Thermal Creep Behavior and Creep Crystallization of Al-Mg-Si Aluminum Alloys
Abstract
:1. Introduction
2. Methods
3. Results
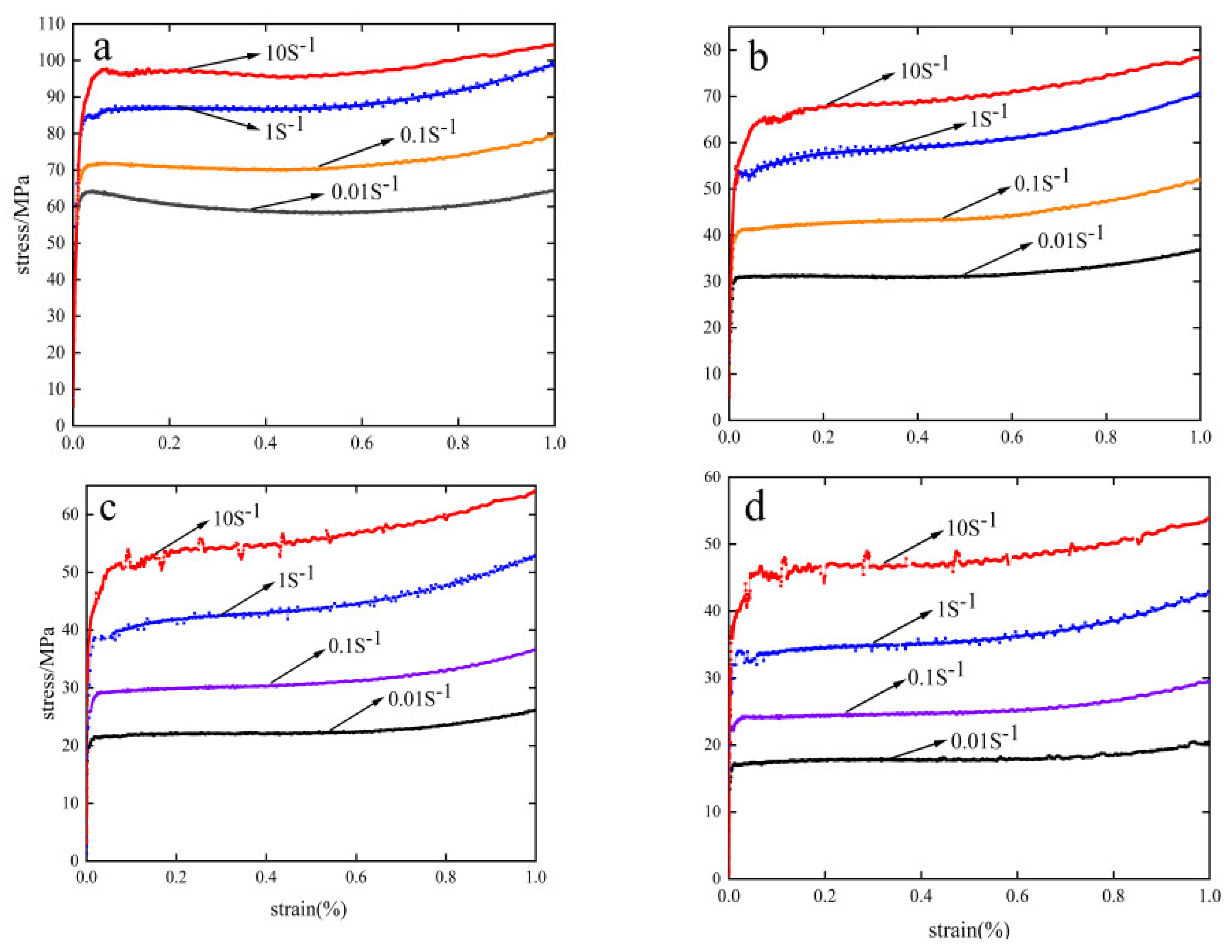
3.1. Stress–Strain Curve
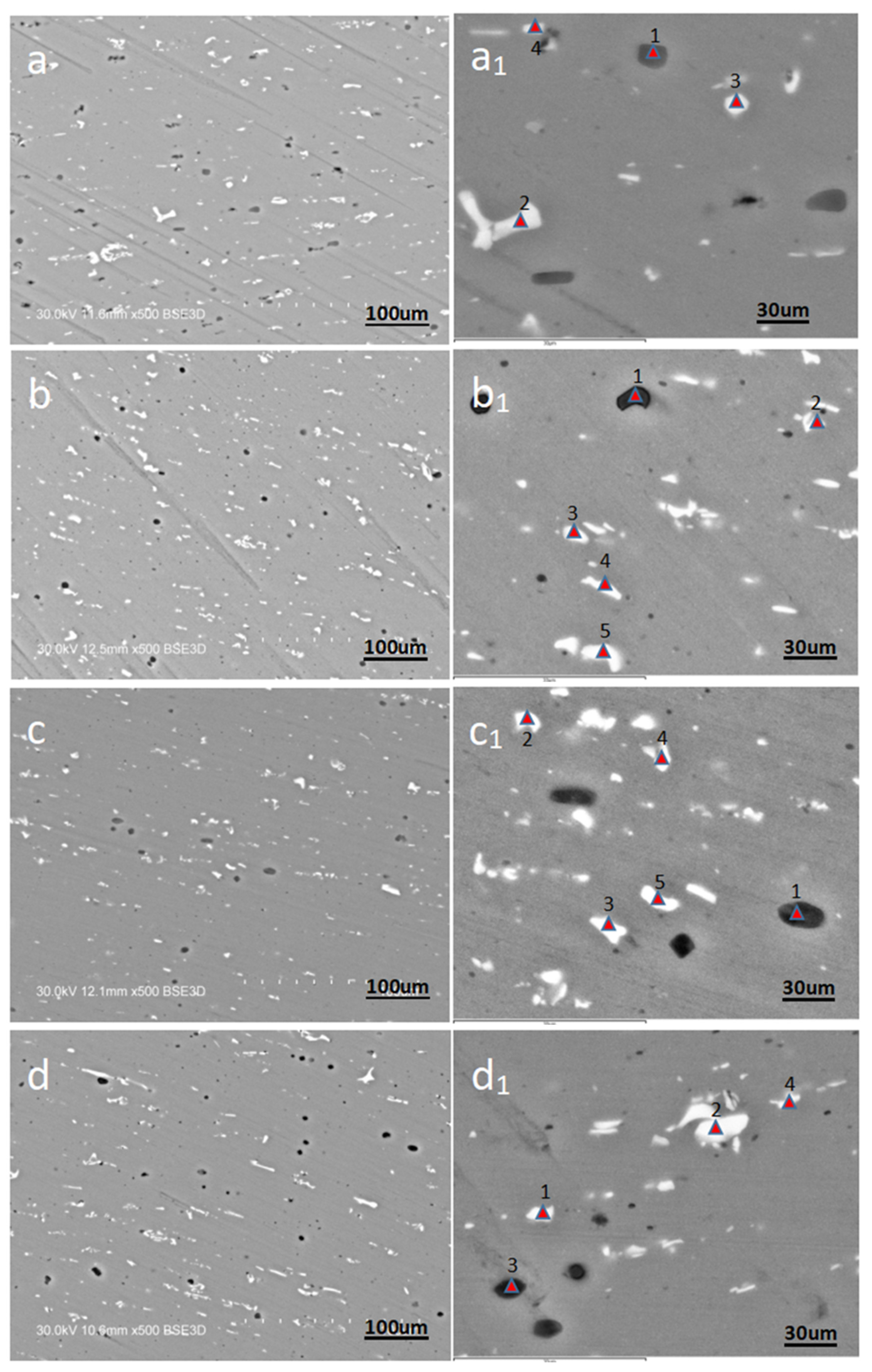
3.2. Micro-Analysis
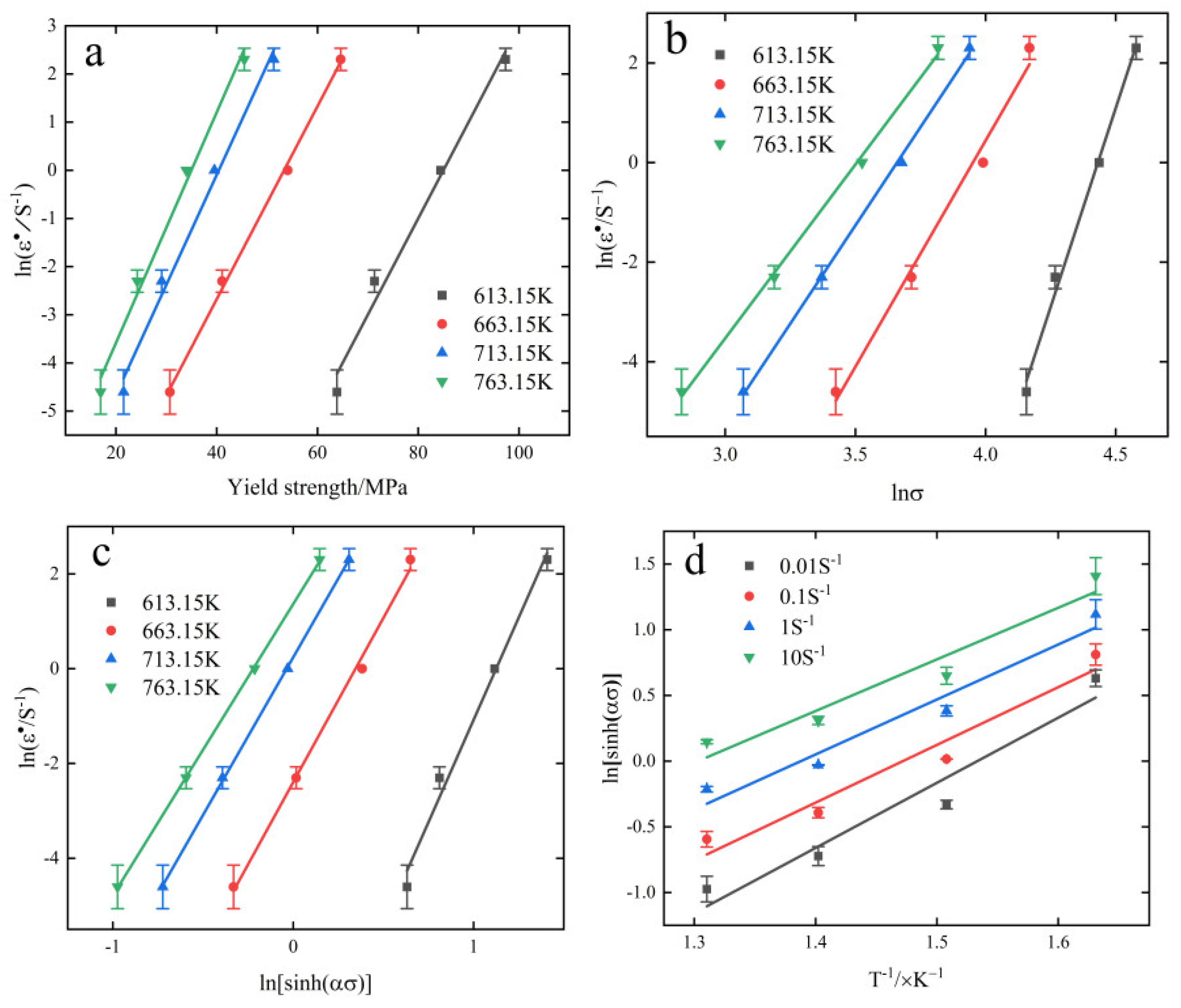
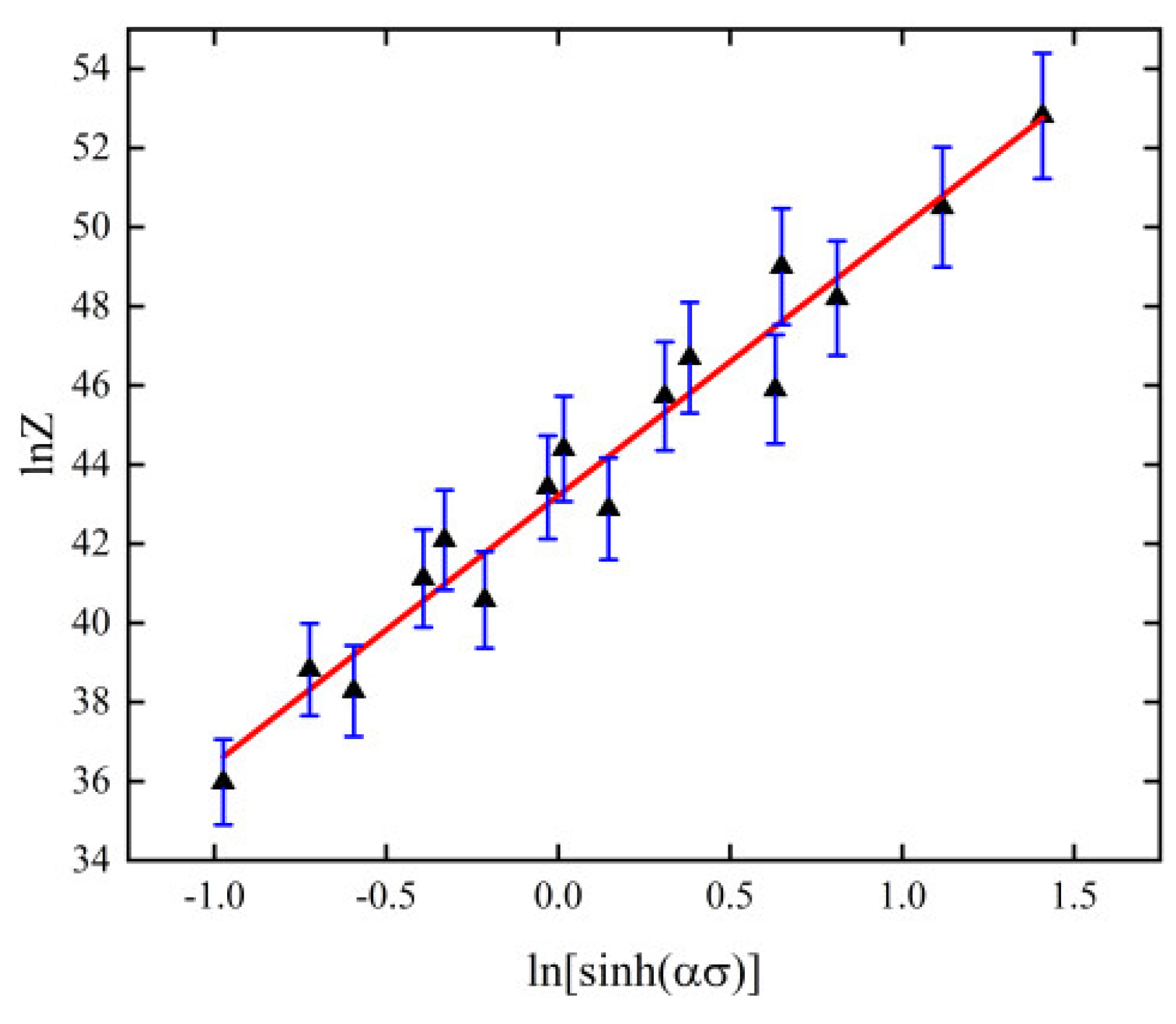
