CuAPO-5 as a Multiphase Catalyst for Synthesis of Verbenone from α-Pinene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CuAPO-5
2.3. Catalyst Characterization
2.4. Catalyst Activity Tests
3. Results
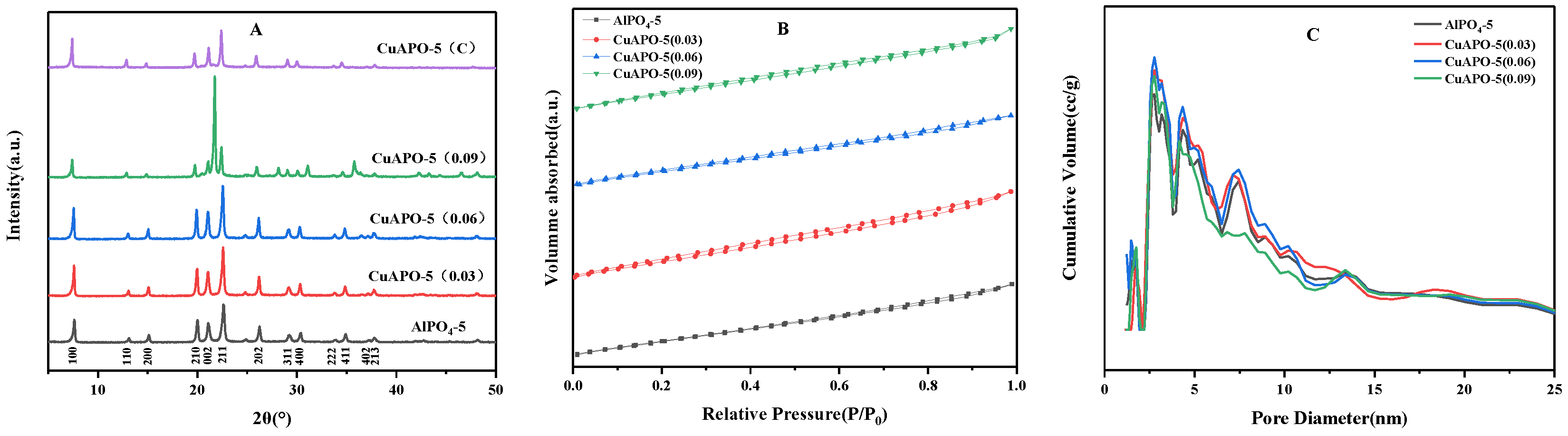
3.1. X-ray Diffraction Analysis
3.2. ICP-OES Analysis
3.3. N2 Sorption Studies
3.4. Electron Microscopic Analyses
3.5. X-ray Photoelectron Spectroscopy
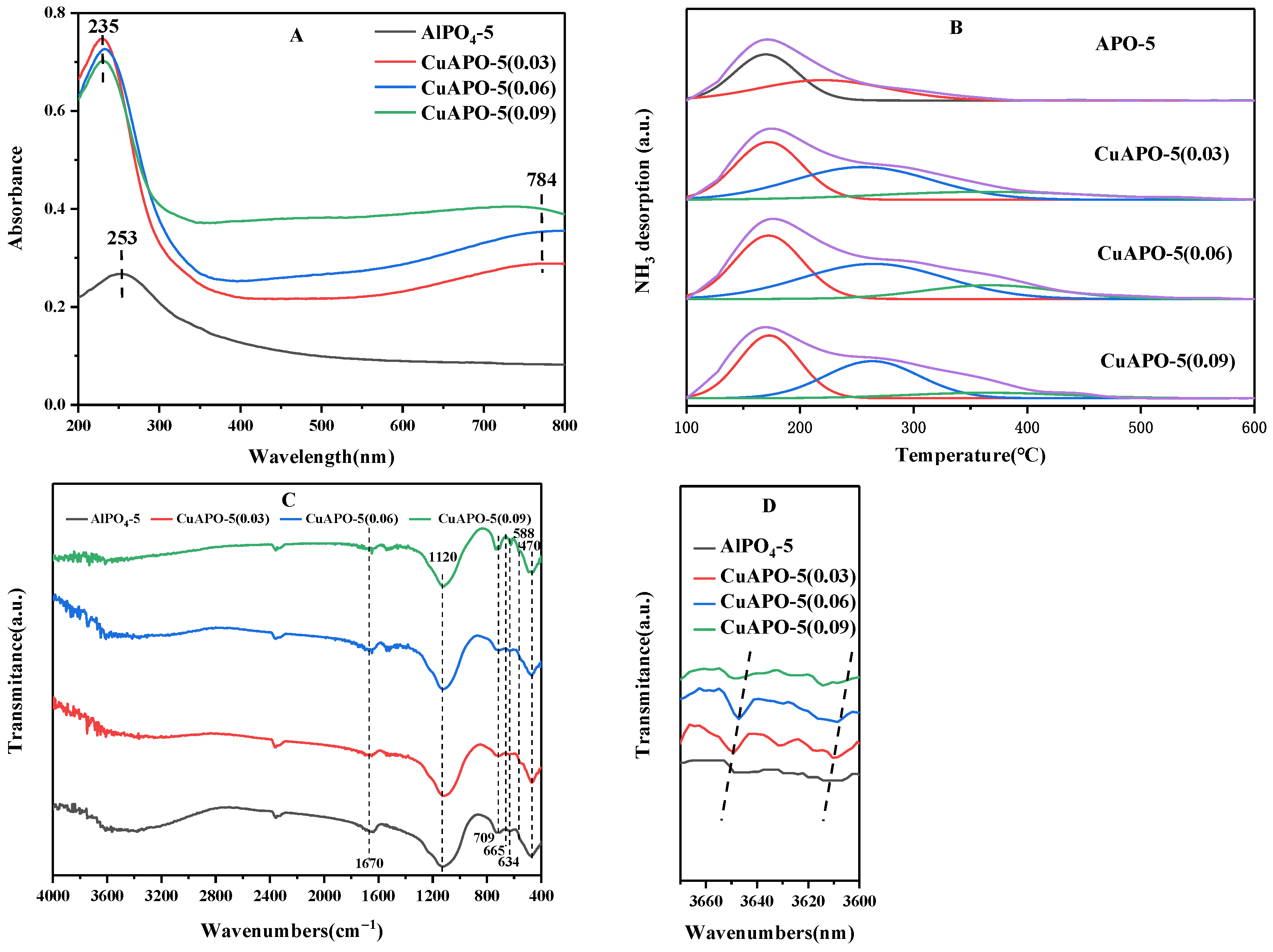
3.6. UV-Vis Spectroscopic Analysis
3.7. Characterization of Acid Sites
3.8. FT-IR Spectroscopy
3.9. Catalytic Activity
3.10. Effect of Different Reaction Parameters on α-Pinene Oxidation Rates
3.10.1. Effect of Reaction Temperature
3.10.2. Effect of Reaction Time
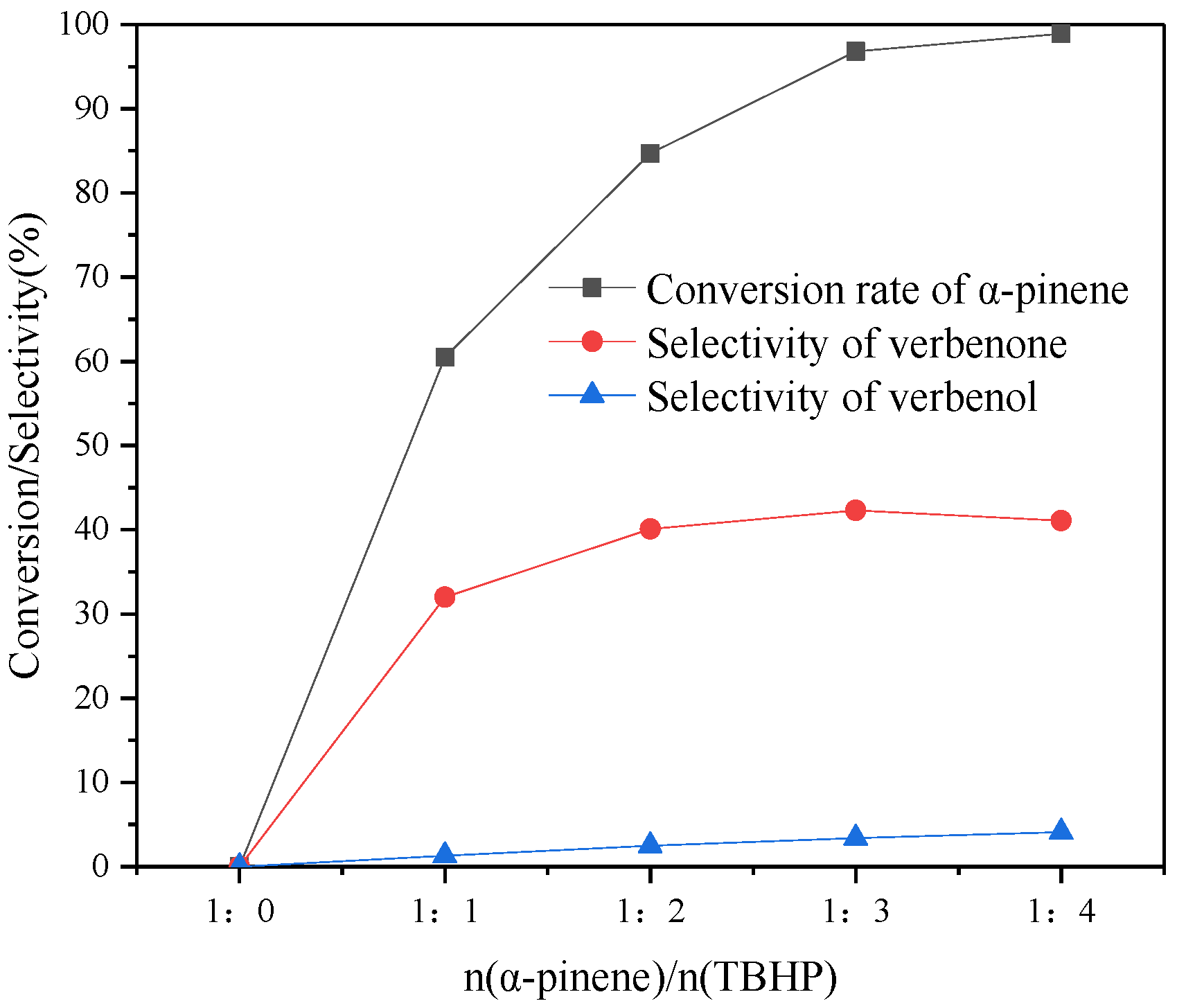
3.10.3. Effect of n(α-Pinene)/n(TBHP)
3.10.4. Effect of Solvent
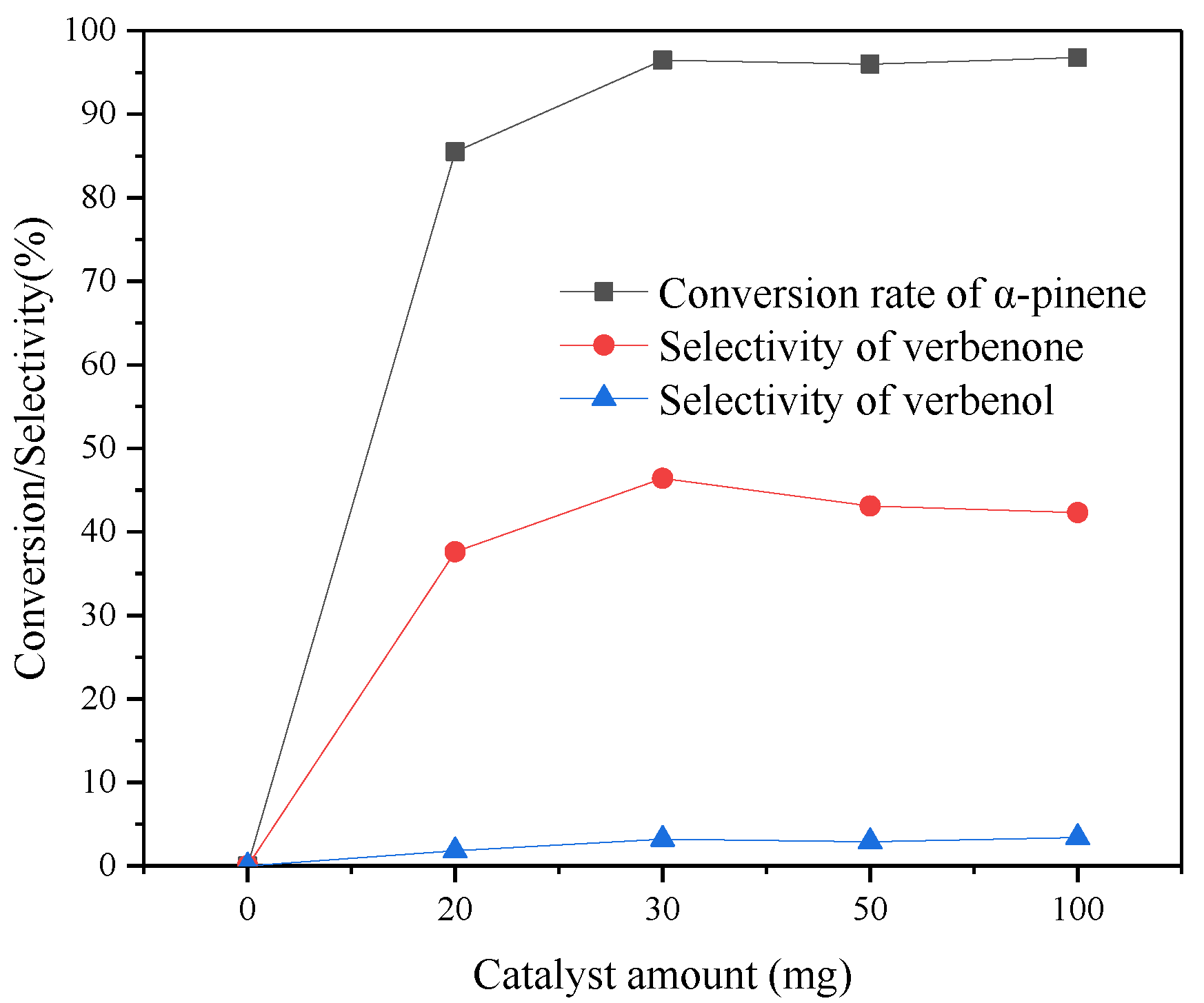
3.10.5. Effect of the Catalyst Amount
3.10.6. Recycling Efficiency of CuAPO-5
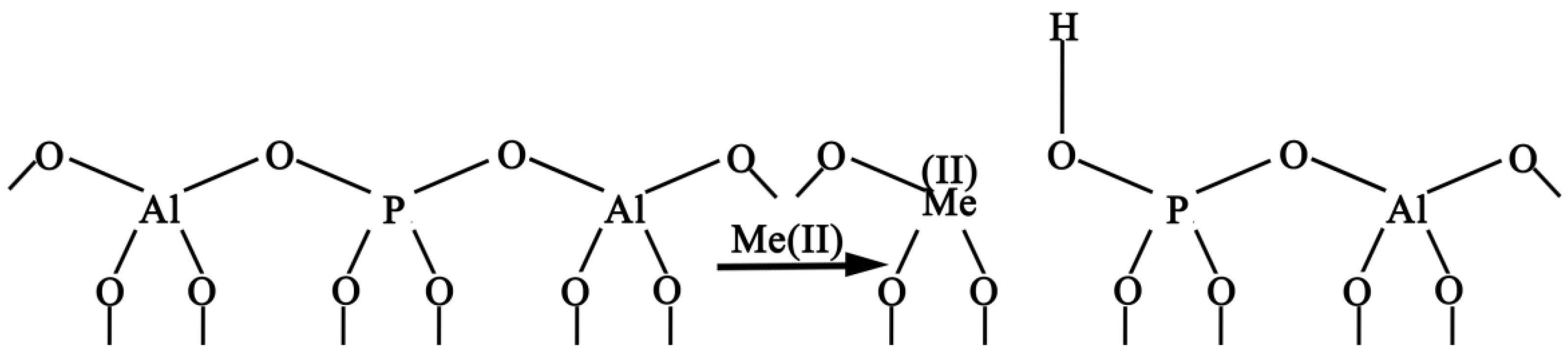
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demko, J.; Machava, J. Tree Resin, a Macroergic Source of Energy, a Possible Tool to Lower the Rise in Atmospheric CO2 Levels. Sustainability 2022, 14, 3506. [Google Scholar] [CrossRef]
- Donoso, D.; Ballesteros, R.; Bolonio, D.; García-Martínez, M.-J.; Lapuerta, M.; Canoira, L. Hydrogenated Turpentine: A Biobased Component for Jet Fuel. Energy Fuels 2020, 35, 1465–1475. [Google Scholar] [CrossRef]
- Wróblewska, A.; Makuch, E.; Miądlicki, P. The studies on the limonene oxidation over the microporous TS-1 catalyst. Catal. Today 2016, 268 (Suppl. S1), 121–129. [Google Scholar] [CrossRef]
