Towards Investigating the Effect of Ammonium Nitrate on the Characteristics and Thermal Decomposition Behavior of Energetic Double Base NC/DEGDN Composite
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
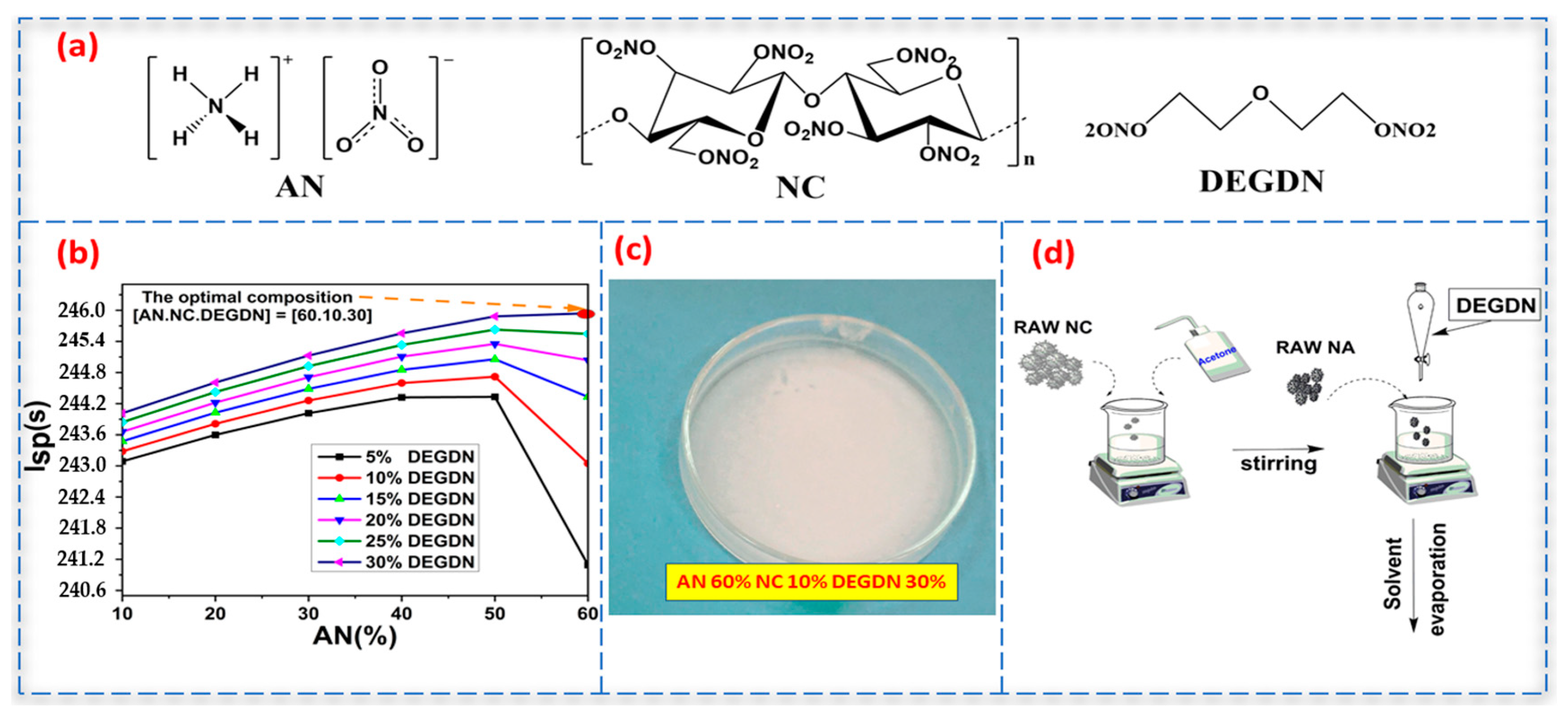
2.2. Preparation of DEGDN Plasticizer
2.3. Evaluation of the Theoretical Performance of CSPs
2.4. Elaboration of AN/NC-DEGDN Propellant
2.5. Characterization Methods
2.6. Kinetic Modeling
3. Results and Discussions
3.1. Evaluation of the Optimal Composition
3.2. Morphology and Chemical Structure
3.3. TGA Characterization
3.4. DSC Characterization
3.5. Evaluation of Thermal Decomposition Kinetics
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benhammada, A.; Trache, D. Thermal decomposition of energetic materials using TG-FTIR and TG-MS: A state-of-the-art review. Appl. Spectrosc. Rev. 2020, 55, 724–777. [Google Scholar] [CrossRef]
- Klapötke, T.M. Chemistry of high-energy materials. In Chemistry of High-Energy Materials; de Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Elbasuney, S.; Fahd, A.; Mostafa, H.E. Combustion characteristics of extruded double base propellant based on ammonium perchlorate/aluminum binary mixture. Fuel 2017, 208, 296–304. [Google Scholar] [CrossRef]
- Elghafour, A.M.; Radwan, M.A.; Mostafa, H.E.; Fahd, A.; Elbasuney, S. Highly energetic nitramines: A novel platonizing agent for double-base propellants with superior combustion characteristics. Fuel 2018, 227, 478–484. [Google Scholar] [CrossRef]
- Damse, R.; Singh, A. Studies on the high-energy gun propellant formulations based on 1, 5-diazido-3-nitrazapentane. J. Hazard. Mater. 2009, 172, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, P.; Liu, Y.; Yin, P.; He, C.; Pang, S. Synthesis and Characterization of Fluorodinitrobenzenes with Tunable Melting Point: Potential Low Sensitive Energetic Plasticizer and Melt-Cast Carrier. Chin. J. Chem. 2020, 38, 1619–1624. [Google Scholar] [CrossRef]
- Reese, D.A.; Groven, L.J.; Son, S.F. Formulation and Characterization of a New Nitroglycerin-Free Double Base Propellant. Propellants Explos. Pyrotech. 2014, 39, 205–210. [Google Scholar] [CrossRef]
- Buszek, R.J.; Soto, D.; Dailey, J.M.; Bolden, S.; Tall, T.L.; Hudgens, L.M.; Marshall, C.A.; Boatz, J.A.; Drake, G.W. Structures and binding energies of nitrate plasticizers DEGDN, TEGDN, and nitroglycerine. Propellants Explos. Pyrotech. 2018, 43, 115–121. [Google Scholar] [CrossRef]
- Yahya, P.K.I.; Moniruzzaman, M.; Gill, P.P. Interaction and thermal studies on graphene oxide in NC/DEGDN/GO nanocomposites. RSC Adv. 2019, 9, 35158–35164. [Google Scholar] [CrossRef]
- Agrawal, J.P. High Energy Materials: Propellants, Explosives and Pyrotechnics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Badgujar, D.; Talawar, M.; Zarko, V.; Mahulikar, P. New directions in the area of modern energetic polymers: An overview. Combust. Explos. Shock. Waves 2017, 53, 371–387. [Google Scholar] [CrossRef]
