Electrochemical Polishing of Ti6Al4V Alloy Assisted by High-Speed Flow of Micro-Abrasive Particles in NaNO3 Electrolyte
Abstract
:1. Introduction
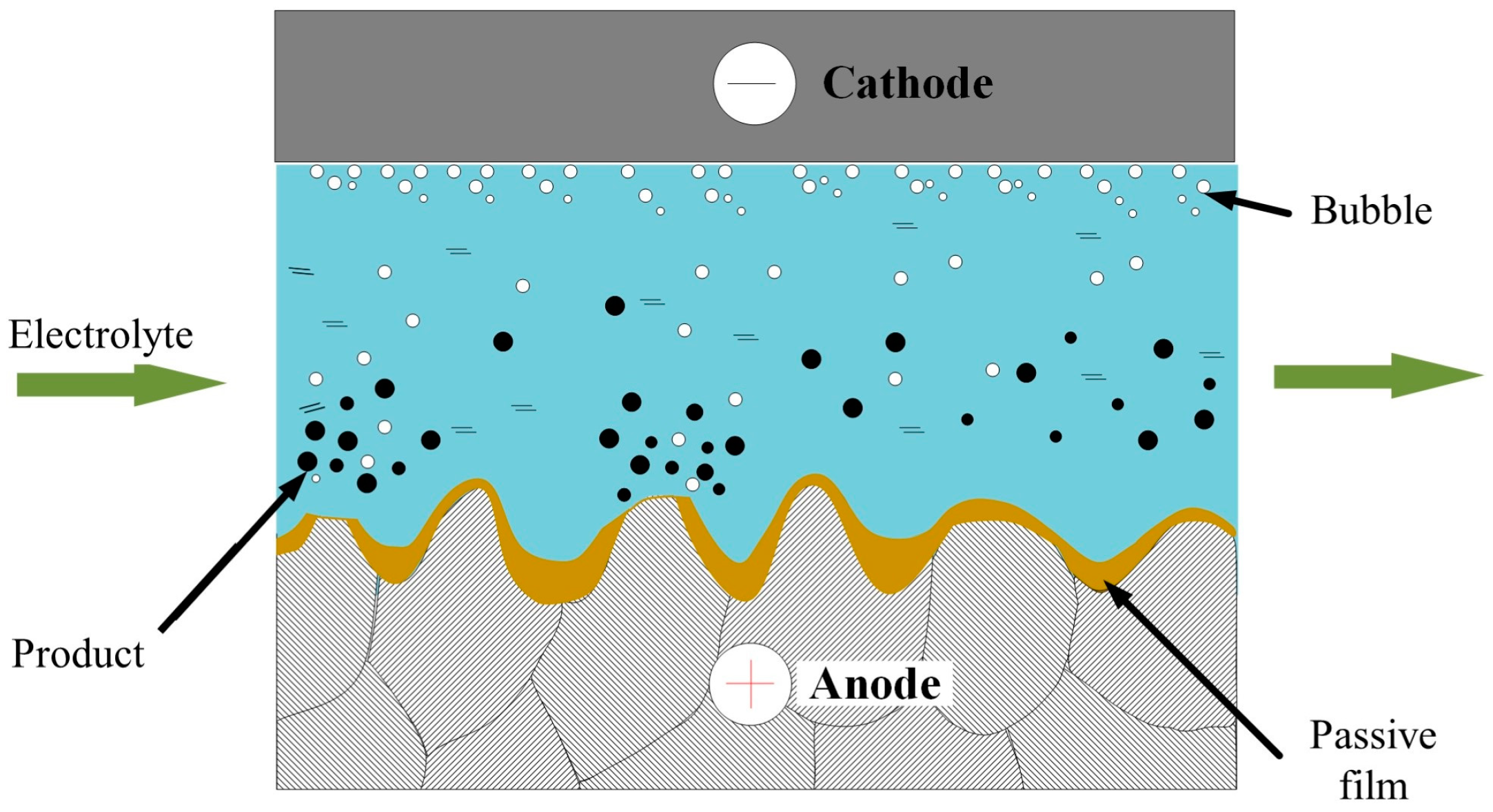
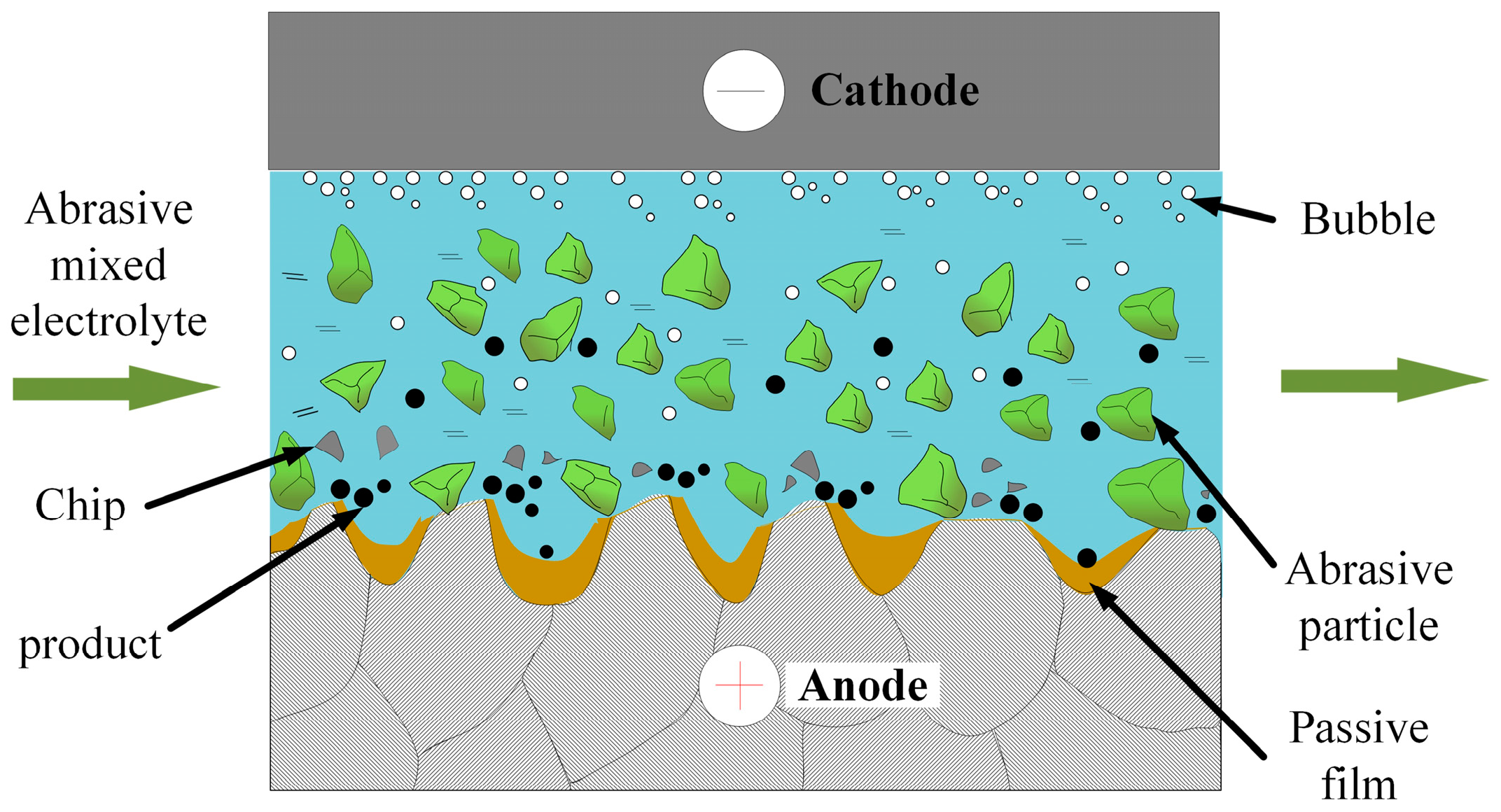
2. Principles of ECP and ECFAP Methods
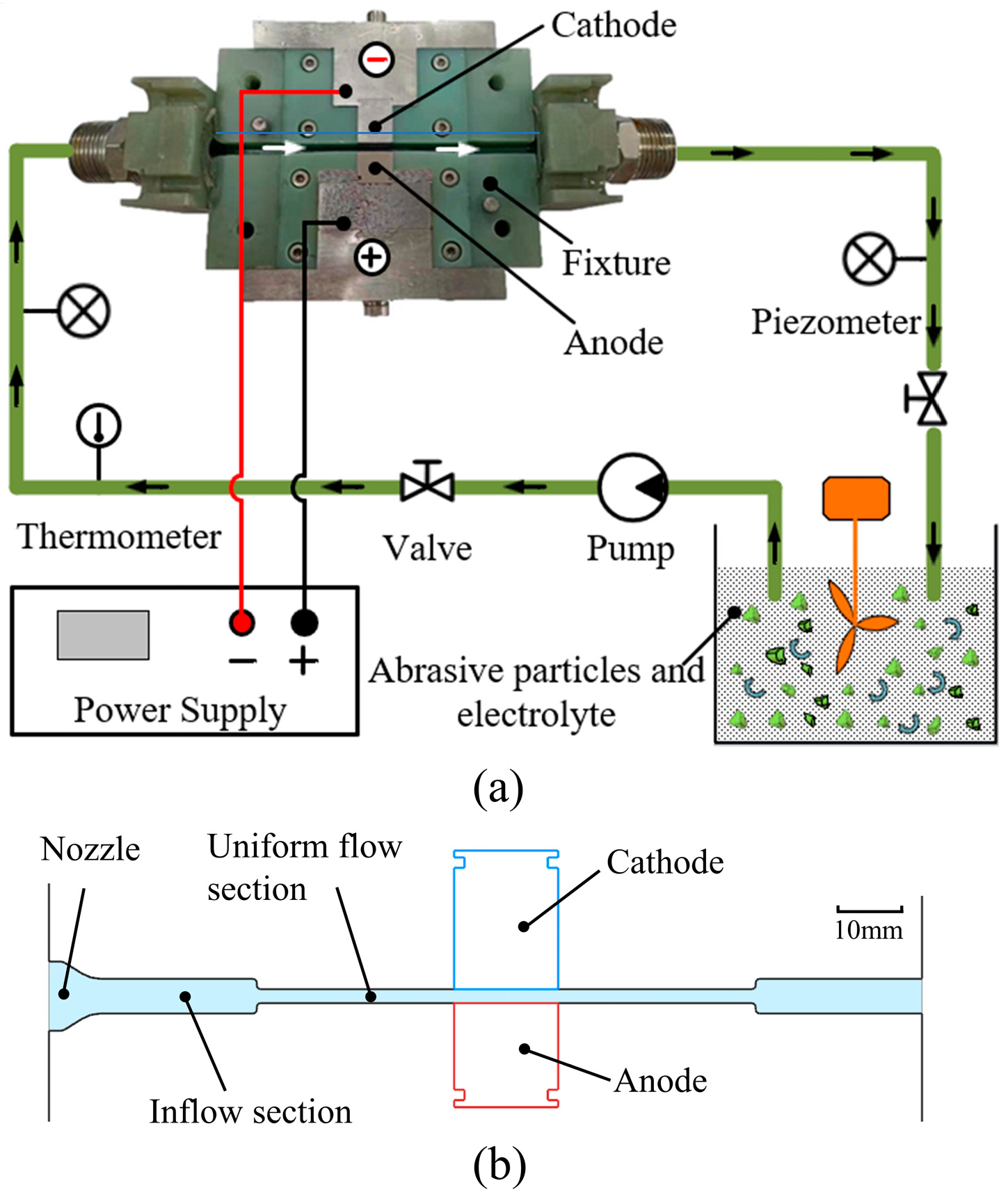
3. Experimental Procedures
3.1. Electrochemical Tests
3.2. ECFAP Experiments
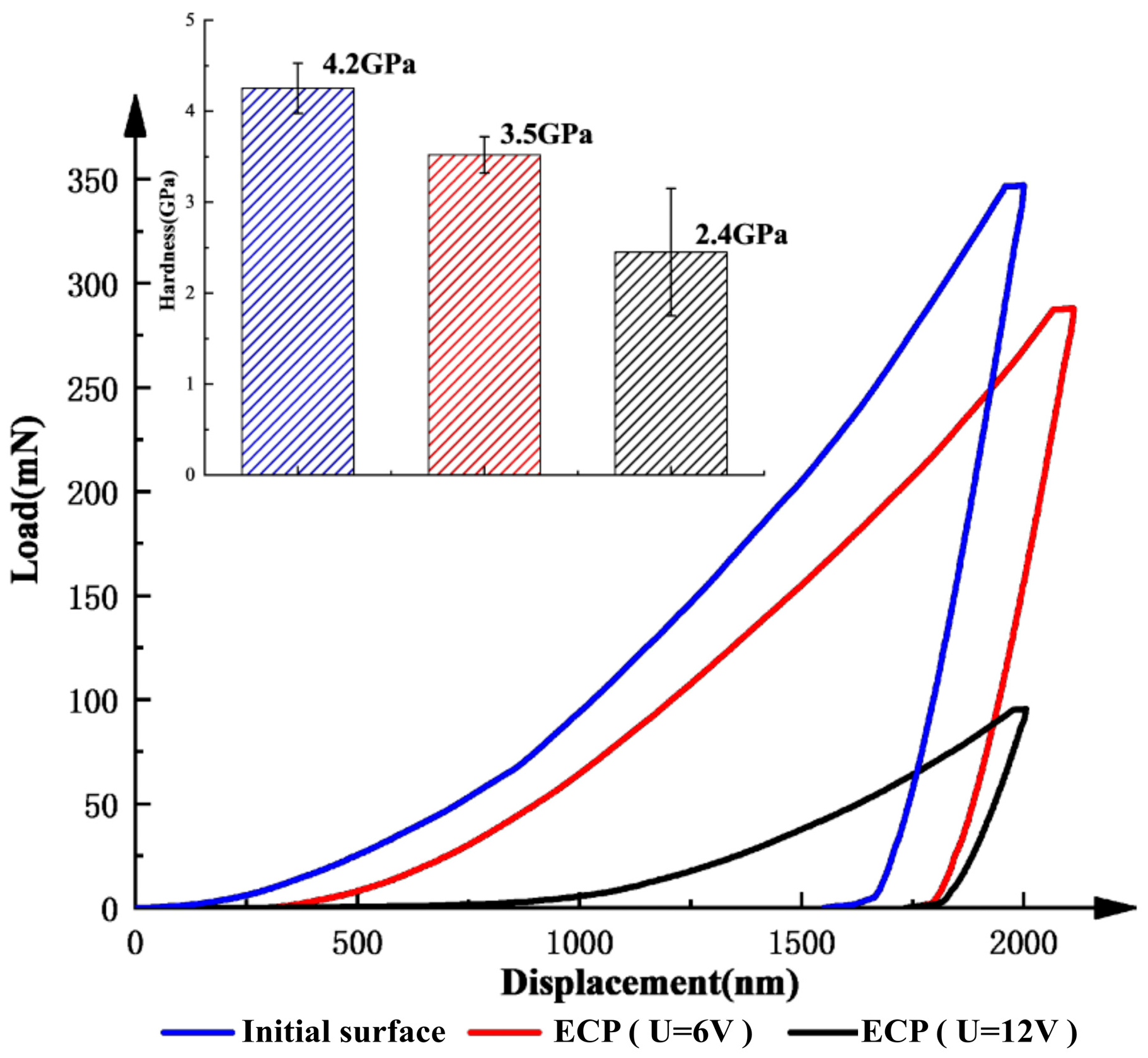
3.3. Surface Integrity Measurements
4. Results
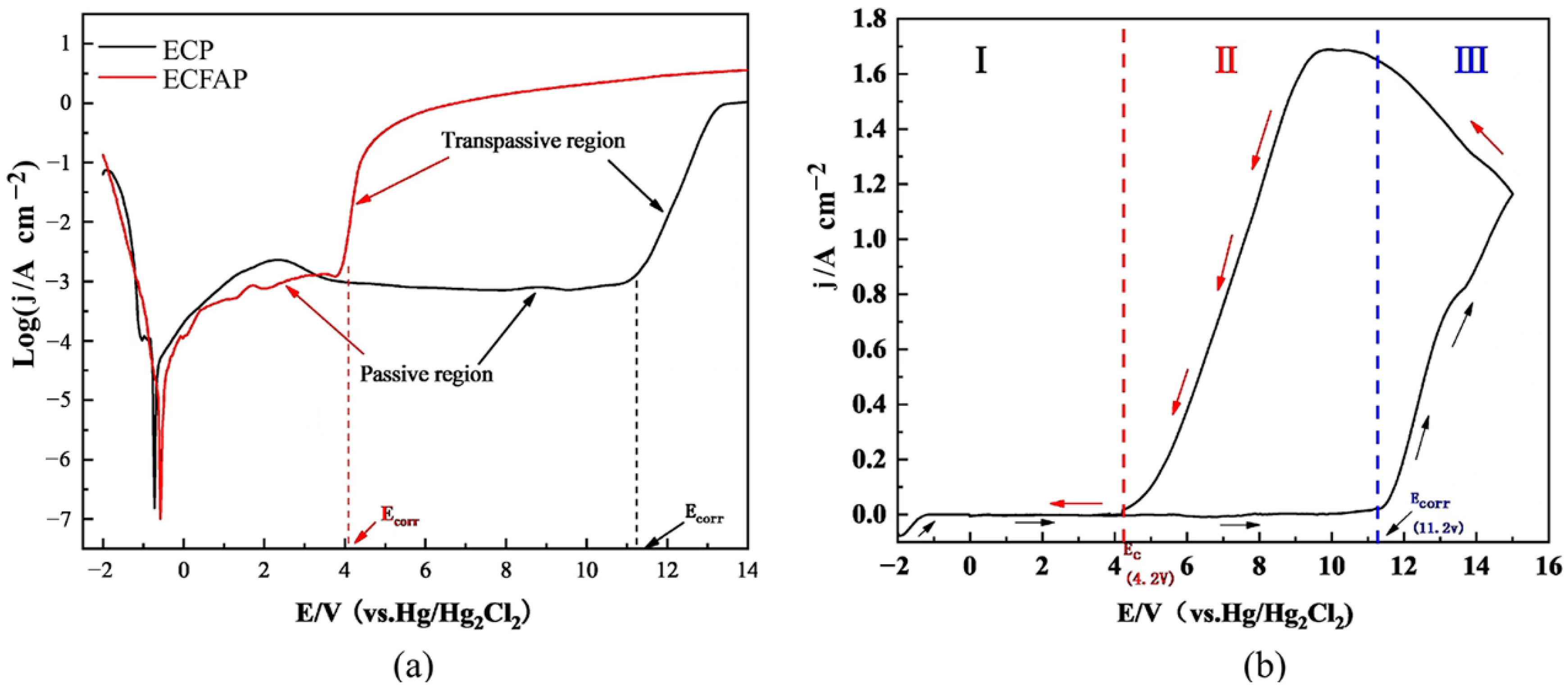
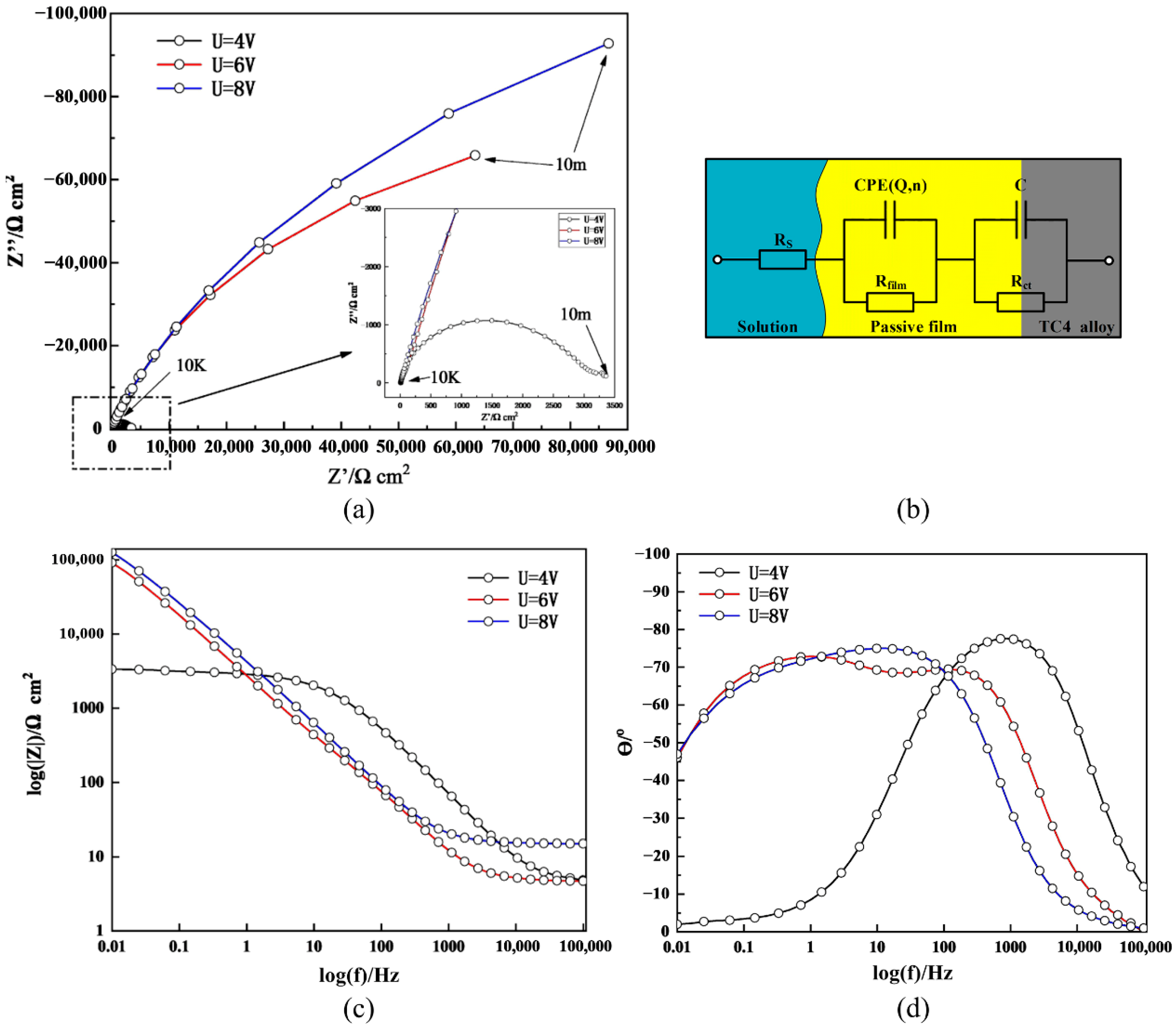
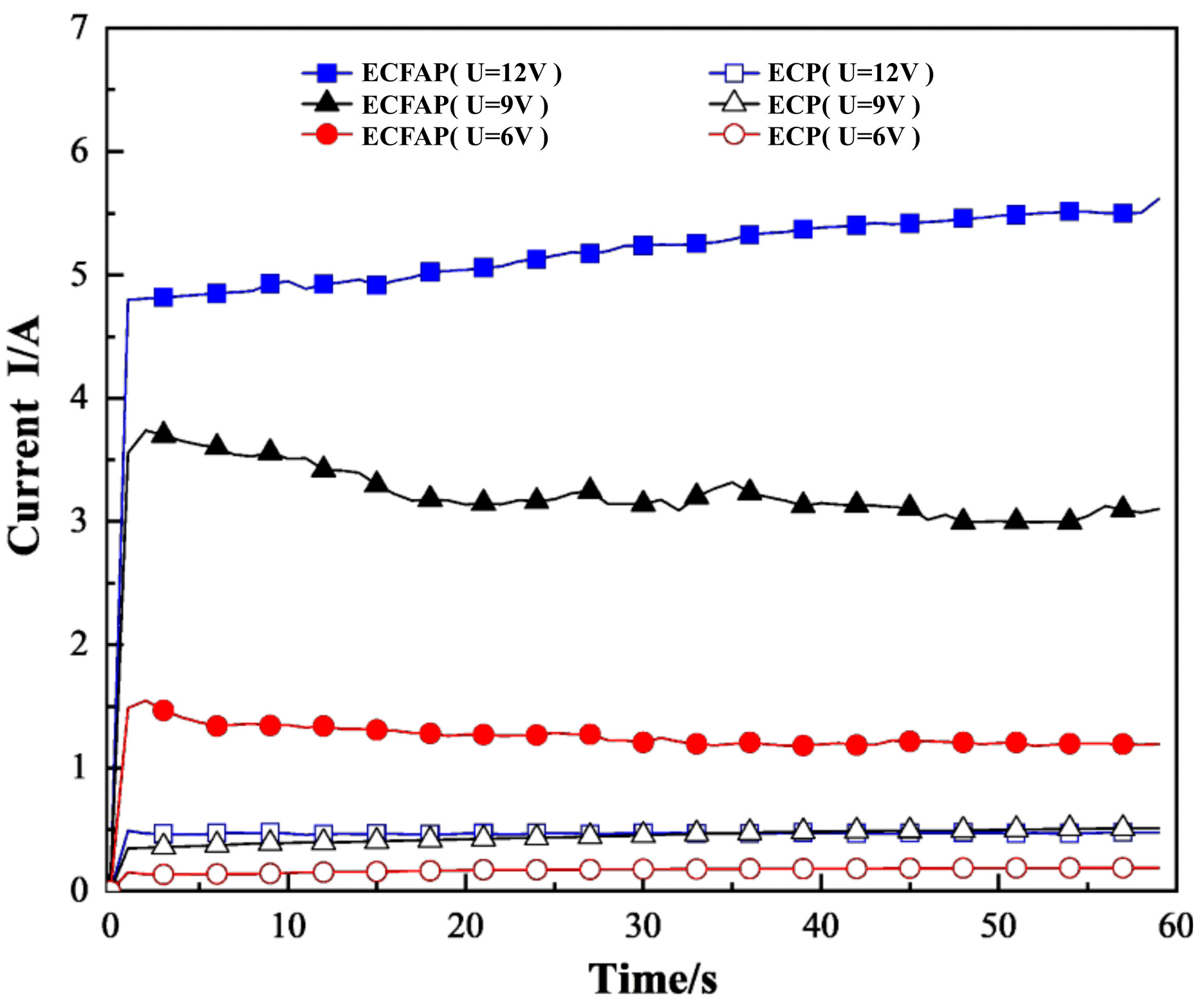
4.1. Polarization and CV Curves
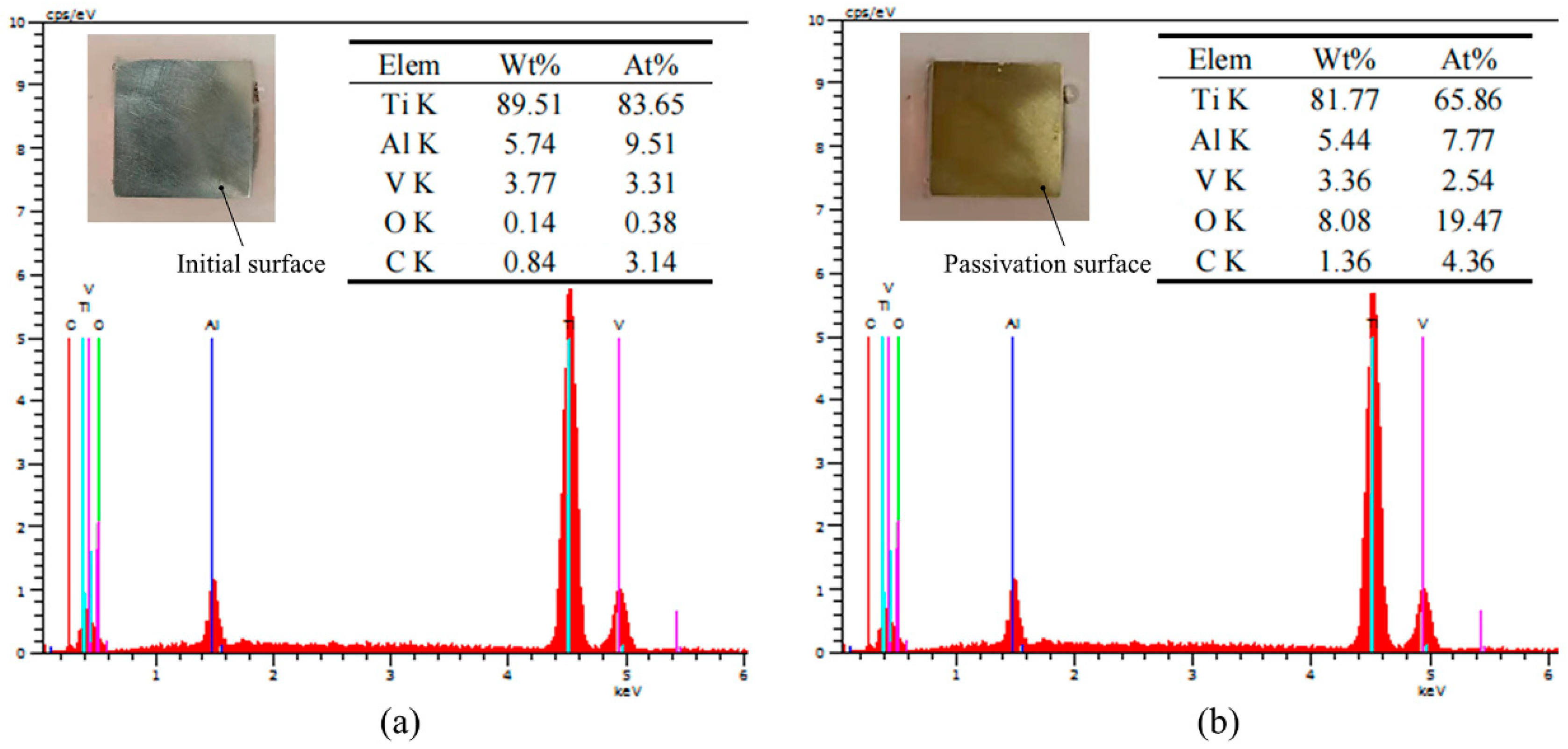
4.2. Analysis of Passivation Film
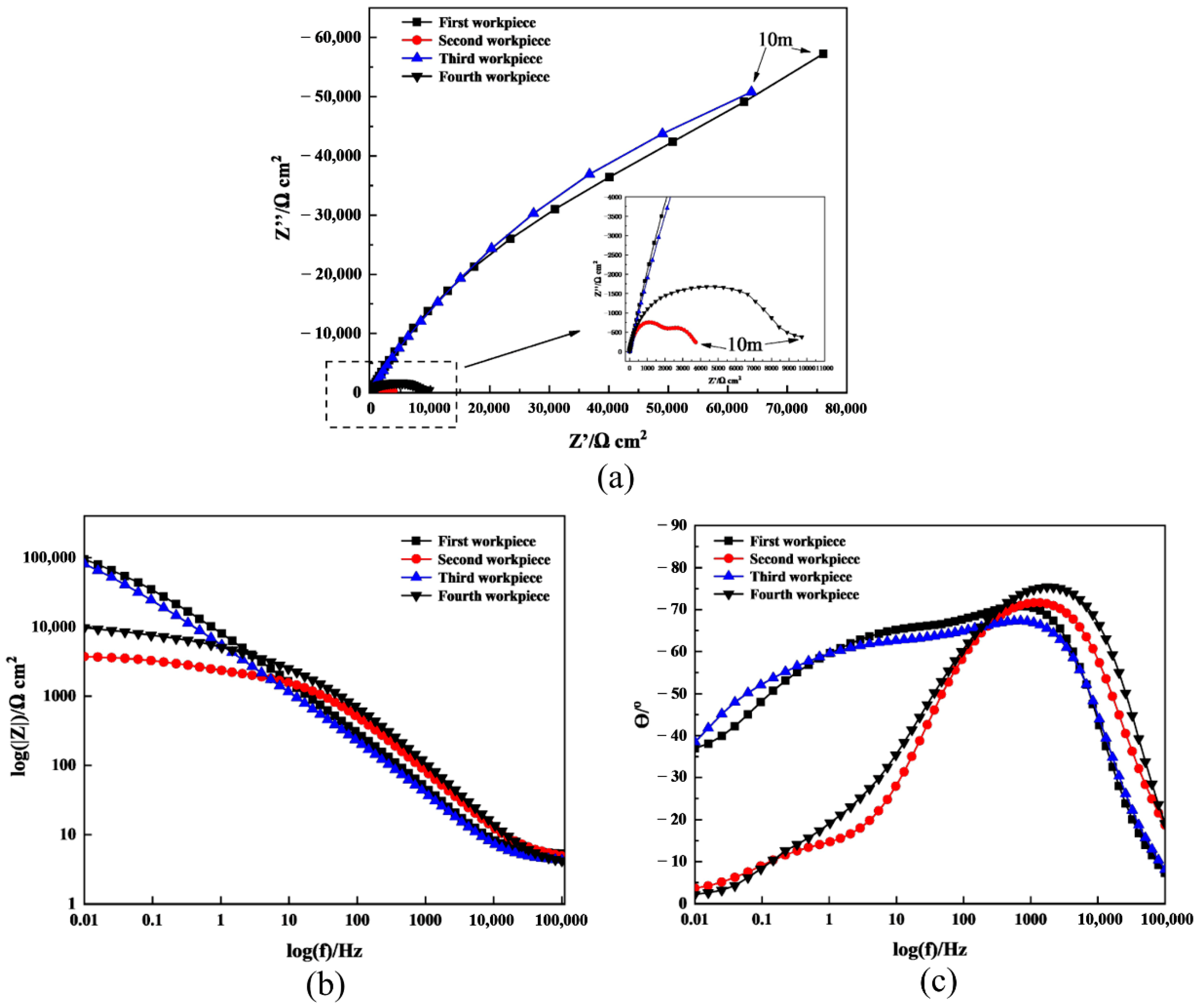
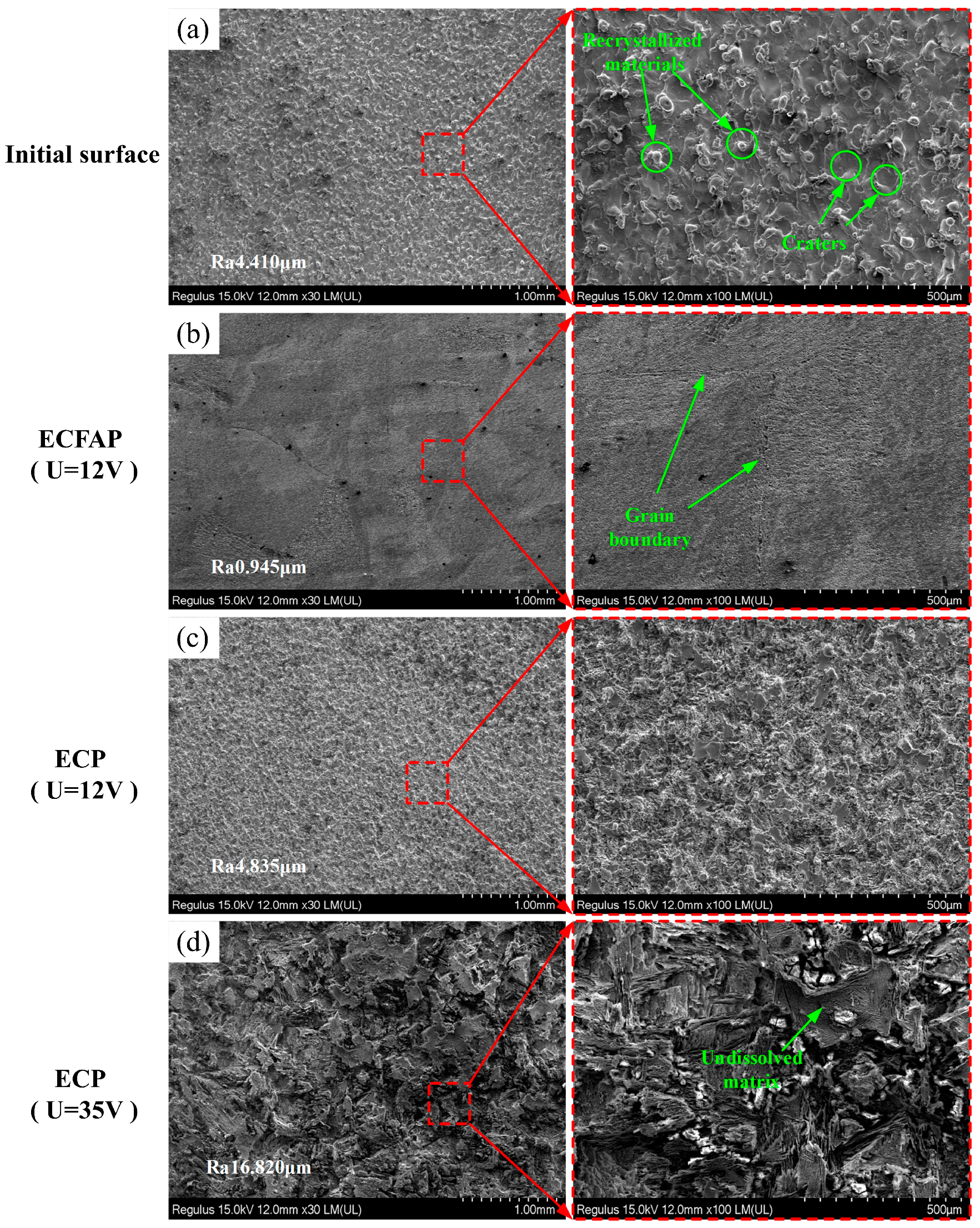
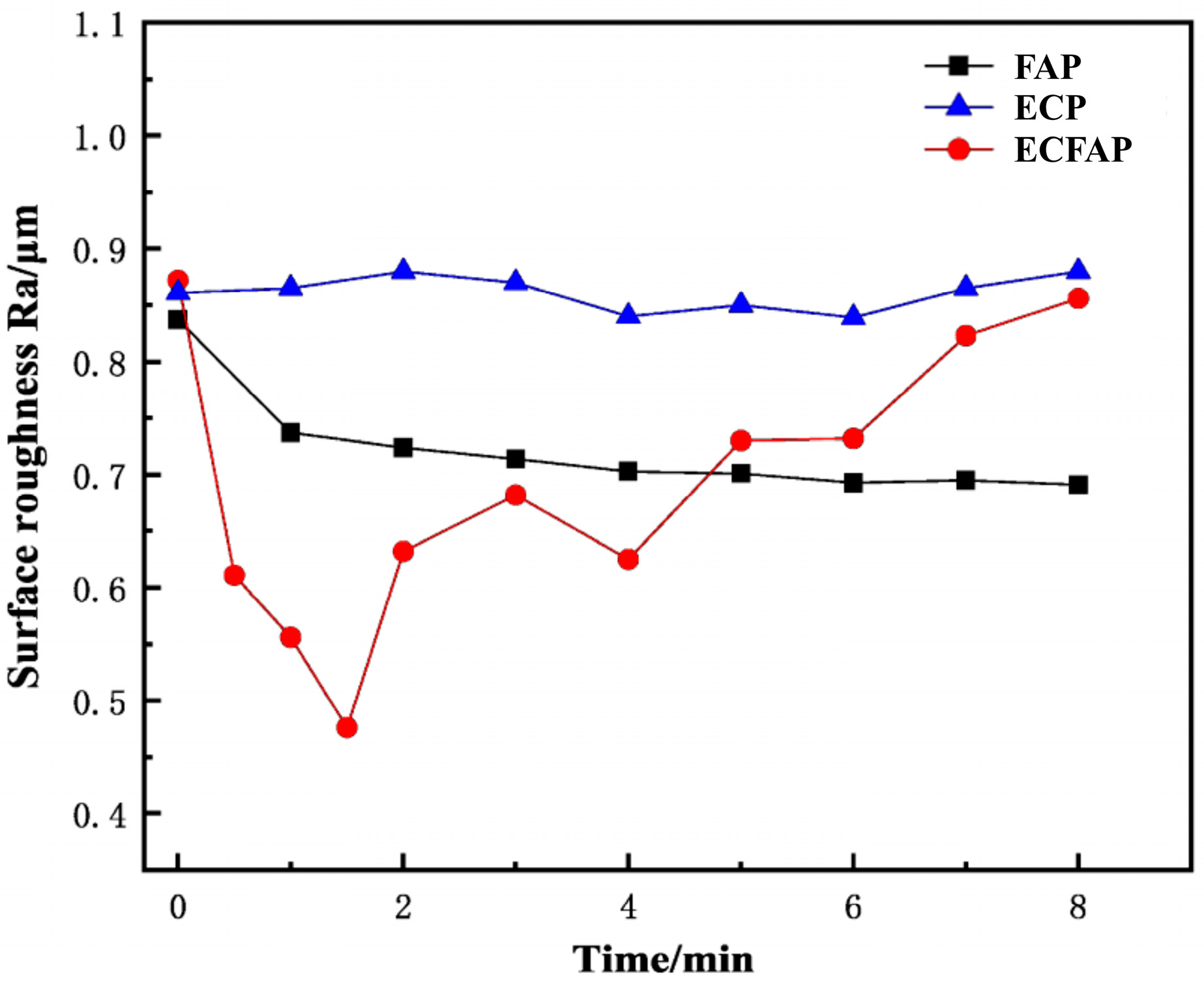
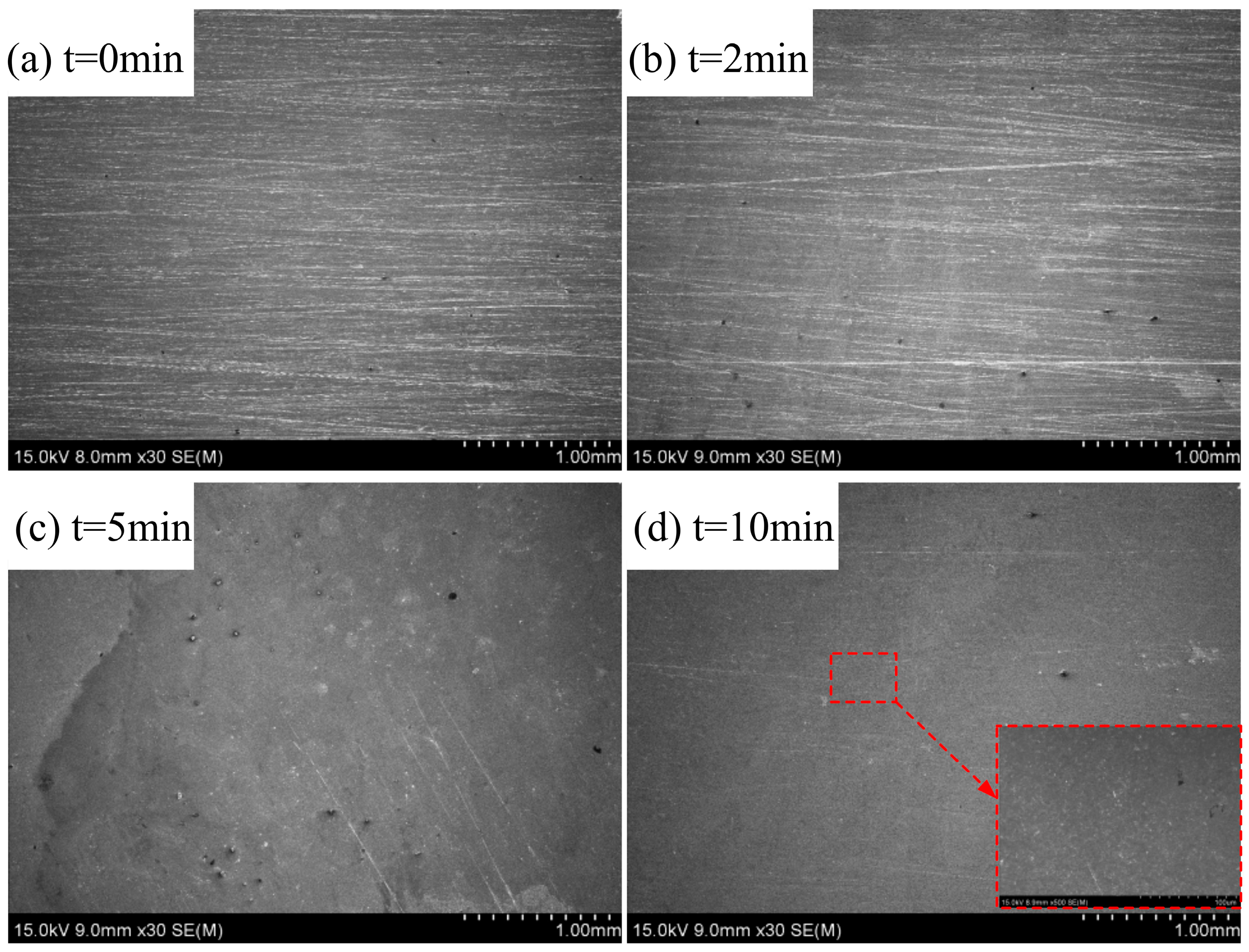
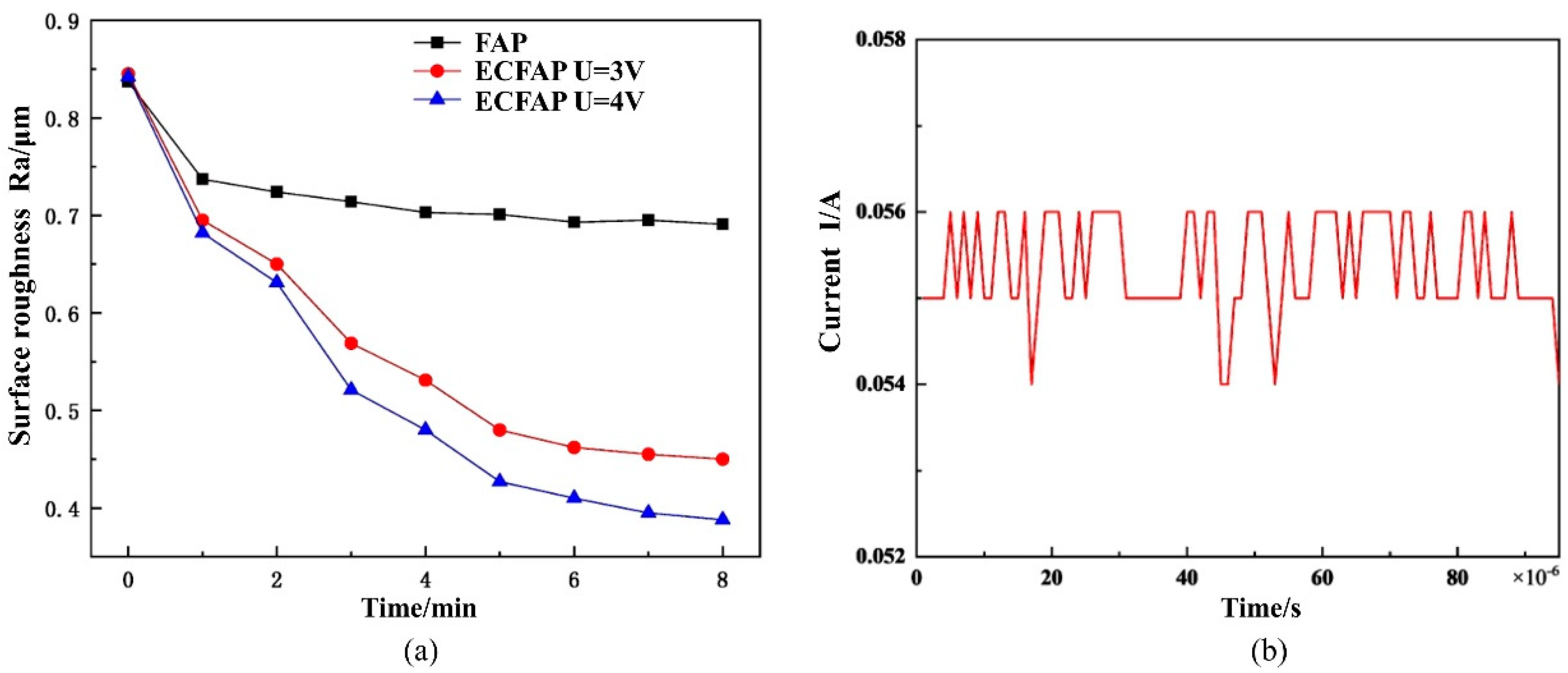
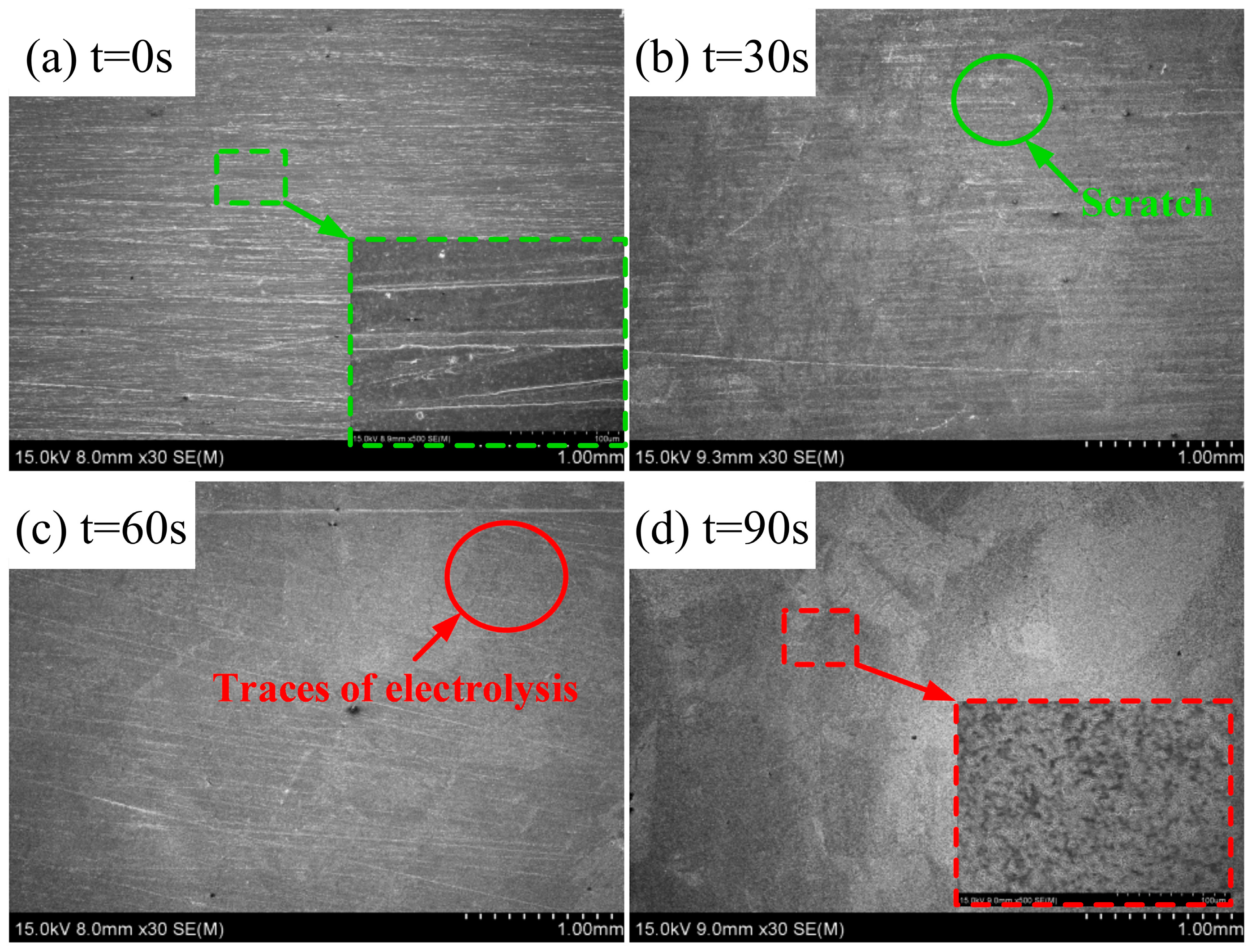
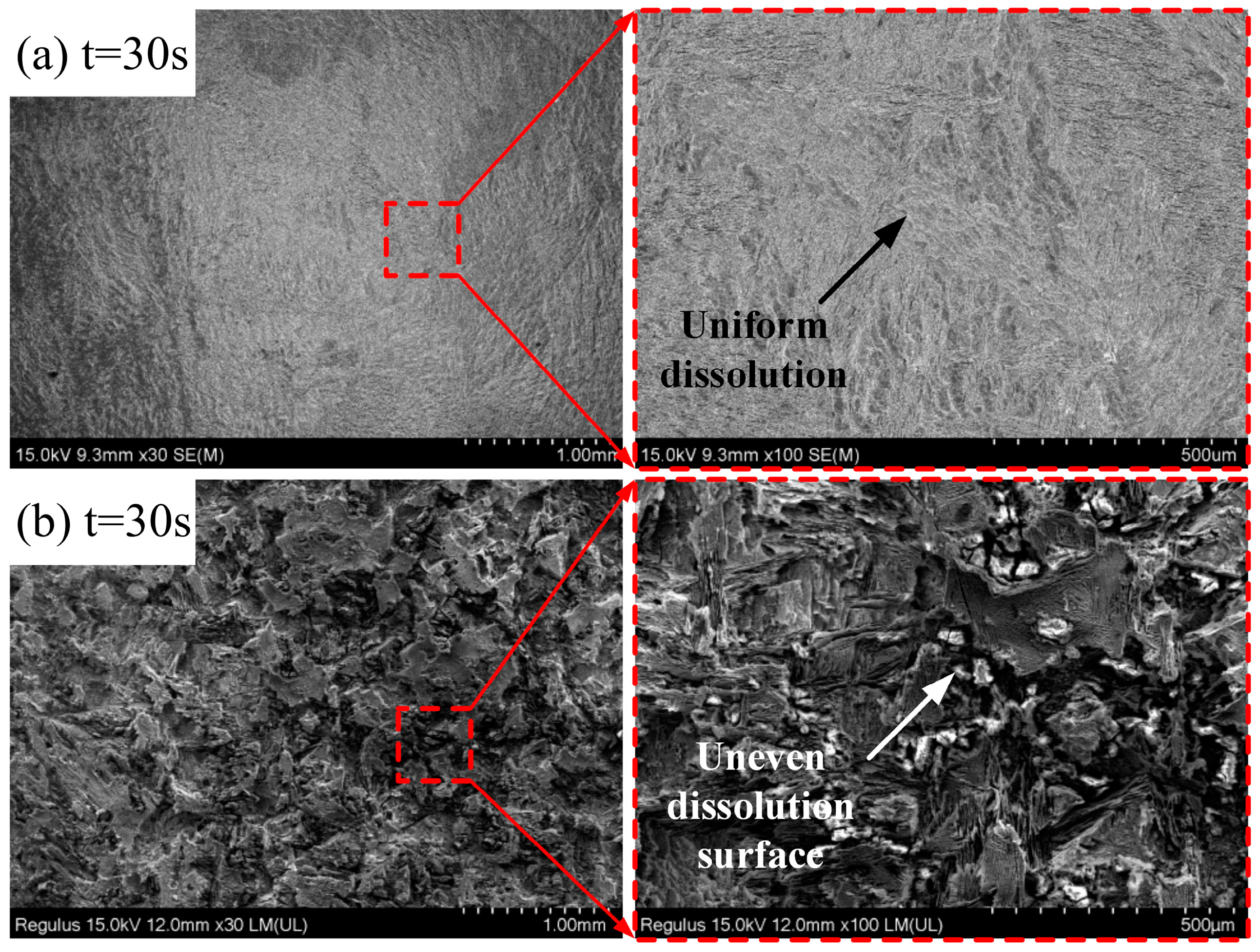
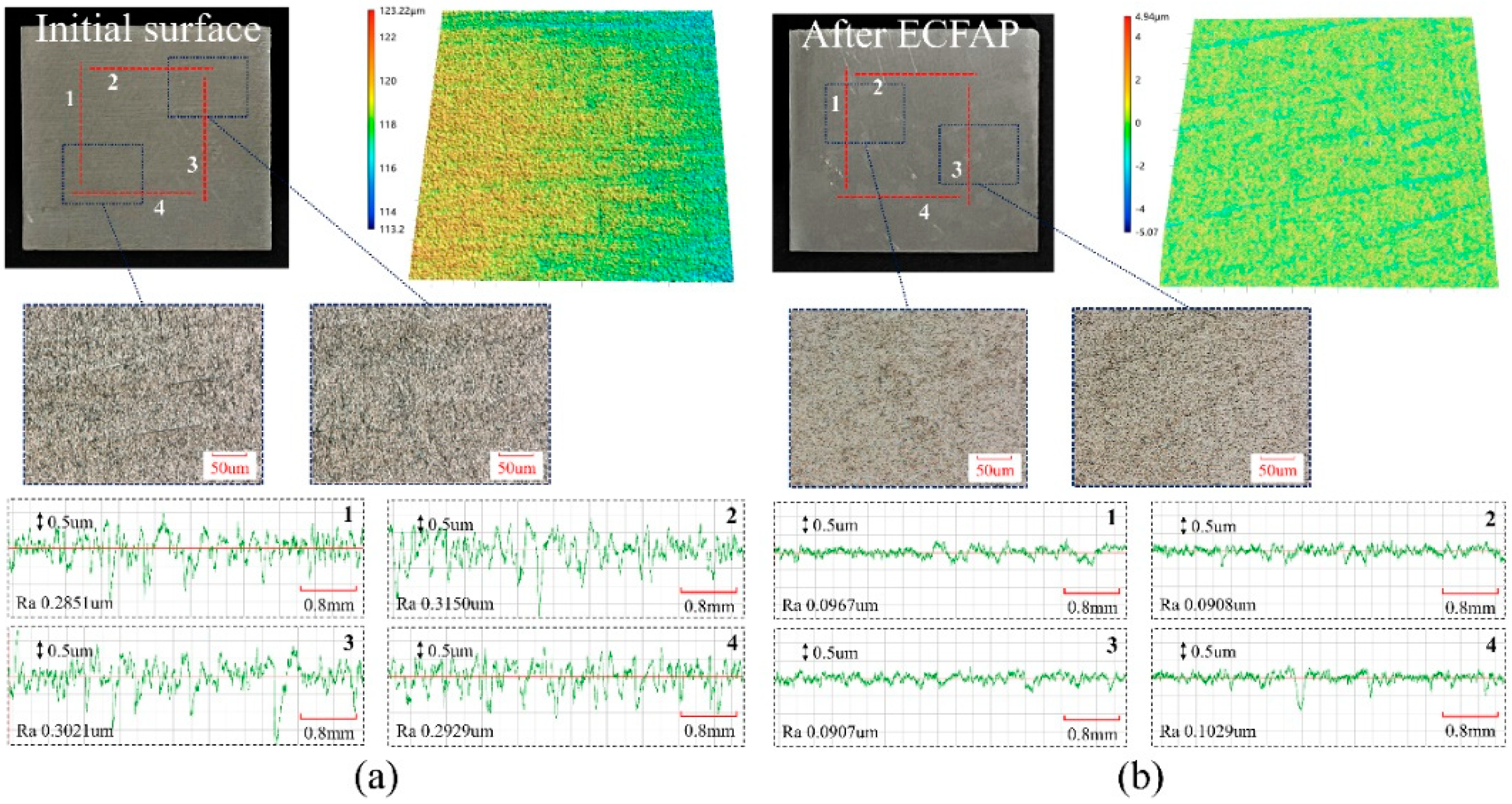
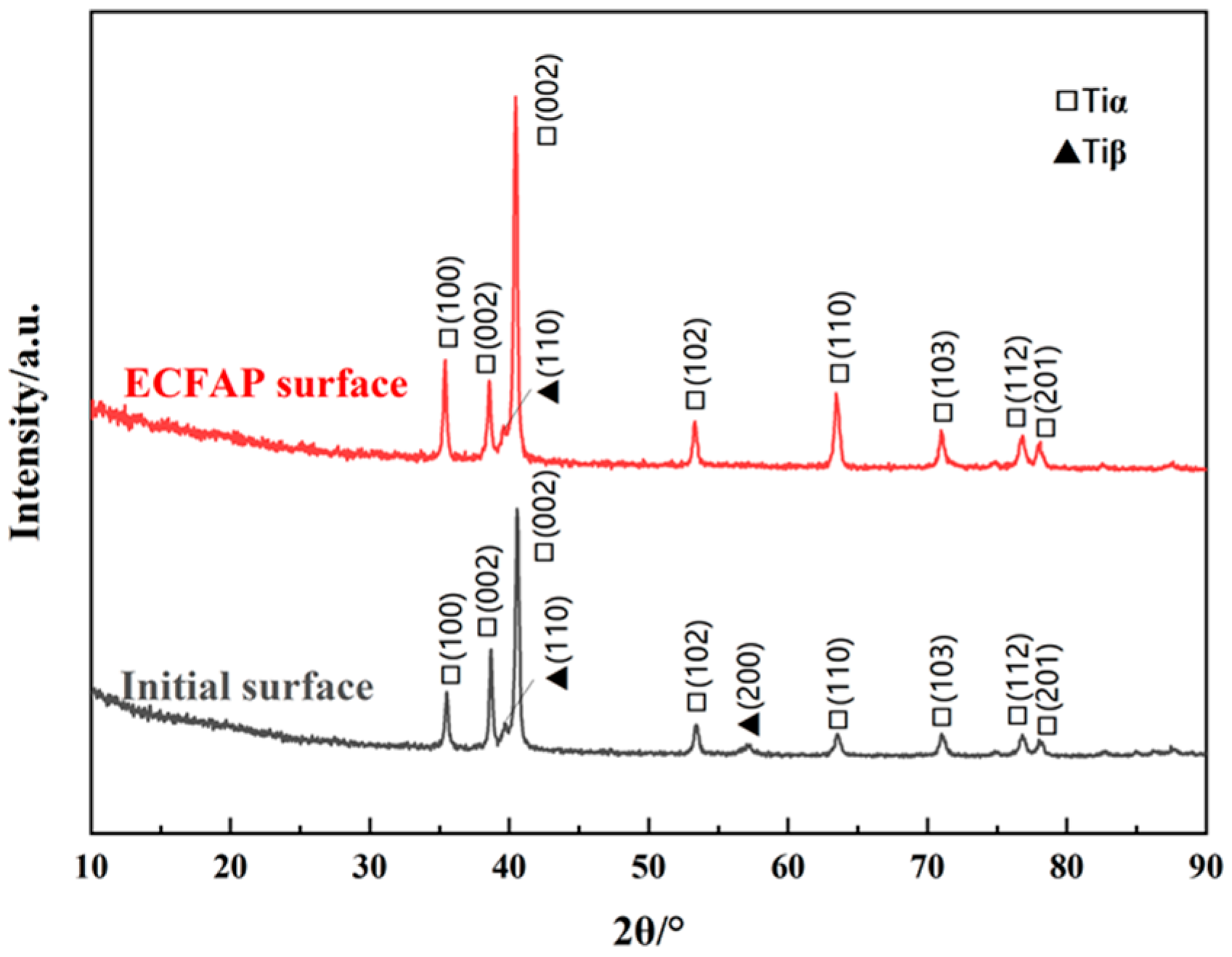
4.3. Results of ECFAP Experiments
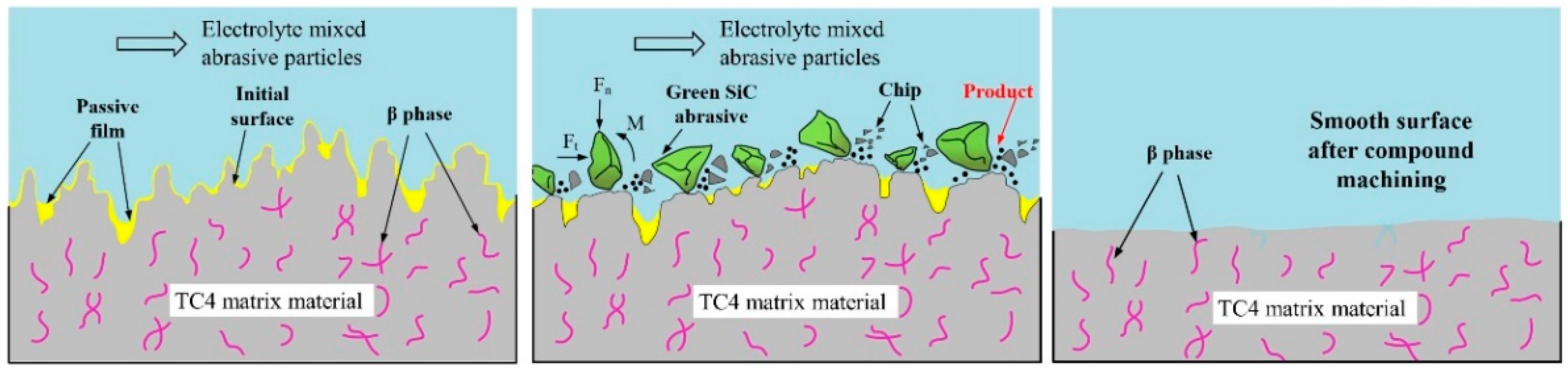
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, W.; Beaucamp, A. Compliant grinding and polishing: A review. Int. J. Mach. Tools Manuf. 2020, 158, 103634. [Google Scholar] [CrossRef]