- Lara-Romero, J.; Ocampo-Macias, T.; Martínez-Suarez, R.; Rangel-Segura, R.; López-Tinoco, J.; Paraguay-Delgado, F.; Alonso-Nuñez, G.; Jiménez-Sandoval, S.; Chiñas-Castillo, F. Parametric Study of the Synthesis of Carbon Nanotubes by Spray Pyrolysis of a Biorenewable Feedstock: α-Pinene. ACS Sustain. Chem. Eng. 2017, 5, 3890–3896. [Google Scholar] [CrossRef]
- Vanek, T.; Halík, J.; Anková, R.; Valterová, I. Formation of trans-Verbenol and Verbenone from α-Pinene Catalysed by Immobilised Picea abies Cells. Biosci. Biotechnol. Biochem. 2005, 69, 321–325. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Huang, K.; Li, C.; Tao, D.-J. Ultralow Loading Cobalt-Based Nanocatalyst for Benign and Efficient Aerobic Oxidation of Allylic Alcohols and Biobased Olefins. ACS Sustain. Chem. Eng. 2018, 7, 1901–1908. [Google Scholar] [CrossRef]
- Becerra, J.; González, L.; Villa, A. Kinetic study of α-pinene allylic oxidation over FePcCl16-NH2-SiO2 catalyst. Mol. Catal. 2016, 423, 12–21. [Google Scholar] [CrossRef]
- Rozenbaum, H.F.; Patitucci, M.L.; Antunes, O.A.C.; Pereira, N., Jr. Production of aromas and fragrances through microbial oxidation of monoterpenes. Braz. J. Chem. Eng. 2006, 23, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Wender, P.A.; Mucciaro, T.P. A New and Practical Approach to the Synthesis of Taxol and Taxol Analogues: The Pinene Path. J. Am. Chem. Soc. 1992, 114, 5878–5879. [Google Scholar] [CrossRef]
- Rivera-Dávila, O.L.; Sánchez-Martínez, G.; Martínez, R.R. Ecotoxicity of pesticides and semiochemicals used for control and prevention of conifer bark beetle (Dendroctonus spp.) outbreaks. Chemosphere 2021, 263, 128375. [Google Scholar] [CrossRef]