- Trache, D.; Klapötke, T.M.; Maiz, L.; Abd-Elghany, M.; DeLuca, L.T. Recent advances in new oxidizers for solid rocket propulsion. Green Chem. 2017, 19, 4711–4736. [Google Scholar] [CrossRef]
- Singh, G. Recent Advances on Energetic Materials; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 1–411. [Google Scholar]
- Oommen, C.; Jain, S. Ammonium nitrate: A promising rocket propellant oxidizer. J. Hazard. Mater. 1999, 67, 253–281. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. A review on the use of nanometals as catalysts for the thermal decomposition of ammonium perchlorate. J. Saudi Chem. Soc. 2013, 17, 135–149. [Google Scholar] [CrossRef]
- Touidjine, S.; Boulkadid, K.M.; Trache, D.; Belkhiri, S.; Mezroua, A. Preparation and Characterization of Polyurethane/Nitrocellulose Blends as Binder for Composite Solid Propellants. Propellants Explos. Pyrotech. 2022, 47, e202000340. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Bekhouche, S.; Boukeciat, H. Exploration of palm fronds as a prominent alternative resource for the production of energetic cellulose-rich biopolymers. Mater. Today Proc. 2022, 53, 31–35. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Slimani, K.; Belouettar, B.-E.; Abdelaziz, A.; Bekhouche, S.; Bessa, W. Valorization of esparto grass cellulosic derivatives for the development of promising energetic azidodeoxy biopolymers: Synthesis, characterization and isoconversional thermal kinetic analysis. Propellants Explos. Pyrotech. 2022, 47, e202100293. [Google Scholar] [CrossRef]
- Chalghoum, F.; Trache, D.; Maggi, F.; Benziane, M. Effect of Complex Metal Hydrides on the Elimination of Hydrochloric Acid Exhaust Products from High-Performance Composite Solid Propellants: A Theoretical Analysis. Propellants Explos. Pyrotech. 2020, 45, 1204–1215. [Google Scholar] [CrossRef]
- Bondarchuk, S.V. Magic of Numbers: A Guide for Preliminary Estimation of the Detonation Performance of C–H–N–O Explosives Based on Empirical Formulas. Ind. Eng. Chem. Res. 2021, 60, 1952–1961. [Google Scholar] [CrossRef]
- Trache, D.; Abdelaziz, A.; Siouani, B. A simple and linear isoconversional method to determine the pre-exponential factors and the mathematical reaction mechanism functions. J. Therm. Anal. Calorim. 2017, 128, 335–348. [Google Scholar] [CrossRef]
- Trache, D.; Maggi, F.; Palmucci, I.; DeLuca, L.T. Thermal behavior and decomposition kinetics of composite solid propellants in the presence of amide burning rate suppressants. J. Therm. Anal. Calorim. 2018, 132, 1601–1615. [Google Scholar] [CrossRef]
- Sbirrazzuoli, N. Determination of pre-exponential factor and reaction mechanism in a model-free way. Thermochim. Acta 2020, 691, 178707. [Google Scholar] [CrossRef]
