Polylactide (PLA) as a Cell Carrier in Mesophilic Anaerobic Digestion—A New Strategy in the Management of PLA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum Sources
2.2. Carrier Source and Preparation
2.3. Anaerobic Digestion
2.3.1. Batch Preparation
2.3.2. Biogas Production and Analysis
2.4. Analysis of Substrate and Digestate Samples
2.5. Analysis of Carrier’s Properties
2.5.1. Microscopy and Microstructural Investigations
2.5.2. Apparent Density of PLA Granules and Powder State
2.5.3. Elemental Analysis
2.5.4. Differential Scanning Calorimetry Analysis
2.6. Bacillus Amyloliquefaciens Biomass Designation
2.7. Analysis of the Biochemical Activity of the Digestate
2.8. Statistical Analysis
3. Results and Discussion
3.1. Substrates and Inoculum Characterisation
3.2. Polylactide Properties and Productivity
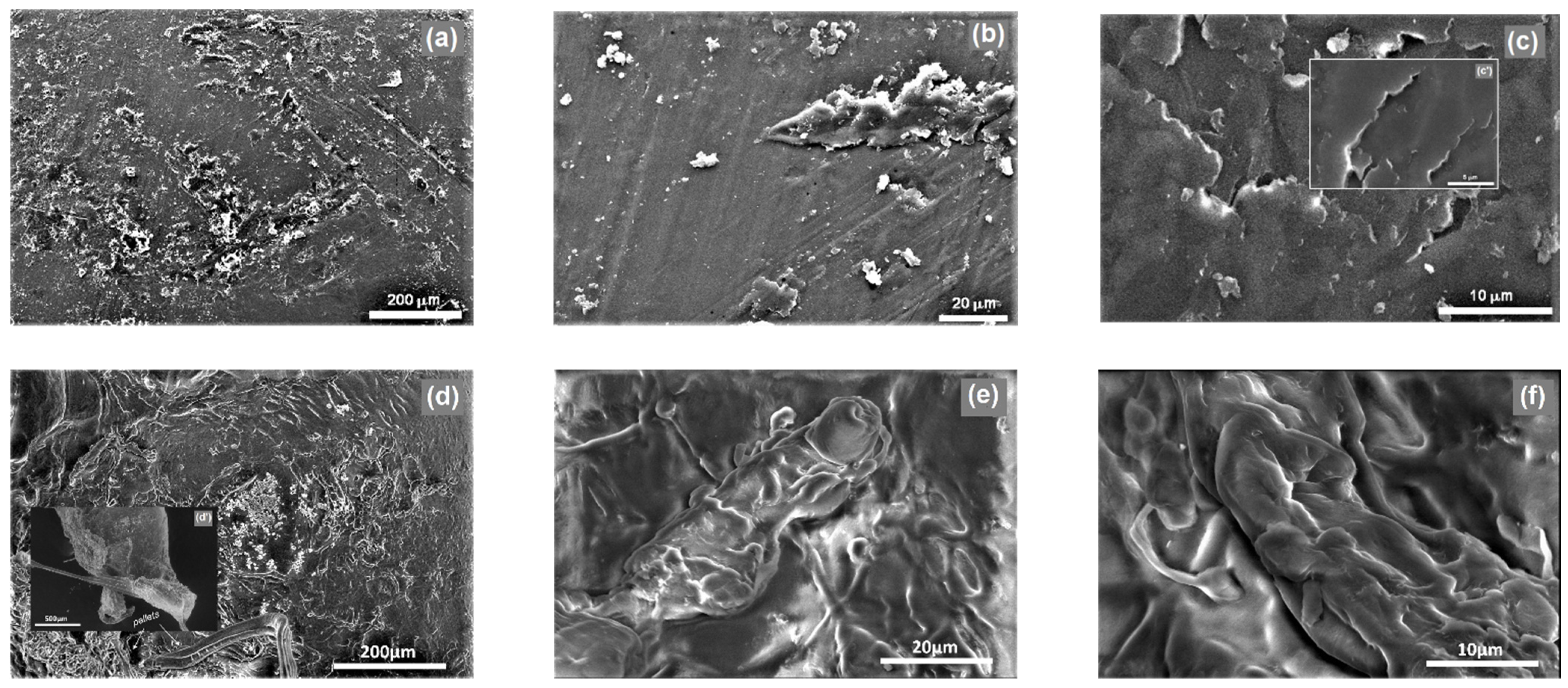
3.2.1. Morphological and Microstructural Properties
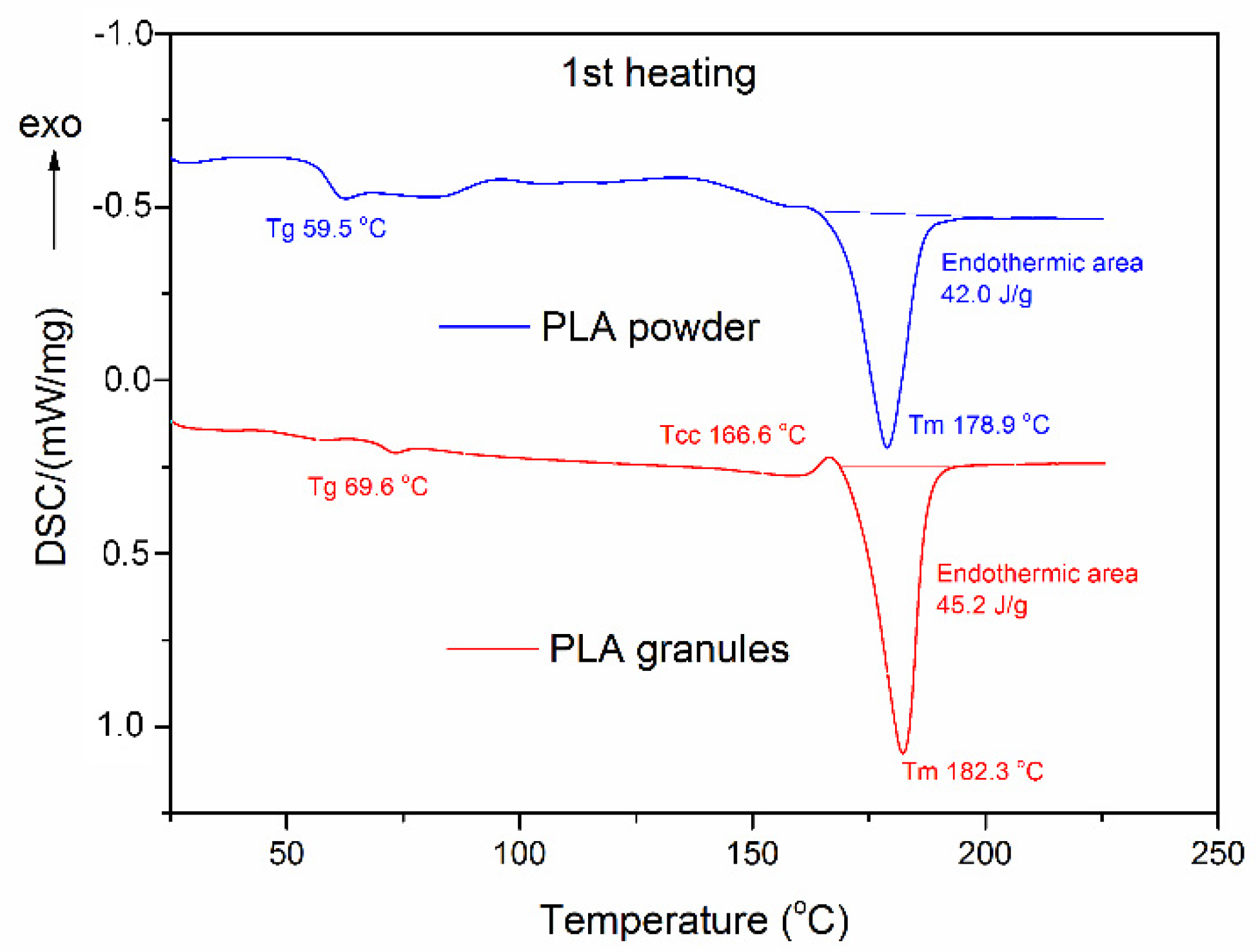
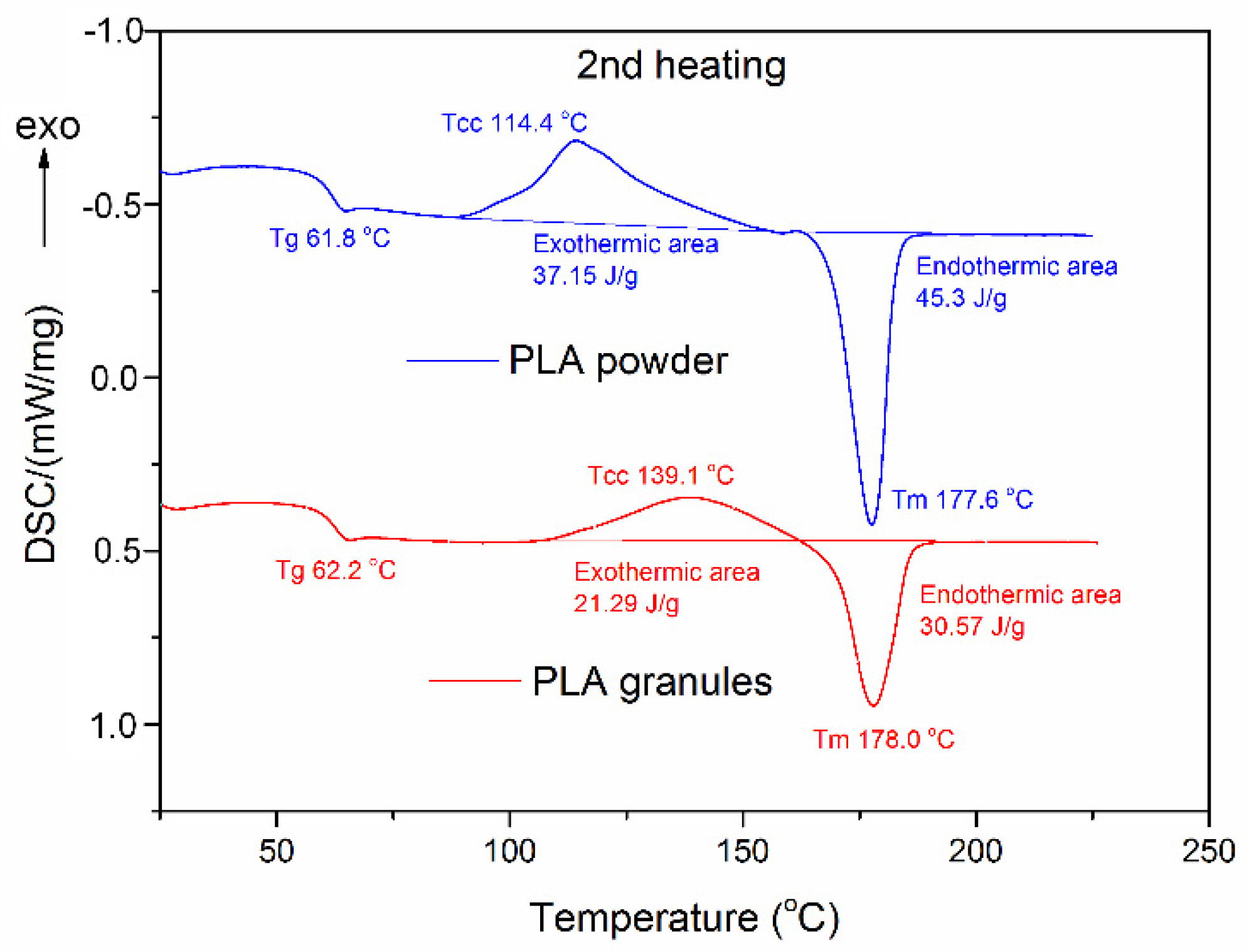
3.2.2. Thermal Properties
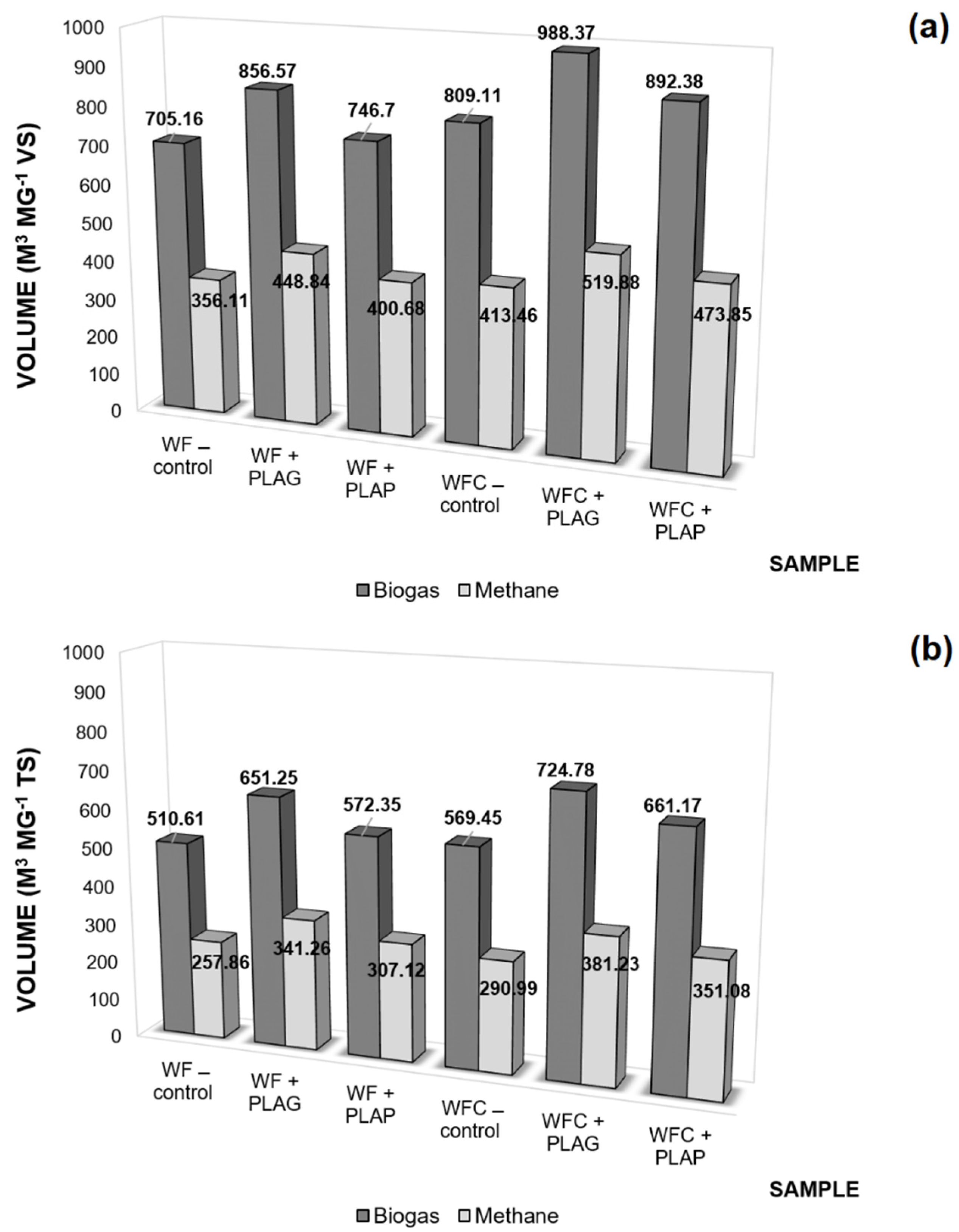
3.2.3. Carrier Productivity
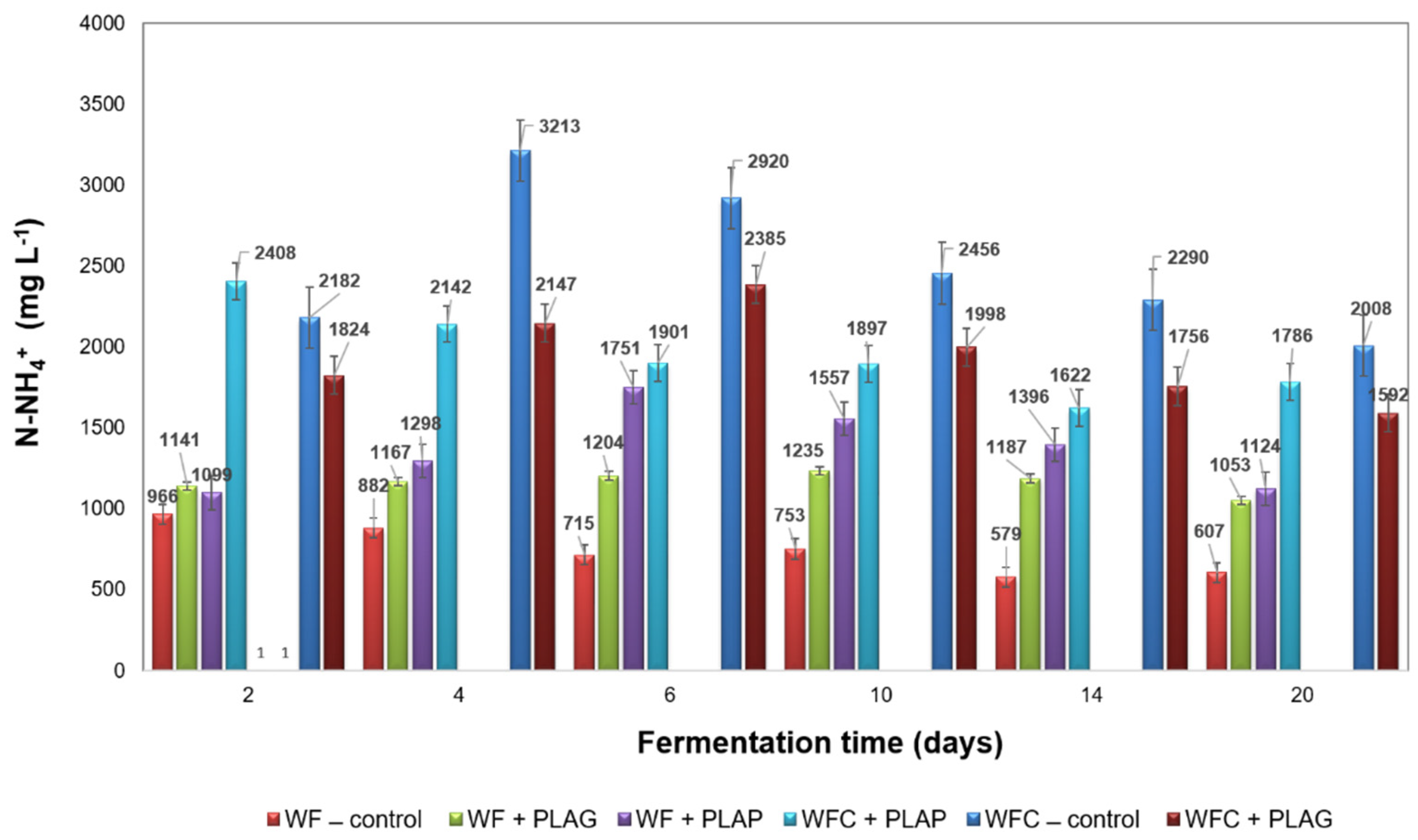
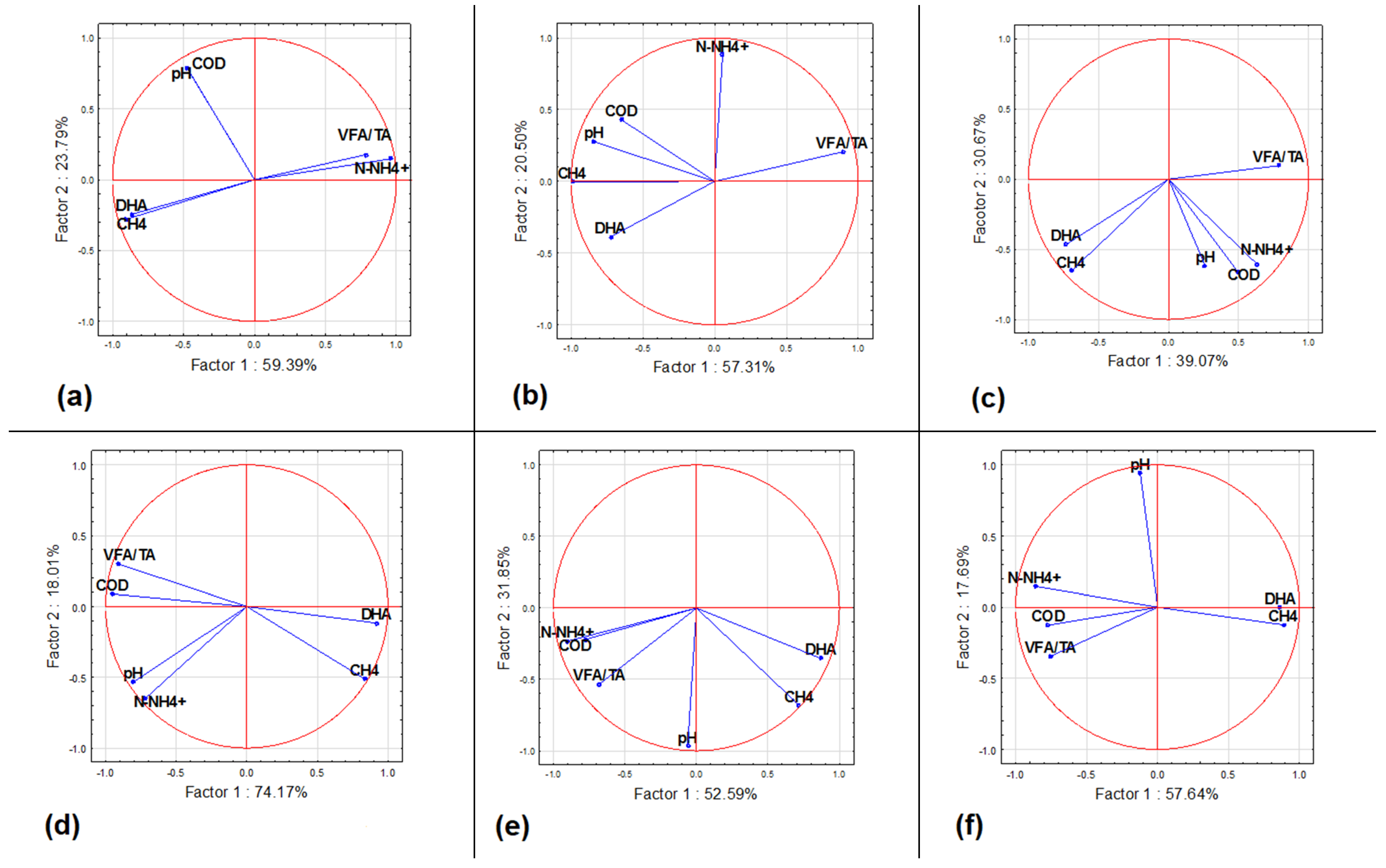
3.3. Anaerobic Digestion Stability and Performance
3.4. Dehydrogenase Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- IEA. Curtailing Methane Emissions from Fossil Fuel Operations Pathways to a 75% Cut by 2030; International energy agency report; IEA: Paris, France, 2022. [Google Scholar]