- Tan, L.; Yao, C.; Zhang, D.; Ren, J.; Zhou, Z.; Zhang, J. Evolution of surface integrity and fatigue properties after milling, polishing, and shot peening of TC17 alloy blades. Int. J. Fatigue 2020, 136, 105630. [Google Scholar] [CrossRef]
- Mohammad, A.E.K.; Wang, D. Electrochemical mechanical polishing technology: Recent developments and future research and industrial needs. Int. J. Adv. Manuf. Technol. 2016, 86, 1909–1924. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y. Electrochemical machining of complex components of aero-engines: Developments, trends, and technological advances. Chin. J. Aeronaut. 2021, 34, 28–53. [Google Scholar] [CrossRef]
- Díaz-Tena, E.; Rodríguez-Ezquerro, A.; de Lacalle Marcaide, L.L.; Bustinduy, L.G.; Sáenz, A.E. A sustainable process for material removal on pure copper by use of extremophile bacteria. J. Clean. Prod. 2014, 84, 752–760. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.; Aggarwal, V.; Singh, S.; Pimenov, D.Y.; Giasin, K.; Nadolny, K. Hand and Abrasive Flow Polished Tungsten Carbide Die: Optimization of Surface Roughness, Polishing Time and Comparative Analysis in Wire Drawing. Materials 2022, 15, 1287. [Google Scholar] [CrossRef]