3.3. Thermal Creep Model
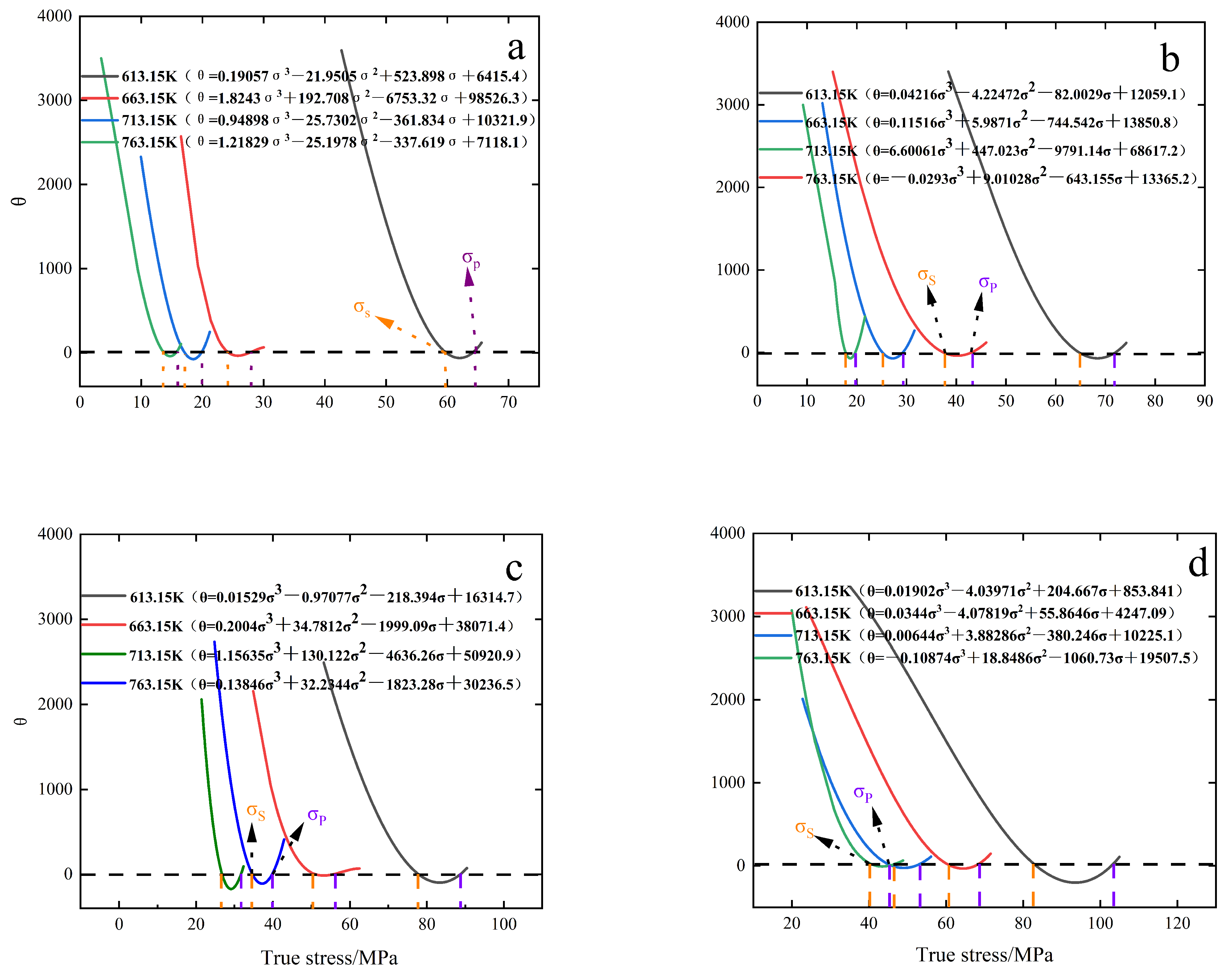
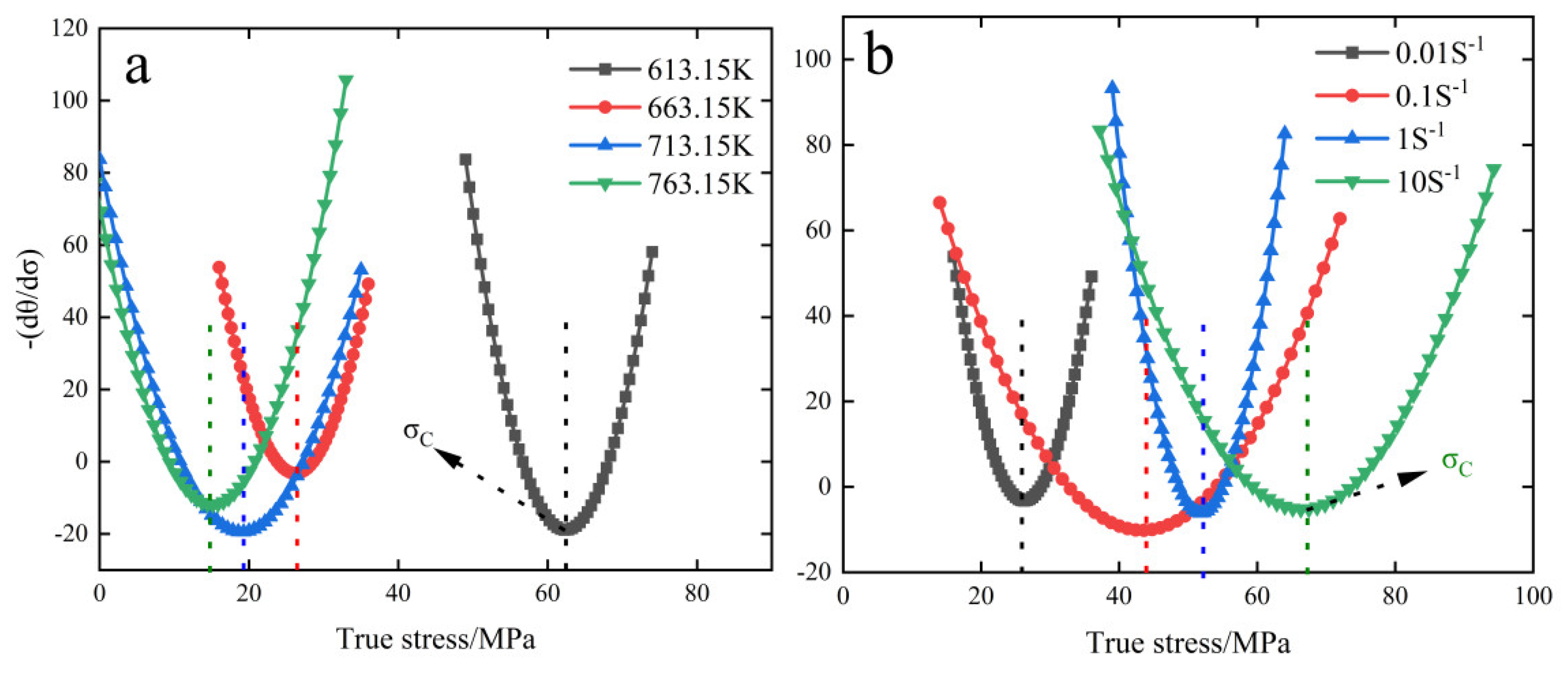
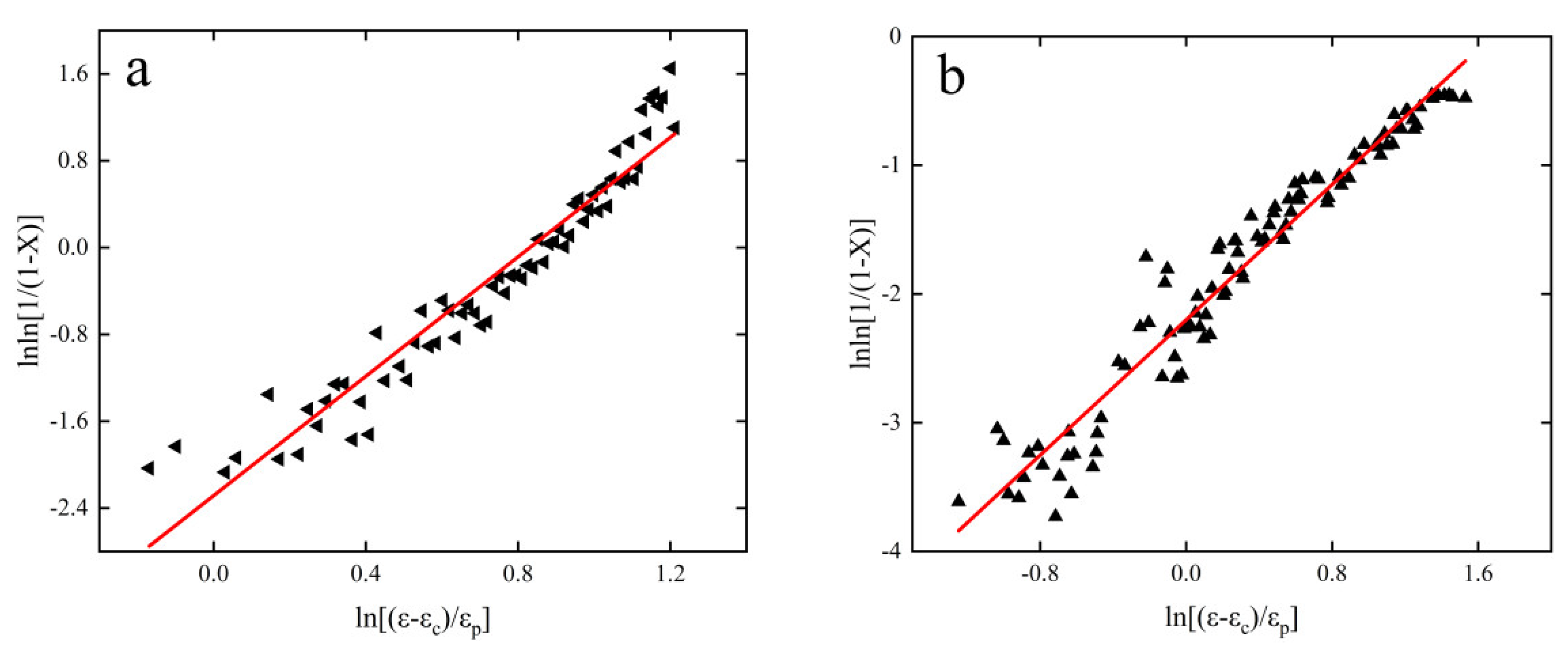
3.4. Creep Crystallization Critical Conditions
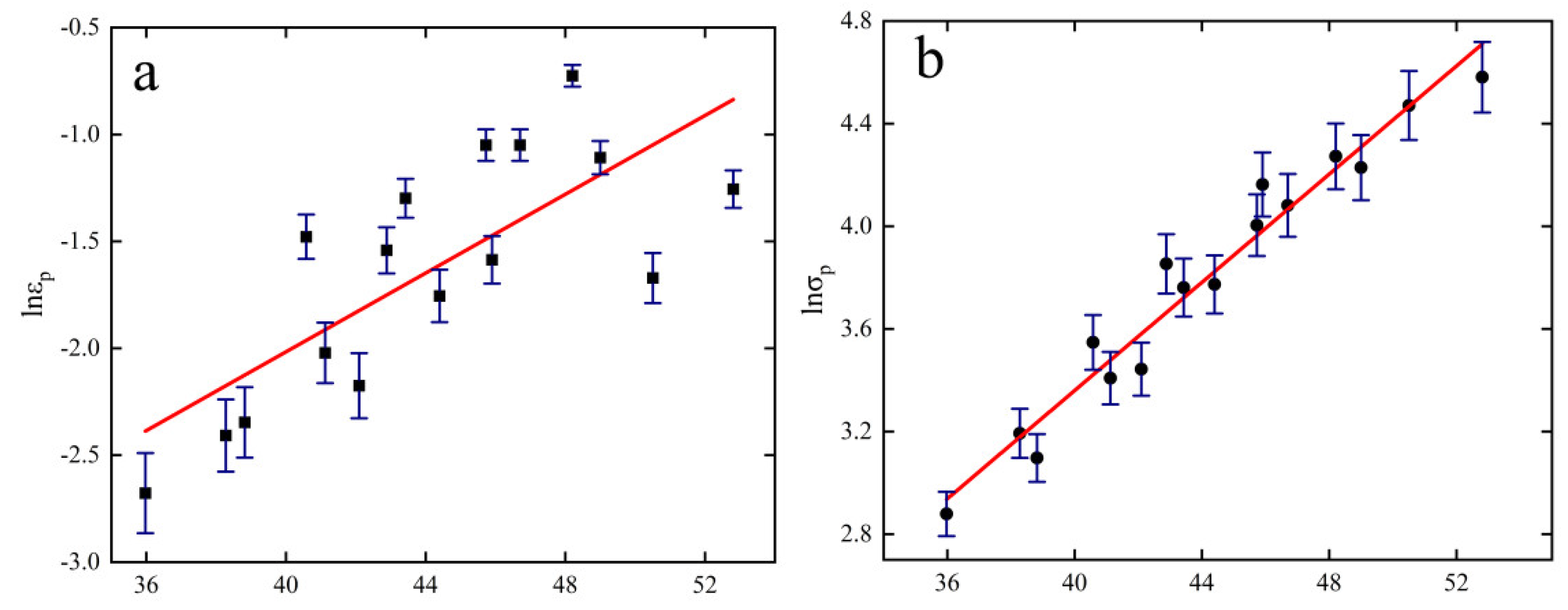
3.5. Creep Recrystallization Volume Model
4. Conclusions
- The value of thermal creep activation energy Q of 6082-T6 aluminum alloy is 254.473 kJ/mol, and the constructed thermal creep principal equation is:
- 2.
- Based on the Avrami model, the kinetic equations for creep crystallization of 6082-T6 aluminum alloy at 613.15 to 763.15 K with strain rates of 0.01 and 10 S−1 are constructed as follows.
- 3.
- Scanning electron microscope analysis shows that DRX crystal blocks have different colors and shapes. When the contents of Mg and Si elements in DRX crystal blocks are far greater than that of Mn elements, the crystal blocks will appear black. When the content of Mn and Si in the DRX crystal block is close, and the content of Mn and Si is far greater than that of MG, the crystal block will appear bright white. When the content of Mn and Si in the DRX crystal block is far greater than that of MG, and the content of Si is greater than that of Mn, the crystal block will appear bright white.