- Barrena, R.; Moral-Vico, J.; Font, X.; Sánchez, A. Enhancement of anaerobic digestion with nanomaterials: A mini review. Energies 2022, 15, 5087. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Pilarski, K.; Janczak, D.; Przybył, K.; Gawrysiak-Witulska, M. The use of lignin as a microbial carrier in the co-digestion of cheese and wafer waste. Polymers 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Li, Y.; Ge, X.; Yang, L.; Li, Y. Anaerobic digestion of food waste—Challenges and opportunities. Bioresour. Technol. 2018, 247, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Pilarski, K.; Wolna-Maruwka, A.; Boniecki, P.; Zaborowicz, M. Use of confectionery waste in biogas production by the anaerobic digestion process. Molecules 2019, 24, 37. [Google Scholar] [CrossRef] [Green Version]
- König, R.; Cuomo, M.; Pianta, E.; Buetti, A.; Mauri, F.; Tanadini, M.; Principi, P. Addition of conductive materials to support syntrophic microorganisms in anaerobic digestion. Fermentation 2022, 8, 354. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Jarosław Grządziel, J.; Gałązka, A.; Paluch, E.; Borowiak, K.; Pilarski, K. Quantitative and qualitative changes in the genetic diversity of bacterial communities in anaerobic bioreactors with the diatomaceous earth/peat cell carrier. Cells 2022, 11, 2571. [Google Scholar] [CrossRef]
- Weiß, S.; Zankel, A.; Lebuhn, M.; Petrak, S.; Somitsch, W.; Guebitz, G.M. Investigation of microorganisms colonising activated zeolites during anaerobic biogas production from grass silage. Bioresour. Technol. 2011, 102, 4353–4359. [Google Scholar] [CrossRef]