- Egea, A.S.; Rodriguez, A.; Celentano, D.; Calleja, A.; de Lacalle, L.L. Joining metrics enhancement when combining FSW and ball-burnishing in a 2050 aluminium alloy. Surf. Coat. Technol. 2019, 367, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Aviles, A.; Aviles, R.; Albizuri, J.; Pallares-Santasmartas, L.; Rodriguez, A. Effect of shot-peening and low-plasticity burnishing on the high-cycle fatigue strength of DIN 34CrNiMo6 alloy steel. Int. J. Fatigue 2019, 119, 338–354. [Google Scholar] [CrossRef]
- Han, S.; Salvatore, F.; Rech, J.; Bajolet, J. Abrasive flow machining (AFM) finishing of conformal cooling channels created by selective laser melting (SLM). Precis. Eng. 2020, 64, 20–33. [Google Scholar] [CrossRef]
- Santhosh, K.S.; Somashekhar, S.H. A review on abrasive flow machining (AFM). Procedia Technol. 2016, 25, 1297–1304. [Google Scholar]
- Gorana, V.K.; Jain, V.K.; Lal, G.K. Experimental investigation into cutting forces and active grain density abrasive flow machining. Int. J. Mach. Tool. Manu. 2004, 44, 201–211. [Google Scholar] [CrossRef]
- Yu, Z.; Zuo, D.; Sun, Y.; Li, G.; Chen, X.; Zhao, J.; Shi, K. Study on the improvement of the surface integrity and efficiency of electrical-discharge-machined TC4 titanium alloy via abrasive flow machining. Proc. Inst. Mech. Eng. B 2021, 235, 6–7. [Google Scholar] [CrossRef]
- Ge, J.Q.; Zhou, H.T.; Li, C.; Li, Z.A.; Li, C.; Qian, P.Y.; Chen, C.W. Soft abrasive flow polishing method and optimization research on the constrained space. Int. J. Adv. Manuf. Technol. 2022, 118, 1673–1688. [Google Scholar] [CrossRef]
