Synthetic Sulfide Concentrate Dissolution Kinetics in HNO3 Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Analysis
3. Results and Discussion
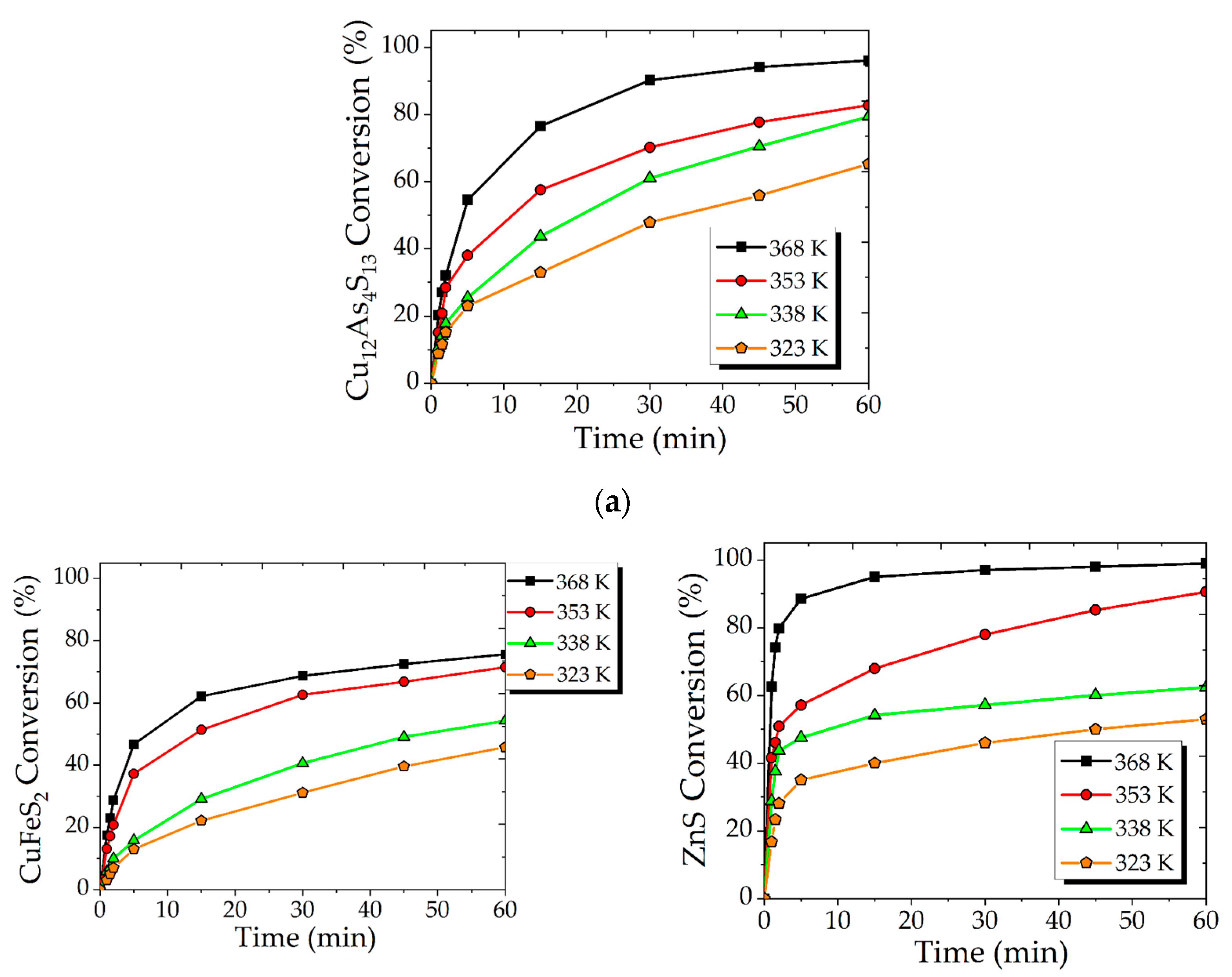