- Chauhan, A.; Ogram, A. Evaluation of support matrices for immobilization of anaerobic consortia for efficient carbon cycling in waste regeneration. Biochem. Biophys. Res. Commun. 2005, 327, 884–893. [Google Scholar] [CrossRef]
- Purnomo, C.W.; Mellyanawaty, M.; Budhijanto, W. Simulation and experimental study on iron impregnated microbial immobilization in zeolite for production of biogas. Waste Biomass Valor. 2017, 8, 2413–2421. [Google Scholar] [CrossRef]
- Yan, W.; Mukherjee, M.; Zhou, Y. Direct interspecies electron transfer (DIET) can be suppressed under ammonia-stressed conditio—Reevaluate the role of conductive materials. Water Res. 2020, 183, 116094. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Che, X.; Li, C.; Liang, D.; Zhou, H.; Liu, L.; Zhao, Z.; Zhang, Y. Biochar stimulates growth of novel species capable of direct interspecies electron transfer in anaerobic digestion via ethanol-type fermentation. Environ. Res. 2020, 189, 109983. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Joshi, C.; Paritosh, K.; Thakur, J.; Pareek, N.; Masakapalli, S.K.; Vivekanand, V. Reprint of Organic waste conversion through anaerobic digestion: A critical insight into the metabolic pathways and microbial interactions. Metab. Eng. 2022, 71, 62–76. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, T.L.; Júnior, J.E.M.; de Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic acid production from biotechnological routes: A review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Murariu, M.; Bonnaud, L.; Yoann, P.; Fontaine, G.; Bourbigot, S.; Dubois, P. New trends in polylactide (PLA)-based materials: “Green” PLA: Calcium sulfate (nano)composites tailored with flame retardant properties. Polym. Degrad. Stab. 2010, 95, 374–381. [Google Scholar] [CrossRef]
- Rahman, M.M.; Afrin, S.; Haque, P.; Islam, M.M.; Islam, M.S.; Gafur, M.A. Preparation and characterization of jute cellulose crystals-reinforced poly(L-lactic acid) biocomposite for biomedical applications. Int. J. Chem. Eng. 2014, 2014, 842147. [Google Scholar] [CrossRef] [Green Version]
- Kosowska, K.; Domalik-Pyzik, P.; Krok-Borkowicz, M.; Chłopek, J. Polylactide/hydroxyapatite nonwovens incorporated into chitosan/graphene materials hydrogels to form novel hierarchical scaffolds. Int. J. Mol. Sci. 2020, 21, 2330. [Google Scholar] [CrossRef] [Green Version]
- Grząbka-Zasadzińska, A.; Klapiszewski, Ł.; Bula, K.; Jesionowski, T.; Borysiak, S. Supermolecular structure and nucleation ability of polylactide based composites with silica/lignin hybrid fillers. J. Therm. Anal. Calorim. 2016, 126, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Garlotta, D. A literature review of poly(lactic acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Rasselet, D.; Ruellan, A.; Guinault, A.; Miquelard-Garnier, G.; Sollogoub, C.; Fayolle, B. Oxidative degradation of polylactide (PLA) and its effects on physical and mechanical properties. Eur. Polym. J. 2014, 50, 109–116. [Google Scholar] [CrossRef]