- Bezuidenhout, M.; Ter Haar, G.; Becker, T.; Rudolph, S.; Damm, O.; Sacks, N. The effect of HF-HNO3 chemical polishing on the surface roughness and fatigue life of laser powder bed fusion produced Ti6Al4V. Mater. Today Commun. 2020, 25, 101396. [Google Scholar] [CrossRef]
- Bae, J.T.; Kim, H.J. Finishing characteristics of Inconel alloy 625 bars in ultra-precision magnetic abrasive finishing using CNC machine center. J. Mech. Sci. Technol. 2021, 35, 2851–2859. [Google Scholar] [CrossRef]
- Jayswal, S.C.; Jain, V.K.; Dixit, P.M. Modeling and simulation of magnetic abrasive finishing process. Int. J. Adv. Manuf. Technol. 2005, 26, 477–490. [Google Scholar] [CrossRef]
- Singh, D.K.; Jain, V.K.; Raghuram, V. Parametric study of magnetic abrasive finishing process. J. Mater. Process. Technol. 2004, 149, 22–29. [Google Scholar] [CrossRef]
- El Hassanin, A.; Obeidi, M.A.; Scherillo, F.; Brabazon, D. CO2 laser polishing of laser-powder bed fusion produced AlSi10Mg parts. Surf. Coat. Technol. 2021, 419, 127291. [Google Scholar] [CrossRef]
- Marimuthu, S.; Triantaphyllou, A.; Antar, M.; Wimpenny, D.; Morton, H.; Beard, M. Laser polishing of selective laser melted components. Int. J. Mach. Tool. Manu. 2015, 95, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Ye, M.; Ye, Z.; Wang, L.; Hao, Y.; Zhu, J.; Xu, P.; Wang, C. Electrochemical polishing of 7075 Al alloy in phosphoric acid. J. Electrochem. Soc. 2021, 168, 123505. [Google Scholar] [CrossRef]