- 4.
- The critical conditions for creep crystallization of 6082-T6 aluminum alloy were described by introducing the work-hardening rate (θ) and parameter Z. The critical conditions for this material were obtained as follows: ; ; ; ; . ; the parameter in the previous equation, it is found that the critical condition is positively correlated with the increase of parameter Z, positively correlated with the increase of strain rate, and negatively correlated with the increase of temperature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Panagopoulos, C.N.; Georgiou, E.P.; Gavras, A.G. Corrosion and wear of 6082 aluminum alloy. Tribol. Int. 2009, 42, 886–889. [Google Scholar] [CrossRef]
- Briottet, L.; Jonas, J.J.; Montheillet, F. A mechanical interpretation of the activation energy of high temperature deformation in two phase materials. Acta Mater. 1996, 44, 1665–1672. [Google Scholar] [CrossRef]
- Liu, W.; Qiu, Q.; Zhou, F.; Chen, Y. Effects of Aging Treatments on the Dynamic Mechanical Properties and Microstructure Evolution of 7N01 Aluminum Alloy at High Temperature. Rare Met. Mater. Eng. 2018, 47, 311. [Google Scholar]
- Sathish, T.; Karthick, S. Wear behaviour analysis on aluminium alloy 7050 with reinforced SiC through taguchi approach. J. Mater. Res. Technol. 2020, 9, 3481–3487. [Google Scholar] [CrossRef]
- Viana, F.; Pinto, A.M.P.; Santos, H.M.C.; Lopes, A.B. Retrogression and re-ageing of 7075 aluminium alloy: Microstructural characterization. J. Mater. Process. Technol. 1999, 92–93, 54–59. [Google Scholar] [CrossRef] [Green Version]
- Vasanthakumar, P.; Sekar, K.; Venkatesh, K. Recent developments in powder metallurgy based aluminium alloy composite for aerospace applications. Mater. Today Proc. 2019, 18, 5400–5409. [Google Scholar] [CrossRef]
- Tan, Q.; Zhang, J.; Sun, Q.; Fan, Z.; Li, G.; Yin, Y.; Liu, Y.; Zhang, M.X. Inoculation treatment of an additively manufactured 2024 aluminium alloy with titanium nanoparticles. Acta Mater. 2020, 196, 1–16. [Google Scholar] [CrossRef]
- Natesan, E.; Ahlström, J.; Eriksson, S.; Persson, C. Effects of Temperature on the Evolution of Yield Surface and Stress Asymmetry in A356–T7 Cast Aluminium Alloy. Materials 2021, 14, 7898. [Google Scholar] [CrossRef]
- Han, L.; Guo, Y.; Lin, P.; Zhang, G.; Zhong, L.; Fu, W.; Liu, C. Study on Weldability of Aluminium Alloy TL091 in Resistance Spot Welding with Intermediate Frequency and Direct Current. In DEStech Transactions on Materials Science and Engineering; DEStech Publications, Inc.: Lancaster, PA, USA, 2021. [Google Scholar]
- Žagar, S.; Markoli, B.; Naglič, I.; Šturm, R. The Influence of Age Hardening and Shot Peening on the Surface Properties of 7075 Aluminium Alloy. Materials 2021, 14, 2220. [Google Scholar] [CrossRef]
- Kok, M. Production and mechanical properties of Al2O3 particle-reinforced 2024 aluminium alloy composites. J. Mater. Process. Technol. 2005, 161, 381–387. [Google Scholar] [CrossRef]
- Guillaumin, V.; Mankowski, G. Localized corrosion of 6056 T6 aluminium alloy in chloride media. Corros. Sci. 2000, 42, 105–125. [Google Scholar] [CrossRef]
- Sahin, Y. Preparation and some properties of SiC particle reinforced aluminium alloy composites. Mater. Des. 2003, 24, 671–679. [Google Scholar] [CrossRef]
- Yan, F.; Wang, R.; Peng, C. Influence of Ga and In on microstructure and electrochemical properties of Mg anodes. Trans. Nonferrous Met. Soc. China 2013, 23, 2650–2656. [Google Scholar]
- Mindivan, H.; Kayali, E.S.; Cimenoglu, H. Tribological behavior of squeeze cast aluminum matrix composites. Wear 2008, 365, 645–654. [Google Scholar] [CrossRef]