- Najafi, N.; Heuzey, M.C.; Carreau, P.J.; Therriault, D.; Park, C.B. Mechanical and morphological properties of injection molded linear and branched-polylactide (PLA) nanocomposite foams. Eur. Polym. J. 2015, 73, 455–465. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.Q.; Wei, Q.Y.; Chen, Y.; Jia, D.Z.; Lin, H.; Zhong, G.J.; Li, Z.M. Light weight, low dielectric constant, super-robust polylactide film based on stress-induced cavitation aided by crystallization. Polymer 2022, 256, 125234. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Cazaudehore, G.; Guyoneaud, R.; Evon, P.; Martin-Closas, L.; Pelacho, A.M.; Raynaud, C.; Monlau, F. Can anaerobic digestion be a suitable end-of-life scenario for biodegradable plastics? A critical review of the current situation, hurdles, and challenges. Biotechnol. Adv. 2022, 56, 107916. [Google Scholar] [PubMed]
- Kolstad, J.J.; Vink, E.T.H.; Wilde, B.D.; Debeer, L. Assessment of anaerobic degradation of IngeoTM polylactides under accelerated landfill conditions. Polym. Degrad. Stab. 2012, 97, 1131–1141. [Google Scholar] [CrossRef]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M. Augmentation of a microbial consortium for enhanced polylactide (PLA) degradation. Indian J. Microbiol. 2016, 56, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Hidaka, T.; Tsuno, H.; Tsubota, J. Co-digestion of polylactide and kitchen garbage in hyperthermophilic and thermophilic continuous anaerobic process. Bioresour. Technol. 