- González-Velasco, H.E.; Pérez-Gutiérrez, M.S.; Alonso-Castro Á, J.; Zapata-Morales, J.R.; Niño-Moreno PD, C.; Campos-Xolalpa, N.; González-Chávez, M.M. Anti-Inflammatory and Antinociceptive Activities of the Essential Oil of Tagetes parryi A. Gray (Asteraceae) and Verbenone. Molecules 2022, 27, 2612. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Joseph, R. Bioconversion of alpha pinene to verbenone by resting cells of Aspergillus niger. Appl. Microbiol. Biotechnol. 2000, 53, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, G.R.; Sora, C.; Ion, S.; Parvulescu, V.I.; Tudorache, M. Cascade Biocatalysis Designed for the Allylic Oxidation of α-Pinene. Catalysts 2021, 11, 134. [Google Scholar] [CrossRef]
- Allal, B.A.; El Firdoussi, L.; Allaoud, S.; Karim, A.; Castanet, Y.; Mortreux, A. Catalytic oxidation of α-pinene by transition metal using t-butyl hydroperoxide and hydrogen peroxide. J. Mol. Catal. A Chem. 2003, 200, 177–184. [Google Scholar] [CrossRef]
- Chatterjee, S.; Bhanja, P.; Paul, L.; Ali, M.; Bhaumik, A. MnAPO-5 as an efficient heterogeneous catalyst for selective liquid phase partial oxidation reactions. Dalton Trans. 2018, 47, 791–798. [Google Scholar] [CrossRef]
- Nan, S.; Zhang, X.; Jin, L.; Hu, B.; Shen, Z.; Hu, X. Recyclable copper-catalyzed ambient aerobic oxidation of primary alcohols to aldehydes in water using water-soluble PEG-functionalized pyridine triazole as ligand. Catal. Commun. 2017, 101, 5–9. [Google Scholar]
- Feng, X.; Lv, P.; Sun, W.; Han, X.; Gao, L.; Zheng, G. Reduced graphene oxide-supported Cu nanoparticles for the selective oxidation of benzyl alcohol to aldehyde with molecular oxygen. Catal. Commun. 2017, 99, 105–109. [Google Scholar] [CrossRef]