- Rokosz, K.; Solecki, G.; Mori, G.; Fluch, R.; Kapp, M.; Lahtinen, J. Effect of polishing on electrochemical behavior and passive layer composition of different stainless steels. Materials 2020, 13, 3402. [Google Scholar] [CrossRef] [PubMed]
- Simka, W.; Mosialek, M.; Nawrat, G.; Nowak, P.; Zak, J.; Szade, J.; Winiarski, A.; Maciej, A.; Szyk-warszynska, L. Electrochemical polishing of Ti-13Nb-13Zr alloy. Surf. Coat. Technol. 2012, 213, 239–246. [Google Scholar] [CrossRef]
- Wang, S.F.; Wang, Y.; Wen, J.C.; Suo, H.L.; Liu, Z.Z.; Suo, W.H.; Zhao, C.C. Study on electrochemical polishing of TC4 alloy. Mater. Res. Express. 2021, 8, 106520. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, X.; Zhou, Z.; Qin, P.; Zhu, D. Electrochemical machining of high-temperature titanium alloy Ti60. Procedia CIRP 2016, 42, 125–130. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Meng, D.; Wang, Z. Obtaining high surface quality in electrochemical machining of TC17 titanium alloy and Inconel 718 with high current densities in NaNO3 solution. J. Electrochem. Soc. 2021, 168, 073502. [Google Scholar] [CrossRef]