2012, 112, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Tsuno, H.; Hidaka, T.; Tsubota, J. Promotion of polylactide degradation by ammonia under hyperthermophilic anaerobic conditions. Bioresour. Technol. 2011, 102, 9933–9941. [Google Scholar] [CrossRef]
- Benn, N.; Zitome, D. Pretreatment and anaerobic co-digestion of selected PHB and PLA bioplastics. Front. Environ. Sci. 2018, 5, 93. [Google Scholar] [CrossRef] [Green Version]
- Alqaralleh, R.M.; Kennedy, K.; Delatolla, R. Improving biogas production from anaerobic co-digestion of Thickened Waste Activated Sludge (TWAS) and fat, oil and grease (FOG) using a dual-stage hyper-thermophilic/thermophilic semi-continuous reactor. J. Environ. Manag. 2018, 217, 416–428. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Sosulski, T.; Stępień, W.; Pilarski, K.; Pilarska, A. Chemical properties and fertilizer value of ten different anaerobic digestates. Fresenius Environ. Bull. 2018, 27, 3425–3432. [Google Scholar]
- Pilarska, A.A.; Pilarski, K.; Waliszewska, B.; Zborowska, M.; Witaszek, K.; Waliszewska, H.; Kolasi´nski, M.; Szwarc-Rzepka, K. Evaluation of bio-methane yields for high-energy organic waste and sewage sludge: A pilot-scale study for a wastewater treatment plant. Environ. Eng. Manag. J. 2019, 18, 2019–2030. [Google Scholar] [CrossRef]
- Norm VDI 4630; Fermentation of Organic Materials Characterization of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Engineers Club: Düsseldorf, Germany, 2006.