- Mdletshe, L.S.; Makgwane, P.R.; Ray, S.S. Fabrication of Bimetal CuFe2O4 Oxide Redox-Active Nanocatalyst for Oxidation of Pinene to Renewable Aroma Oxygenates. Nanomaterials 2019, 9, 1140. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.M.; Luan, H.M.; Xiao, F.S. Targeted synthesis of zeolites from calculated interaction between zeolite structure and organic template. Natl. Sci. Rev. 2022, 9. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Pan, J.; Sun, F.; Huang, K.; He, M.; Chen, Q. Catalytic oxidation of 4-tert-butyltoluene to 4-tert-butylbenzaldehyde over cobalt modified APO-5 zeolite. React. Kinet. Mech. Catal. 2016, 117, 789–799. [Google Scholar] [CrossRef]
- Du, Y.; Feng, B.; Jiang, Y.; Yuan, L.; Huang, K.; Li, J. Solvent-Free Synthesis and n-Hexadecane Hydroisomerization Performance of SAPO-11 Catalyst. Eur. J. Inorg. Chem. 2018, 2018, 2599–2606. [Google Scholar] [CrossRef]
- Ng, E.; Ghoy, J.-P.; Awala, H.; Vicente, A.; Adnan, R.; Ling, T.C.; Mintova, S. Ionothermal synthesis of FeAPO-5 in the presence of phosphorous acid. CrystEngComm 2015, 18, 257–265. [Google Scholar] [CrossRef]
- Sundaravel, B.; Babu, C.M.; Palanisamy, B.; Palanichamy, M.; Shanthi, K.; Murugesan, V. Praseodymium incorporated AIPO-5 molecular sieves for aerobic oxidation of ethylbenzene. J. Nanosci. Nanotechnol. 2013, 13, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Sundaravel, B.; Babu, C.M.; Vinodh, R.; Cha, W.S.; Jang, H.-T. Synthesis of campholenic aldehyde from α-pinene using bi-functional PrAlPO-5 molecular sieves. J. Taiwan Inst. Chem. Eng. 2016, 63, 157–165. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, J.; Chen, C.; Wang, F.; Li, X. Synthesis of Fe, Co, and Mn substituted AlPO-5 molecular sieves and their catalytic activities in the selective oxidation of cyclohexane. J. Porous Mater. 2008, 15, 7–12. [Google Scholar] [CrossRef]