- Law, C.K.; Makino, A.; Lu, T.F. On the off-stoichiometric peaking of adiabatic flame temperature. Combustion 2006, 145, 808–819. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Solid propellants: AP/HTPB composite propellants. Arab. J. Chem. 2019, 12, 2061–2068. [Google Scholar] [CrossRef]
- Wang, H.; Kline, D.J.; Rehwoldt, M.; Wu, T.; Zhao, W.; Wang, X.; Zachariah, M.R. Architecture can significantly alter the energy release rate from nanocomposite energetics. ACS Appl. Polym. Mater. 2019, 1, 982–989. [Google Scholar] [CrossRef]
- Huang, X.; Luo, Q.; Zhu, J.; Li, Z.; Li, C.; Pei, C. The preparation and rheological properties of novel energetic composites TEGDN/NBC. Propellants Explos. Pyrotech. 2020, 45, 101–110. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, X.; Lu, L.; Yang, X.; Wang, X. Study on the catalytic effect of NiO nanoparticles on the thermal decomposition of TEGDN/NC propellant. J. Hazard. Mater. 2009, 168, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, Y.; Cong, K.; He, J.; Yang, R. Curing behaviors of alkynyl-terminated copolyether with glycidyl azide polymer in energetic plasticizers. Polymers 2020, 12, 1199. [Google Scholar] [CrossRef]
- Trache, D.; Khimeche, K. Study on the influence of ageing on thermal decomposition of double-base propellants and prediction of their in-use time. Fire Mater. 2013, 37, 328–336. [Google Scholar] [CrossRef]
- Zhao, W.; Ren, H.; Ou, Y.; Jiao, Q. Nanocomposites with Al and Ti binary fuels and potassium oxysalts for energetic applications. Mater. Lett. 2020, 262, 127189. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Dong, J.; Li, B.; Shen, J.; Chen, L.; Fu, Y.; He, W. Three-dimensional network structure nitramine gun propellant with nitrated bacterial cellulose. J. Mater. Res. Technol. 2020, 9, 15094–15101. [Google Scholar] [CrossRef]
- Wei, R.; Huang, S.; Huang, Q.; Ouyang, D.; Chen, Q.; Yuen, R.; Wang, J. Experimental study on the fire characteristics of typical nitrocellulose mixtures using a cone calorimeter. J. Therm. Anal. Calorim. 2018, 134, 1471–1480. [Google Scholar] [CrossRef]
- Gunawan, R.; Zhang, D. Thermal stability and kinetics of decomposition of ammonium nitrate in the presence of pyrite. J. Hazard. Mater. 2009, 165, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Vargeese, A.A.; Muralidharan, K. Anatase–brookite mixed phase nano TiO2 catalyzed homolytic decomposition of ammonium nitrate. J. Hazard. Mater. 2011, 192, 1314–1320. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elghany, M.; Klapötke, T.M.; Elbeih, A. Investigation of 2, 2, 2-trinitroethyl-nitrocarbamate as a high energy dense oxidizer and its mixture with Nitrocellulose (thermal behavior and decomposition kinetics). J. Anal. Appl. Pyrolysis 2017, 128, 397–404. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Sayah, Z.B.D.; Trache, D.; Klapötke, T.M.; Belmerabt, M.; Abdelaziz, A.; Bekhouche, S. Towards investigating the characteristics and thermal kinetic behavior of emergent nanostructured nitrocellulose prepared using various sulfonitric media. J. Nanostructure Chem. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Wang, Y.; Song, X.; Song, D.; Liang, L.; An, C.; Wang, J. Synthesis, thermolysis, and sensitivities of HMX/NC energetic nanocomposites. J. Hazard. Mater. 2016, 312, 73–83. [Google Scholar] [CrossRef]
- Hanafi, S.; Trache, D.; Meziani, R.; Boukciat, H.; Mezroua, A.; Tarchoun, A.F.; Derradji, M. Synthesis, characterization and thermal decomposition behavior of a novel HNTO/AN co-crystal as a promising rocket propellant oxidizer. Chem. Eng. J. 2021, 417, 128010. [Google Scholar] [CrossRef]
- Vyazovkin, S. How much is the accuracy of activation energy affected by ignoring thermal inertia? Int. J. Chem. Kinet. 2020, 52, 23–28. [Google Scholar] [CrossRef]
- Burnham, A.K.; Stanford, V.L.; Vyazovkin, S.; Kahl, E.M. Effect of pressure on TATB and LX-17 thermal decomposition. Thermochim. Acta 2021, 699, 178908. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Sbirrazzuoli, N. Determination of pre-exponential factors and of the mathematical functions f (α) or G (α) that describe the reaction mechanism in a model-free way. Thermochim. Acta 2013, 564, 59–69. [Google Scholar] [CrossRef]
- Vara, J.A.; Dave, P.N. Metal oxide nanoparticles as catalyst for thermal behavior of AN based composite solid propellant. Chem. Phys. Lett. 2019, 730, 600–607. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Cheng, Y.; Li, Y.; Mei, X. Thermal decomposition properties of double-base propellant and ammonium perchlorate. J. Therm. Anal. Calorim. 2014, 115, 887–894. [Google Scholar] [CrossRef]
- Jain, S.; Chakraborty, S.; Qiao, L. Burn rate enhancement of ammonium perchlorate–nitrocellulose composite solid propellant using copper oxide–graphene foam micro-structures. Combust. Flame 2019, 206, 282–291. [Google Scholar] [CrossRef]
- Babrauskas, V.; Leggett, D. Thermal decomposition of ammonium nitrate. Fire Mater. 2020, 44, 250–268. [Google Scholar] [CrossRef]
- Liu, Z. Review and prospect of thermal analysis technology applied to study thermal properties of energetic materials. FirePhysChem 2021, 1, 129–138. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Pyrolysis, A. Review of thermal decomposition, kinetics parameters and evolved gases during pyrolysis of energetic materials using different techniques. J. Anal. 2022, 161, 105364. [Google Scholar] [CrossRef]





| Sample | 1st Decomposition Stage | 2nd Decomposition Stage | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Tonset (°C) | Tpeak (°C) | ∆T * (°C) | ∆H (J/g) | Tonset (°C) | Tpeak (°C) | ∆T * (°C) | ∆H (J/g) | ∆HT (J/g) | |