- Tixier, N.; Guibaud, G.; Baudu, M. Determination of some rheological parameters for the characterization of activated sludge. Bioresour. Technol. 2003, 90, 215–220. [Google Scholar] [CrossRef]
- Markis, F.; Baudez, J.; Parthasarathy, R.; Slatter, P.; Eshtiaghi, N. Rheological characterisation of primary and secondary sludge: Impact of solids concentration. Chem. Eng. J. 2014, 253, 526–537. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Pilarski, K.; Witaszek, K.; Waliszewska, H.; Zborowska, M.; Waliszewska, B.; Kolasiński, M.; Szwarc-Rzepka, K. Treatment of dairy waste by anaerobic digestion with sewage sludge. Ecol. Chem. Eng. 2016, 23, 99–115. [Google Scholar] [CrossRef] [Green Version]
- DIN Guideline 38 414-S8; Characterisation of the Substrate, Sampling, Collection of Material Data, Fermentation Tests. German Institute for Standardization: Berlin, Germany, 1985.
- Pilarska, A.A.; Pilarski, K.; Adamski, M.; Zaborowicz, M.; Dorota Cais-Sokolińska, D.; Wolna-Maruwka, A.; Niewiadomska, A. Eco-friendly and effective diatomaceous earth/peat (DEP) microbial carriers in the anaerobic biodegradation of food waste products. Energies 2022, 15, 3442. [Google Scholar] [CrossRef]
- EN ISO 60:2000 (The British Standard); Plastics—Determination of Apparent Density of Material That Can Be Poured from a Specified Funnel. British Standards Institution (BSI): London, UK, 2000.
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarski, K.; Olesienkiewicz, A. A Comparison of the influence of kraft lignin and the kraft lignin/silica system as cell carriers on the stability and efficiency of the anaerobic digestion process. Energies 2020, 13, 5803. [Google Scholar] [CrossRef]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of dehydrogenase activity in acid soilsrich in organic matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Rico, C.; Muñoz, N.; Fernández, J.; Rico, J.L. High-load anaerobic co-digestion of cheese whey and liquid fraction of dairy manure in a one-stage UASB process: Limits in co-substrates ratio and organic loading rate. Chem. Eng. J. 2015, 262, 794–802. [Google Scholar] [CrossRef]
- Boonmee, C.; Kositanont, C.; Leejarkpai, T. Degradation of poly (lactic acid) under simulated landfill conditions. Environ. Natural Res. J. 2016, 14, 1–9. [Google Scholar]
- Liu, Q.; Zhao, M.; Zhou, Y.; Yang, Q.; Shen, Y.; Gong, R.H.; Zhou, F.; Li, Y.; Deng, B. Polylactide single-polymer composites with a wide melt-processing window based on core-sheath PLA fibers. Mater. Des. 2018, 139, 36–44. [Google Scholar] [CrossRef]
- Coltelli, M.B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin nanofibrils in poly(lactic acid) (PLA) nanocomposites: Dispersion and thermo-mechanical properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Wepf, R.; Fischer, K.; Schmidt, M.; Besse, S.; Lindner, P.; King, B.T.; Sigel, R.; Schurtenberger, P.; Talmon, Y.; et al. Dieter SchlüterThe largest synthetic structure with molecular precision: Towards a molecular object. Angew. Chem. (Int. Ed.) 2011, 50, 737–740. [Google Scholar] [CrossRef]
- Teixeira, S.; Eblagon, K.M.; Miranda, M.; Pereira, M.F.R.; Figueiredo, J.L. Towards controlled degradation of poly(lactic) acid in technical applications. C J. Carbon Res. 2021, 7, 42. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Zhu, T.; Wang, Z.; Chen, L.; Fu, L. Morphology, crystallization and thermal behaviors of PLA-based composites: Wonderful effects of hybrid GO/PEG via dynamic impregnating. Polymers 2017, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Deb, P.; Talukdar, S.A.; Mohsina, K.; Sarker, P.K.; Sayem, S.M.A. Production and partial characterization of extracellular amylase enzyme from Bacillus amyloliquefaciens P-001. SpringerPlus 2013, 2, 154. [Google Scholar] [CrossRef] [Green Version]
- Daas, M.S.; Acedo, J.Z.; Rosana, A.R.R.; Orata, F.D.; Reiz, B.; Zheng, J.; Nateche, F.; Case, R.J.; Kebbouche-Gana, S.; Vederas, J.C. Bacillus amyloliquefaciens ssp. plantarum F11 isolated from Algerian salty lake as a source of biosurfactants and bioactive lipopeptides. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska; Pilarski, K.; Adamski, M.; Grzyb, A.; Grządziel, J.; Gałązka, A. Silica/lignin carrier as a factor increasing the process performance and genetic diversity of microbial communities in laboratory-scale anaerobic digesters. Energies 2021, 14, 4429. [Google Scholar] [CrossRef]