- Muñoz, T.; Prakash, A.M.; Kevan, L.; Balkus, K.J. Synthesis and Characterization of CuAPO-5 Molecular Sieves: Evidence for the Framework Incorporation of Cu(II) Ions. J. Phys. Chem. B 1998, 102, 1379–1386. [Google Scholar] [CrossRef]
- Dang, T.T.H.; Zubowa, H.-L.; Bentrup, U.; Richter, M.; Martin, A. Microwave-assisted synthesis and characterization of Cu-containing AlPO4-5 and SAPO-5. Microporous Mesoporous Mater. 2009, 123, 209–220. [Google Scholar] [CrossRef]
- Chow, L.; Lupan, O.; Chai, G.; Khallaf, H.; Ono, L.; Cuenya, B.R.; Tiginyanu, I.; Ursaki, V.; Sontea, V.; Schulte, A. Synthesis and characterization of Cu-doped ZnO one-dimensional structures for miniaturized sensor applications with faster response. Sens. Actuators A 2013, 189, 399–408. [Google Scholar] [CrossRef]
- Xingyi, Q.; Lili, Z.; Wenhua, X.; Tianhao, J.; Rongguang, L. Synthesis of copper-substituted aluminophosphate molecular sieves (CuAPO-11) and their catalytic behavior for phenol hydroxylation. Appl. Catal. A Gen. 2004, 276, 89–94. [Google Scholar] [CrossRef]
- Ke, Q.P.; Wu, M.; Yu, H.; Lu, G. Superior Catalytic Performance of Hierarchically Micro-Meso-Macroporous CuAlPO-5 for the Oxidation of Aromatic Amines under Mild Conditions. ChemCatChem 2017, 9, 733–737. [Google Scholar] [CrossRef]
- Zhang, H.X.; Chokkalingam, A.; Subramaniam, P.V.; Joseph, S.; Takeuchi, S.; Wei, M.D.; Al-Enizi, A.M.; Jang, H.-G.; Kim, J.-H.; Seo, G.; et al. The isopropylation of biphenyl over transition metal substituted aluminophosphates: MAPO-5 (M: Co and Ni). J. Mol. Catal. A Chem. 2015, 412, 117–124. [Google Scholar] [CrossRef]
