum-Killed Steel. Metall. Mater. Trans. B 2020, 51, 589–600. [Google Scholar] [CrossRef]
- Wang, J.Q.; Huang, J.R. Solidification of Metal and its Control; Machine Press: Beijing, China, 1977; pp. 9–35. [Google Scholar]
- Du, T. Thermodynamics of rare-earth elements in iron-base solutions. J. Iron Steel Res. 1994, 3, 6–12. [Google Scholar]
- Vahed, A.; Kay, D. Thermodynamics of rare earths in steelmaking. Metall. Mater. Trans. B 1976, 7, 375–383. [Google Scholar] [CrossRef]
- Wagner, C. Thermodynamics of Alloys; Addison-Wesley Press: Boston, MA, USA, 1952; p. 51. [Google Scholar]
- Chen, J.X. Manual of Chart Data for Steelmaking, 10th ed.; Liu, X.F., Ed.; Metallurgical Industry Press: Beijing, China, 1984; pp. 753–761. [Google Scholar]
- Wu, P.; Pelton, A.D. Coupled thermodynamic-phase diagram assessment of the rare earth oxide-aluminium oxide binary systems. J. Alloys Compd. 1992, 179, 259–287. [Google Scholar] [CrossRef]
- Dwivedi, K.R. Determination of the Thermodynamic Properties of Rare Earth-Oxygen-Sulfur Systems at High Temperatures. Ph.D. Thesis, McMaster University, Hamilton, ON, Canada, July 1982. [Google Scholar]






| Sample | T.O | C | Al | Si | Mn | S | Cr | La |
|---|---|---|---|---|---|---|---|---|
| NO. 1 | 0.0076 | 0.1740 | 0.0179 | 0.1346 | 1.56 | 0.0030 | 0.0549 | - |
| NO. 2 | 0.0076 | 0.1611 | 0.0203 | 0.1327 | 1.47 | 0.0028 | 0.0566 | 0.0075 |
| NO. 3 | 0.0076 | 0.1657 | 0.0192 | 0.1344 | 1.47 | 0.0023 | 0.0555 | 0.0184 |
| NO. 4 | 0.0076 | 0.1791 | 0.0325 | 0.2079 | 1.59 | 0.0022 | 0.0554 | 0.0425 |
| Reaction Equation of Various Inclusions | ||
|---|---|---|
| A | B | |
| −158,365 | 93.966 | |
| −122,500 | 393.8 | |
| −1,443,880 | 337 | |
| −1,341,200 | 301 | |
| −445,180 | 141.5 | |
| −801,616 | 28.9 | |
| C | Si | Mn | P | S | O | Cr | Ni | Mo | Cu | Al | La | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O | −0.45 | −0.131 | −0.021 | 0.07 | −0.133 | −0.2 | −0.04 | 0.006 | 0.0035 | −0.013 | −3.9 | −12.1 |
| S | 0.110 | 0.063 | −0.026 | 0.029 | −0.028 | −0.27 | −0.011 | 0 | 0.0027 | −0.0084 | 0.035 | −2.79 |
| Al | 0.091 | 0.0056 | 0.056 | 0.033 | 0.03 | −6.6 | 0.025 | 0.008 | - | 0.008 | 0.045 | −0.511 |
| Mn | −0.07 | 0.39 | 0 | −0.0035 | −0.048 | −0.083 | 0.003 | −0.007 | 0.0045 | - | 0.07 | - |
| La | −0.279 | - | 0.28 | 1.734 | −12.130 | −105 | - | - | - | - | −2.649 | −0.013 |
| No. | O | S | Al | Mn | La |
|---|---|---|---|---|---|
| 1 | 0.6290 | 0.9668 | 1.140 | 1.0981 | - |
| 2 | 0.5103 | 0.9214 | 1.131 | 1.098 | 0.3484 |
| 3 | 0.3764 | 0.8589 | 1.116 | 1.0982 | 0.3483 |
| 4 | 0.1924 | 0.7358 | 1.085 | 1.0982 | 0.3480 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, N.; Yang, W.; Chen, D.; Lu, W.; Zhang, X.; Chen, S.; Xu, M.; Pan, B.; Han, L.; Zhang, X.; et al. Effect of Lanthanum Addition on Formation Behaviors of Inclusions in Q355B Weathering Steel. Materials 2022, 15, 7952. https://doi.org/10.3390/ma15227952
Mao N, Yang W, Chen D, Lu W, Zhang X, Chen S, Xu M, Pan B, Han L, Zhang X, et al. Effect of Lanthanum Addition on Formation Behaviors of Inclusions in Q355B Weathering Steel. Materials. 2022; 15(22):7952. https://doi.org/10.3390/ma15227952
Chicago/Turabian StyleMao, Ning, Wensheng Yang, Dehong Chen, Wenli Lu, Xiaowei Zhang, Shiying Chen, Minglei Xu, Bo Pan, Liguo Han, Xiaoqiang Zhang, and et al. 2022. "Effect of Lanthanum Addition on Formation Behaviors of Inclusions in Q355B Weathering Steel" Materials 15, no. 22: 7952. https://doi.org/10.3390/ma15227952