- Ryder, D.S. Immobilized yeast in brewing: To immobilize or not to immobilize—That is the question? In Cell Physiology and Interactions of Biomaterials and Matrices; COST 840 and X International BRG Workshop on Bioencapsulation, Physiology and Morphology of Immobilized Cells: Prague, Czech Republic, 2002. [Google Scholar]
- Elhaj, M.A.; Hossain, M.H.; Imtiaz, S.A.; Naterer, G.F. Hysteresis of wettability in porous media: A review. J. Petrol. Explor. Prod. Technol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Jung, I.D.; Ko, J.S. The effect of the surface wettability of nanoprotrusions formed on network-type microstructures. Micromech. Microeng. 2008, 18, 125007. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Liu, X.; Fu, B.; Chen, J.; Yu, H.Q. Acidogenic fermentation of proteinaceous sewage sludge: Effect of pH. Water Res. 2012, 46, 799–807. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Nghiem, L.D.; Hai, F.I.; Deng, L.J.; Wang, J.; Wu, Y. Optimization of process parameters for production of volatile fatty acid, biohydrogen and methane from anaerobic digestion. Bioresour. Technol. 2016, 219, 738–748. [Google Scholar] [CrossRef]
- Morales-Polo, C.; Cledera-Castro, M.M.; Soria, B.Y.M. Reviewing the anaerobic digestion of food waste: From waste generation and anaerobic process to its perspectives. Appl. Sci. 2018, 8, 1804. [Google Scholar] [CrossRef] [Green Version]
- Boniecki, P.; Nowakowski, K.; Ślósarz, P.; Dach, J.; Pilarski, K. Neural image analysis for estimating aerobic and anaerobic decomposition of organic matter based on the example of straw decomposition. In Proceedings of the 4th International Conference on Digital Image Processing, Kuala Lumpur, Malaysia, 7–8 April 2012; Volume 8334. [Google Scholar]
- Järvan, M.; Edesi, L.; Adamson, A.; Võsa, T. Soil microbial communities and dehydrogenase activity depending on farming systems. Plant Soil Environ. 2014, 60, 459–463. [Google Scholar] [CrossRef]






| Materials | pH | Cond. | TS | VS | C/N Ratio | C | N | N-NH4 |
|---|---|---|---|---|---|---|---|---|
| - | (mS cm−1) | (wt %) | (wt %TS) | - | (wt %TS) | (wt %TS) | (wt %TS) | |
| Waste wafers | 7.12 | 2.24 | 74.85 | 98.24 | 54.83 | 45.51 | 0.83 | 0.25 |
| Waste cheese | 5.38 | 68.55 | 42.05 | 91.36 | 4.25 | 47.78 | 11.24 | 0.39 |
| Inoculum | 6.93 | 29.87 | 2.96 | 73.29 | 3.04 | 24.42 | 8.02 | 4.12 |
| Batches | WF (g) | CE (g) | Carrier (g) | Inoculum (g) | pH | TS (%) | VS (%TS) |
|---|---|---|---|---|---|---|---|
| WF − control | 9.80 | - | - | 830.0 | 7.05 | 3.86 | 75.78 |
| WF + PLAG | 9.80 | - | 20.0 | 830.0 | 7.13 | 3.84 | 76.03 |
| WF + PLAP | 9.80 | - | 20.0 | 830.0 | 7.08 | 3.98 | 76.64 |
| WFC − control | 5.50 | 2.90 | - | 832.0 | 6.96 | 3.47 | 72.93 |
| WFC + PLAP | 5.50 | 2.90 | 20.0 | 832.0 | 6.85 | 3.58 | 74.09 |
| WFC + PLAG | 5.50 | 2.90 | 20.0 | 832.0 | 6.92 | 3.37 | 72.74 |
| Element | Weight (%) | Atomic (%) | Error (%) | Net Intensity |
|---|---|---|---|---|
| C K | 56.34 | 63.87 | 12.68 | 2983.87 |
| O K | 40.84 | 34.76 | 17.47 | 1924.51 |
| Si K | 2.82 | 1.37 | 2.56 | 308.03 |
| Carriers | First Scan | Second Scan | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg1 (°C) | Tm1 (°C) | ∆Hm1 (J/g) | Tg2 (°C) | Tcc (°C) | ∆Hcc (J/g) | Tm2 (°C) | ∆Hm2 (J/g) | Xc2 (%) | |
| PLA granules | 69.6 | 182.3 | 45.2 | 62.2 | 139.1 | 21.29 | 178.0 | 30.57 | 9.9 |
| PLA powder | 59.5 | 178.9 | 42.0 | 61.8 | 114.4 | 37.15 | 177.6 | 45.30 | 8.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilarska, A.A.; Bula, K.; Pilarski, K.; Adamski, M.; Wolna-Maruwka, A.; Kałuża, T.; Magda, P.; Boniecki, P. Polylactide (PLA) as a Cell Carrier in Mesophilic Anaerobic Digestion—A New Strategy in the Management of PLA. Materials 2022, 15, 8113. https://doi.org/10.3390/ma15228113
Pilarska AA, Bula K, Pilarski K, Adamski M, Wolna-Maruwka A, Kałuża T, Magda P, Boniecki P. Polylactide (PLA) as a Cell Carrier in Mesophilic Anaerobic Digestion—A New Strategy in the Management of PLA. Materials. 2022; 15(22):8113. https://doi.org/10.3390/ma15228113
Chicago/Turabian StylePilarska, Agnieszka A., Karol Bula, Krzysztof Pilarski, Mariusz Adamski, Agnieszka Wolna-Maruwka, Tomasz Kałuża, Przemysław Magda, and Piotr Boniecki. 2022. "Polylactide (PLA) as a Cell Carrier in Mesophilic Anaerobic Digestion—A New Strategy in the Management of PLA" Materials 15, no. 22: 8113. https://doi.org/10.3390/ma15228113