- Hartmann, M.; Kevan, L. Substitution of transition metal ions into aluminophosphates and silicoaluminophosphates: Characterization and relation to catalysis. Res. Chem. Intermed. 2002, 28, 625–695. [Google Scholar] [CrossRef]
- Devika, S.; Palanichamy, M.; Murugesan, V. Selective oxidation of ethylbenzene over CeAlPO-5. Appl. Catal. A 2011, 407, 76–84. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Efficient valorization of biomass-derived furfural to fuel bio-additive over aluminum phosphate. Appl. Catal. B Environ. 2021, 298, 120575. [Google Scholar] [CrossRef]
- Dongare, M.K.; Sabde, D.; Shaikh, R.; Kamble, K.; Hegde, S. Synthesis, characterization and catalytic properties of ZrAPO-5. Catal. Today 1999, 49, 267–276. [Google Scholar] [CrossRef]
- Lajunen, M.K. Co(II) catalysed oxidation of α-pinene by molecular oxygen: Part III. J. Mol. Catal. A Chem. 2001, 169, 33–40. [Google Scholar] [CrossRef]
- Modén, B.; Oliviero, L.; Dakka, J.; Santiesteban, A.J.G.; Iglesia, E. Structural and Functional Characterization of Redox Mn and Co Sites in AlPO Materials and Their Role in Alkane Oxidation Catalysis. J. Phys. Chem. B 2004, 108, 5552–5563. [Google Scholar] [CrossRef] [Green Version]
- Ajaikumar, S.; Ahlkvist, J.; Larsson, W.; Shchukarev, A.; Leino, A.-R.; Kordas, K.; Mikkola, J.-P. Oxidation of α-pinene over gold containing bimetallic nanoparticles supported on reducible TiO2 by deposition-precipitation method. Appl. Catal. A 2011, 392, 11–18. [Google Scholar] [CrossRef]
- Casuscelli, S.G.; Eimer, G.A.; Canepa, A.; Heredia, A.C.; Poncio, C.E.; Crivello, M.E.; Perez, C.F.; Aguilar, A.; Herrero, E.R. Ti-MCM-41 as catalyst for α-pinene oxidation. Catal. Today 2008, 133–135, 678–683. [Google Scholar] [CrossRef]