- Hyniewicz, T.; Rokosz, K.; Rokicki, R. Electrochemical and XPS studies of AISI 316L stainless steel after electropolishing in a magnetic field. Corros. Sci. 2008, 50, 2676–2681. [Google Scholar] [CrossRef]
- Hryniewicz, T.; Rokicki, R.; Rokosz, K. Surface characterization of AISI 316L biomaterials obtained by electropolishing in a magnetic field. Surf. Coat. Technol. 2008, 202, 1668–1673. [Google Scholar] [CrossRef]
- An, L.; Wang, D.; Zhu, D. Combined electrochemical and mechanical polishing of interior channels in parts made by additive manufacturing. Addit. Manuf. 2022, 51, 102638. [Google Scholar] [CrossRef]
- Doroshenko, Y.; Doroshenko, J.; Zapukhliak, V.; Poberezhny, L.; Maruschak, P. Modeling computational fluid dynamics of multiphase flows in elbow and T-junction of the main gas pipeline. Transport 2019, 34, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Oh, M.C.; Kim, J.T.; Srivastava, A.K.; Ahn, B.X. Investigation of electrochemical corrosion behavior of additive manufactured Ti–6Al–4V alloy for medical implants in different electrolytes. J. Alloys Compd. 2020, 830, 154620. [Google Scholar] [CrossRef]
- Yang, X.; Yang, X.; Kawai, K.; Arima, K.; Yamamura, K. Novel SiC wafer manufacturing process employing three-step slurryless electrochemical mechanical polishing. J. Manuf. Process. 2021, 70, 350–360. [Google Scholar] [CrossRef]