| NC-DEGDN | 182.5 | 200.4 | 17.9 | 1624.6 | / | / | / | / | 1624.6 |
| AN/NC-DEGDN | 187.5 | 192.3 | 4.8 | 1961.8 | 204.4 | 212.4 | 8 | 935.04 | 2896.84 |
| AN | / | / | / | / | 300.04 | 318.2 | 24 | 805 | 803–805 [35] |
| Samples | Isoconversional Methods | Eα (kJ/mol) | Log(A(s−1)) | g(α) | |
|---|---|---|---|---|---|
| AN/NC-DEGDN 1st Pic | TAS | 118.9 ± 16.6 | 12.2 ± 2.1 | P1/4 = a1/4 | |
| it-KAS | 118.8 ± 16.5 | 12.2 ± 2.0 | P1/4 = a1/4 | ||
| VYA/CE | β = 5 °C/min | 118.8 ± 16.4 | 12.3 ± 1.4 | / | |
| β = 10 °C/min | 12.6 ± 1.4 | / | |||
| β = 15 °C/min | 12.7 ± 1.4 | / | |||
| β = 20 °C/min | 13.8 ± 1.4 | / | |||
| AN/NC-DEGDN 2nd pic | TAS | 119.2 ± 23.3 | 13.4 ± 2.8 | A4 = [− ln (1 − α)]1/4 | |
| it-KAS | 119.2 ± 23.3 | 13.2 ± 2.8 | P1/2 = a1/2 | ||
| VYA/CE | β = 5 °C/min | 119.1 ± 20.9 | 13.3 ± 1.4 | / | |
| β = 10 °C/min | 13.4 ± 1.4 | / | |||
| β = 15 °C/min | 13.3 ± 1.4 | / | |||
| β = 20 °C/min | 13.3 ± 1.4 | / | |||
| NC-DEGDN | TAS | 134.5 ± 23.9 | 12.9 ± 3.2 | A5/2 = [− ln (1 − α)]2/5 | |
| it-KAS | 134.5 ± 23.9 | 12.7 ± 3.2 | G7 = [1 − (1 − α)1/2]1/2 | ||
| VYA/CE | β = 5 °C/min | 134.4 ± 23.9 | 13.3 ± 3.3 | / | |
| β = 10 °C/min | 13.4 ± 3.3 | / | |||
| β = 15 °C/min | 13.5 ± 3.3 | / | |||
| β = 20 °C/min | 13.6 ± 3.3 | / | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukeciat, H.; Tarchoun, A.F.; Trache, D.; Abdelaziz, A.; Ahmed Hamada, R.; Bouhantala, A.; Bousstila, C.; Hanafi, S.; Dourari, M.; Klapötke, T.M. Towards Investigating the Effect of Ammonium Nitrate on the Characteristics and Thermal Decomposition Behavior of Energetic Double Base NC/DEGDN Composite. Materials 2022, 15, 8138. https://doi.org/10.3390/ma15228138
Boukeciat H, Tarchoun AF, Trache D, Abdelaziz A, Ahmed Hamada R, Bouhantala A, Bousstila C, Hanafi S, Dourari M, Klapötke TM. Towards Investigating the Effect of Ammonium Nitrate on the Characteristics and Thermal Decomposition Behavior of Energetic Double Base NC/DEGDN Composite. Materials. 2022; 15(22):8138. https://doi.org/10.3390/ma15228138
Chicago/Turabian StyleBoukeciat, Hani, Ahmed Fouzi Tarchoun, Djalal Trache, Amir Abdelaziz, Rania Ahmed Hamada, Ayemen Bouhantala, Chamseddine Bousstila, Sabrina Hanafi, Mohammed Dourari, and Thomas M. Klapötke. 2022. "Towards Investigating the Effect of Ammonium Nitrate on the Characteristics and Thermal Decomposition Behavior of Energetic Double Base NC/DEGDN Composite" Materials 15, no. 22: 8138. https://doi.org/10.3390/ma15228138
APA StyleBoukeciat, H., Tarchoun, A. F., Trache, D., Abdelaziz, A., Ahmed Hamada, R., Bouhantala, A., Bousstila, C., Hanafi, S., Dourari, M., & Klapötke, T. M. (2022). Towards Investigating the Effect of Ammonium Nitrate on the Characteristics and Thermal Decomposition Behavior of Energetic Double Base NC/DEGDN Composite. Materials, 15(22), 8138. https://doi.org/10.3390/ma15228138