- Desai, N.C.; Chudasama, J.A.; Patel, B.Y.; Jadeja, K.A.; Karkar, T.J.; Mehta, J.P.; Godhani, D.R. Catalysis by the entangled complexes in matrix structure of zeolite-Y over α-pinene. Microporous Mesoporous Mater. 2017, 242, 245–255. [Google Scholar] [CrossRef]
- Parmar, D.K.; Butani, P.M.; Thumar, N.J.; Jasani, P.M.; Padaliya, R.V.; Sandhiya, P.R.; Nakum, H.D.; Khan, N.; Makwana, D. Oxy-functionalization of olefins with neat and heterogenized binuclear V(IV)O and Fe(II) complexes: Effect of steric hindrance on product selectivity and output in homogeneous and heterogeneous phase. Mol. Catal. 2019, 474, 110424. [Google Scholar] [CrossRef]





| Catalyst | Surface Area, ABET (m2·g−1) | Pore Volume, Vp (cm3·g−1) | Cu, wt% | Number of Acid Sites (mmol·g−1) | |||
|---|---|---|---|---|---|---|---|
| Weak 1 | Moderate 2 | Strong 3 | Total Acid | ||||
| AlPO4-5 | 359 | 0.44 | 0.10 | 0.09 | 0 | 0.19 | |
| CuAPO-5(0.03) | 357 | 0.48 | 0.86 | 0.11 | 0.13 | 0.05 | 0.29 |
| CuAPO-5(0.06) | 359 | 0.48 | 1.88 | 0.12 | 0.14 | 0.05 | 0.31 |
| CuAPO-5(0.09) | 353 | 0.45 | 3.20 | 0.13 | 0.13 | 0.04 | 0.30 |
| Entry | Catalysts | Conv. (%) α-Pinene | Selectivity (%) | |
|---|---|---|---|---|
| Verbenol | Verbenone | |||
| 1 | Blank | 0 | 0 | 0 |
| 2 | CuAPO-5 (no TBHP) | 0 | 0 | 0 |
| 3 | CuAPO-5(0.03) | 81.4 | 3.0 | 30.6 |
| 4 | CuAPO-5(0.06) | 83.2 | 3.5 | 36.4 |
| 5 | CuAPO-5(0.09) | 75.0 | 1.7 | 25.6 |
| Entry | Time (h) | Conv. (%) α-Pinene | Selectivity (%) | |
|---|---|---|---|---|
| Verbenol | Verbenone | |||
| 1 | 6 | 64.4 | 2.7 | 23.9 |
| 2 | 8 | 75.2 | 3.2 | 31.8 |
| 3 | 10 | 83.2 | 3.5 | 36.4 |
| 4 | 12 | 84.7 | 2.5 | 40.1 |
| 5 | 14 | 87.5 | 1.9 | 39.2 |
| Entry | Solvent | Conv. (%) α-Pinene | Selectivity (%) | |
|---|---|---|---|---|
| Verbenol | Verbenone | |||
| 1 | Chloroform | 96.8 | 3.4 | 42.3 |