- Evdokimov, A.; Doynov, N.; Ossenbrink, R.; Obrosov, A.; Weiß, S.; Michailov, V. Thermomechanical laser welding simulation of dissimilar steel-aluminum overlap joints. Int. J. Mech. Sci. 2021, 190, 106019. [Google Scholar] [CrossRef]
- Krolo, J.; Lela, B.; Švagelj, Z.; Jozić, S. Adaptive neuro-fuzzy and regression models for predicting microhardness and electrical conductivity of solid-state recycled EN AW 6082. Int. J. Adv. Manuf. Technol. 2019, 100, 2981–2993. [Google Scholar] [CrossRef]
- Garcia, R.F.; Feix, E.C.; Mendel, H.T.; Gonzalez, A.R.; Souza, A.J. Optimization of cutting parameters for finish turning of 6082-T6 aluminum alloy under dry and RQL conditions. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 317. [Google Scholar] [CrossRef]
- Shao, Z.; Lee, J.; Wang, J.; Lin, J.; Jiang, J. A study of various heating effects on the microstructure and mechanical properties of AA6082 using EBSD and CPFE. J. Alloys Compd. 2020, 818, 152921. [Google Scholar] [CrossRef]
- Zhen, L.; Hu, W.H.; Shao, W.Z.; Chen, J.Z.; Zhang, X.M.; Zhang, B.Y. DRX in 7050 Aluminum Alloy during Constraint Deformation Processing at High Temperature. In Key Engineering Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2007; Volume 353, pp. 647–650. [Google Scholar]
- Hu, J.L.; Wu, X.J.; Bo, H.; Jiao, Z.T.; Huang, S.Q.; Jin, M. Dislocation density model and microstructure of 7A85 aluminum alloy during thermal deformation. J. Cent. South Univ. 2021, 28, 2999–3007. [Google Scholar] [CrossRef]
- Li, S.; Li, L.; He, H.; Liu, Z.; Zhang, L. Effect of dynamic recrystallization on the organization and mechanical properties of the weld zone of Al-Mg-Si aluminum alloy extruded profiles by manifold. Trans. Nonferrous Met. Soc. China 2019, 29, 1803–1815. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Gong, H.; Li, S.; Ahmad, A.S. A physically based constitutive model and continuous dynamic recrystallization behavior analysis of 2219 aluminum alloy during hot deformation process. Materials 2018, 11, 1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, G.; Chen, L.; Zhao, G.; Zhang, C.; Cui, W. Microstructure analysis of an Al-Zn-Mg alloy during porthole die extrusion based on modeling of constitutive equation and dynamic recrystallization. J. Alloys Compd. 2017, 710, 80–91. [Google Scholar] [CrossRef]
- Yang, S.; Shen, J.; Yan, X.; Li, X.; Sun, B.; Zhang, F. Heat deformation rheological behavior and instantonal equations of Al-Cu-Li-Mg-Mn-Zn-Ag alloys. Chin. J. Nonferrous Met. 2015, 25, 8. [Google Scholar]
- Yuan, X.; Chen, L. Hot deformation at elevated temperature and recrystallization behavior of a high manganese austenitic twip steel. Acta Metall. Sin. 2015, 51, 651–658. [Google Scholar]
- Richardson, G.; Sellars, C.M.; Tegart, W. Recrystallization during creep of nickel. Acta Metall. 1966, 14, 1225–1236. [Google Scholar] [CrossRef]
- Mao, M.; Luan, B.; Li, F.; Liao, Z.; Qiu, R. Thermal deformation behavior and tissue evolution of β-T51Z alloy. Rare Met. Mater. Eng. 2020, 49, 9. [Google Scholar]
- Medina, S.F.; Hernandez, C.A. General expression of the Zener-Hollomon parameter as a function of the chemical composition of low alloy and microalloyed steels. Acta Mater. 1996, 44, 137–148. [Google Scholar] [CrossRef]
- Najafizadeh, A.; Jonas, J. Predicting the Critical Stress for Initiation of Dynamic Recrystallization. ISIJ Int. 2006, 46, 1679–1684. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, M.; Li, H. Plastic deformation behavior of GH696 alloy during high temperature deformation. Chin. J. Nonferrous Met. Engl. Ed. 2016, 3, 712–721. [Google Scholar] [CrossRef]
- Yang, L.; Li, Q.; Wang, Y.; Chen, Y.; Guo, X.; Wu, Z.; Chen, G.; Zhong, B.; Xiang, W.; Zhong, Y. A review of cathode materials in lithium-sulfur batteries. Ionics 2020, 26, 5299–5318. [Google Scholar] [CrossRef]