- Chen, Z.; Zhan, S.; Zhao, Y. Electrochemical jet-assisted precision grinding of single-crystal SiC using soft abrasive wheel. Int. J. Mech. Sci. 2021, 195, 106239. [Google Scholar] [CrossRef]

| Processing Technologies | Structural Adaptability | Environmental Friendliness | Equipment Complexity | Polishing Efficiency |
|---|---|---|---|---|
| MB | Low | High | Simple | Low |
| AFM | High | High | Complex | High |
| CP | High | Low | Simple | Low |
| MAF | High | High | Complex | Low |
| LP | Low | High | Complex | Low |
| ECP | High | Low | Simple | High |
| Alloy | Ti | Al | V | Fe | C |
|---|---|---|---|---|---|
| TC4 | Balance | 5.5–6.8 | 3.5–4.5 | 0.3 | ≤0.3 |
| Parameter | Value |
|---|---|
| Electrolyte | 10 wt.% NaNO3 solution |
| Abrasive particles | 10% (v/v) 500-mesh SiC abrasive particles |
| Machining gap | 2 mm |
| Initial pressure | 0.8 MPa |
| Flow speed | 9.7 m/s |
| Voltage | 3, 4, 6, 9, 12, 35 V |
| Parameter | Rs [Ω cm2] | Rfilm [Ω cm2] | Q [μF/cm2] | n | Rct [Ω cm2] | C [μF/cm2] | χ2 |
|---|---|---|---|---|---|---|---|
| U = 4 V | 4.624 | 2378 | 16.374 | 0.8469 | 752.2 | 5.026 | 0.003680 |
| U = 6 V | 4.638 | 173,800 | 79.381 | 0.8298 | 40.48 | 103.82 | 0.000509 |
| U = 8 V | 14.91 | 231,190 | 57.808 | 0.8427 | 1343 | 180.32 | 0.001282 |
| Workpiece No. | Pretreatment Mode |
|---|---|
| 1 | ECP (5 s) |
| 2 | ECP (5 s) → FAP (5 s) |
| 3 | ECP (5 s) → FAP (5 s) → ECP (5 s) |