| 2 | Acetonirile | 91.4 | 1.3 | 38.7 |
| 3 | Acetone | 13 | 0 | 3.1 |
| 4 | Ethylacetate | 25 | 2 | 5.4 |
| 5 | DMF | 85.4 | 9.2 | 33.1 |
| Entry | Catalyst | α-Pinene/Catalyst (mmol/mg) | Conditions | Solvent | Conv. (%) α-Pinene | Selectivity (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Verbenone | |||||||
| 1 | AuCu/TiO2 | 5/100 | TBHP (5 mmol), 24 h, 70 °C | acetonitrile | 97 | 47.9 | [38] |
| 2 | Ti-MCM-41 | 8.4/63 | H2O2 (2.1 mmol), 7 h, 70 °C | acetonitrile | 16.04 | 39 | [39] |
| 3 | FePcCl16-NH2-SiO2 | 0.1875/8.4 | TBHP (0.4875 mmol), 23 h, 40 °C | acetone | 83.7 | 23 | [40] |
| 4 | [VO(HMIMMPP)(H2O)]-Y | 30/100 | TBHP (75 mmol), 12 h, 80 °C | acetonitrile | 45.28 | 40.69 | [7] |
| 5 | CuFe2O4 | 1/100 | TBHP (2 mmol), 20 h, 90 °C | acetonitrile | 46.6 | 38.9 | [18] |
| 6 | Neat [Fe(sal2bz)(H2O)2]2·2H2O | 10/15 | H2O2 (20 mmol), 24 h, 80 °C | acetonitrile | 100 | 79 | [41] |
| 7 | CuCl2 | 7.3/9.8 | TBHP (44 mmol), 6 h, 70 °C | acetonitrile | 100 | 78 | [14] |
| 8 | CuAPO-5(0.06) | 1/30 | TBHP (3 mmol), 12 h, 85 °C | chloroform | 96.8 | 46.4 | This work 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Cheng, H.; Lai, F.; Xiong, D. CuAPO-5 as a Multiphase Catalyst for Synthesis of Verbenone from α-Pinene. Materials 2022, 15, 8097. https://doi.org/10.3390/ma15228097
Wang H, Cheng H, Lai F, Xiong D. CuAPO-5 as a Multiphase Catalyst for Synthesis of Verbenone from α-Pinene. Materials. 2022; 15(22):8097. https://doi.org/10.3390/ma15228097
Chicago/Turabian StyleWang, Hongyun, Haijun Cheng, Fang Lai, and Deyuan Xiong. 2022. "CuAPO-5 as a Multiphase Catalyst for Synthesis of Verbenone from α-Pinene" Materials 15, no. 22: 8097. https://doi.org/10.3390/ma15228097