- Poliak, E.I.; Jonas, J.J. A one-parameter approach to determining the critical conditions for the initiation of dynamic recrystallization. Acta Mater. 1996, 44, 127–136. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Zhang, X.; Dai, H.; Jia, J.; Bai, Z. Dynamic recrystallization kinetics and microstructure evolution of an AZ91D magnesium alloy during hot compression. Mater. Charact. 2018, 145, 39–52. [Google Scholar] [CrossRef]
- Kim, S.I.; Yoo, Y.C. Dynamic recrystallization behavior of AISI 304 stainless steel. Mater. Sci. Eng. A 2001, 311, 108–113. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. II Transformation-Time Relations for Random Distribution of Nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]
- Deng, Y.; Yang, Y.; Cao, J.; Qian, H. Research on Dynamic Recrystallization Behavior of 23Cr-2.2 Ni-6.3 Mn-0.26 N Low Nickel TypeDuplex Stainless Steel. Acta Metall. Sin. 2018, 55, 445–456. [Google Scholar]
- Zhou, H.; Zhang, H.; Liu, J.; Qin, S.; Lv, Y. Prediction of flow stresses for a typical nickel-based superalloy during hot deformation based on dynamic recrystallization kinetic equation. Rare Met. Mater. Eng. 2018, 47, 3329–3337. [Google Scholar]
- Ding, S.; Khan, S.A.; Yanagimoto, J. Flow behavior and dynamic recrystallization mechanism of A5083 aluminum alloys with different initial microstructures during hot compression. Mater. Sci. Eng. A 2020, 787, 139522. [Google Scholar] [CrossRef]
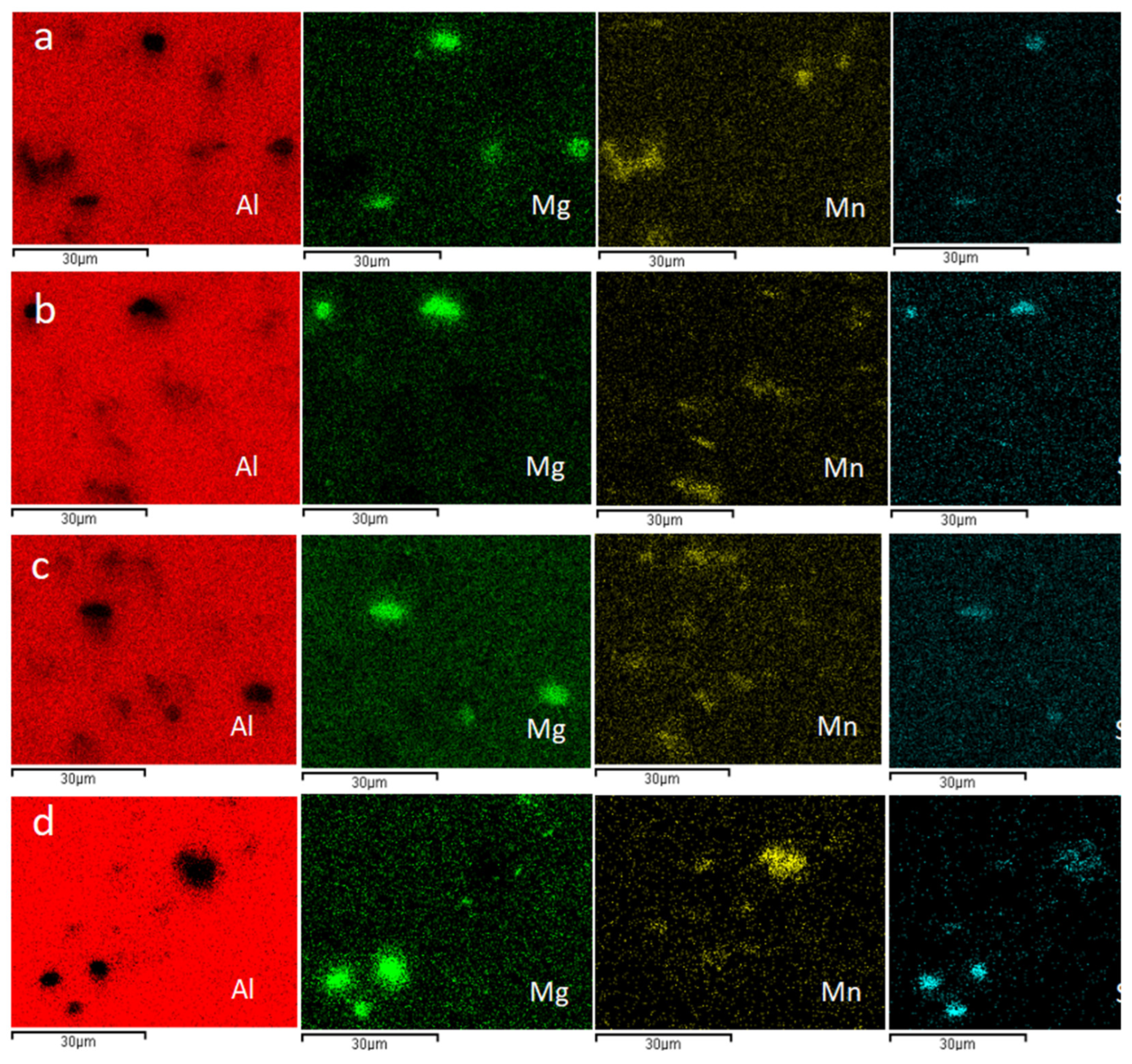



| Element | Mg | Al | Si | Mn | Mg | Al | Si | Mn | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 | weight | Point 1 | 18.37 | 58.54 | 22.93 | 0.16 | Point 2 | 0.07 | 80.73 | 9.57 | 9.63 |
| atom | 20.18 | 57.94 | 21.81 | 0.08 | 0.08 | 85.22 | 9.71 | 4.99 | |||
| weight | Point 3 | 0.33 | 80.76 | 9.16 | 9.75 | Point 4 | 0.32 | 87.64 | 7.46 | 4.58 | |
| atom | 0.39 | 85.27 | 9.29 | 5.05 | 0.37 | 89.97 | 7.36 | 2.31 | |||
| b1 | weight | Point 1 | 15.65 | 67.51 | 16.53 | 0.31 | Point 2 | 0.21 | 86.73 | 8.15 | 4.92 |
| atom | 17.22 | 66.90 | 15.73 | 0.15 | 0.24 | 89.22 | 8.05 | 2.48 | |||
| weight | Point 3 | 0.21 | 87.96 | 7.17 | 4.66 | Point 4 | 0.21 | 86.06 | 8.28 | 5.45 | |
| atom | 0.24 | 90.33 | 7.08 | 2.35 | 0.24 | 88.79 | 8.20 | 2.76 | |||
| weight | Point 5 | 0.39 | 85.30 | 8.36 | 5.94 | ||||||
| atom | 0.45 | 88.22 | 8.31 | 3.02 | |||||||
| c1 | weight | Point 1 | 13.25 | 67.18 | 19.32 | 0.24 | Point 2 | 0.20 | 85.54 | 7.90 | 6.35 |
| atom | 14.62 | 66.80 | 18.45 | 0.12 | 0.23 | 88.66 | 7.87 | 3.23 | |||
| weight | Point 3 | 0.41 | 88.19 | 7.47 | 3.93 | Point 4 | 0.28 | 86.98 | 8.09 | 4.65 | |
| atom | 0.47 | 90.21 | 7.34 | 1.98 | 0.32 | 89.35 | 7.99 | 2.34 | |||
| weight | Point 5 | 0.28 | 86.27 | 8.33 | 5.13 | ||||||
| atom | 0.32 | 88.85 | 8.24 | 2.59 | |||||||
| d1 | weight | Point 1 | 0.62 | 89.04 | 6.27 | 4.07 | Point 2 | 0.21 | 75.53 | 11.23 | 13.04 |
| atom | 0.71 | 91.09 | 6.16 | 2.04 | 0.25 | 81.26 | 11.60 | 6.89 | |||
| weight | Point 3 | 11.40 | 68.18 | 20.15 | 0.27 | Point 4 | 0.40 | 89.26 | 6.72 | 3.62 | |
| atom | 12.61 | 67.96 | 19.30 | 0.13 | 0.46 | 91.14 | 6.59 | 1.82 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Huang, X.; Guo, R.; Chen, D. Thermal Creep Behavior and Creep Crystallization of Al-Mg-Si Aluminum Alloys. Materials 2022, 15, 8117. https://doi.org/10.3390/ma15228117
Zhang Q, Huang X, Guo R, Chen D. Thermal Creep Behavior and Creep Crystallization of Al-Mg-Si Aluminum Alloys. Materials. 2022; 15(22):8117. https://doi.org/10.3390/ma15228117
Chicago/Turabian StyleZhang, Qinmin, Xiaomin Huang, Ran Guo, and Dongyu Chen. 2022. "Thermal Creep Behavior and Creep Crystallization of Al-Mg-Si Aluminum Alloys" Materials 15, no. 22: 8117. https://doi.org/10.3390/ma15228117