| 4 | ECP (5 s) → FAP (5 s) → ECP (5 s) → FAP (5 s) |
| Rs [Ω cm2] | Rfilm [Ω cm2] | Q [μF/cm2] | n | Rct [Ω cm2] | C [μF/cm2] | χ2 | |
|---|---|---|---|---|---|---|---|
| 1 | 4.533 | 113,890 | 32.458 | 0.77232 | 518.3 | 20.362 | 0.015096 |
| 2 | 4.201 | 1983 | 8.147 | 0.8399 | 1487 | 271.1 | 0.004976 |
| 3 | 3.601 | 116,820 | 48.886 | 0.74406 | 216.4 | 26.829 | 0.009161 |
| 4 | 3.385 | 3074 | 5.314 | 0.8654 | 4894 | 48.61 | 0.018525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wang, Z.; Xu, Z. Electrochemical Polishing of Ti6Al4V Alloy Assisted by High-Speed Flow of Micro-Abrasive Particles in NaNO3 Electrolyte. Materials 2022, 15, 8148. https://doi.org/10.3390/ma15228148
Liu J, Wang Z, Xu Z. Electrochemical Polishing of Ti6Al4V Alloy Assisted by High-Speed Flow of Micro-Abrasive Particles in NaNO3 Electrolyte. Materials. 2022; 15(22):8148. https://doi.org/10.3390/ma15228148
Chicago/Turabian StyleLiu, Jia, Zhen Wang, and Zhengyang Xu. 2022. "Electrochemical Polishing of Ti6Al4V Alloy Assisted by High-Speed Flow of Micro-Abrasive Particles in NaNO3 Electrolyte" Materials 15, no. 22: 8148. https://doi.org/10.3390/ma15228148
APA StyleLiu, J., Wang, Z., & Xu, Z. (2022). Electrochemical Polishing of Ti6Al4V Alloy Assisted by High-Speed Flow of Micro-Abrasive Particles in NaNO3 Electrolyte. Materials, 15(22), 8148. https://doi.org/10.3390/ma15228148