3.1. Effect of Temperature
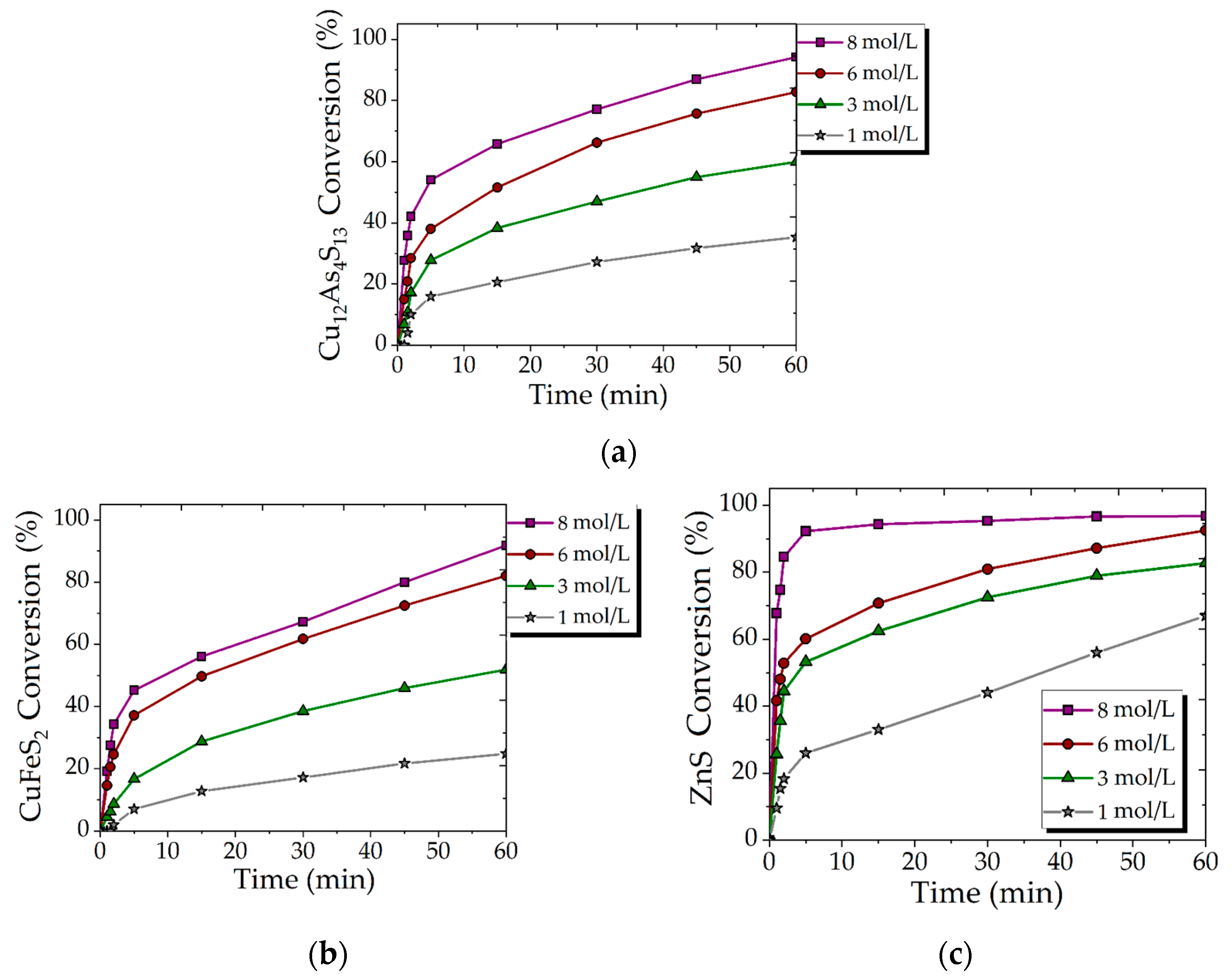
3.2. Effect of HNO3 Concentration
3.3. Effect of Fe(III) Concentration
3.4. Effect of FeS2 Additive
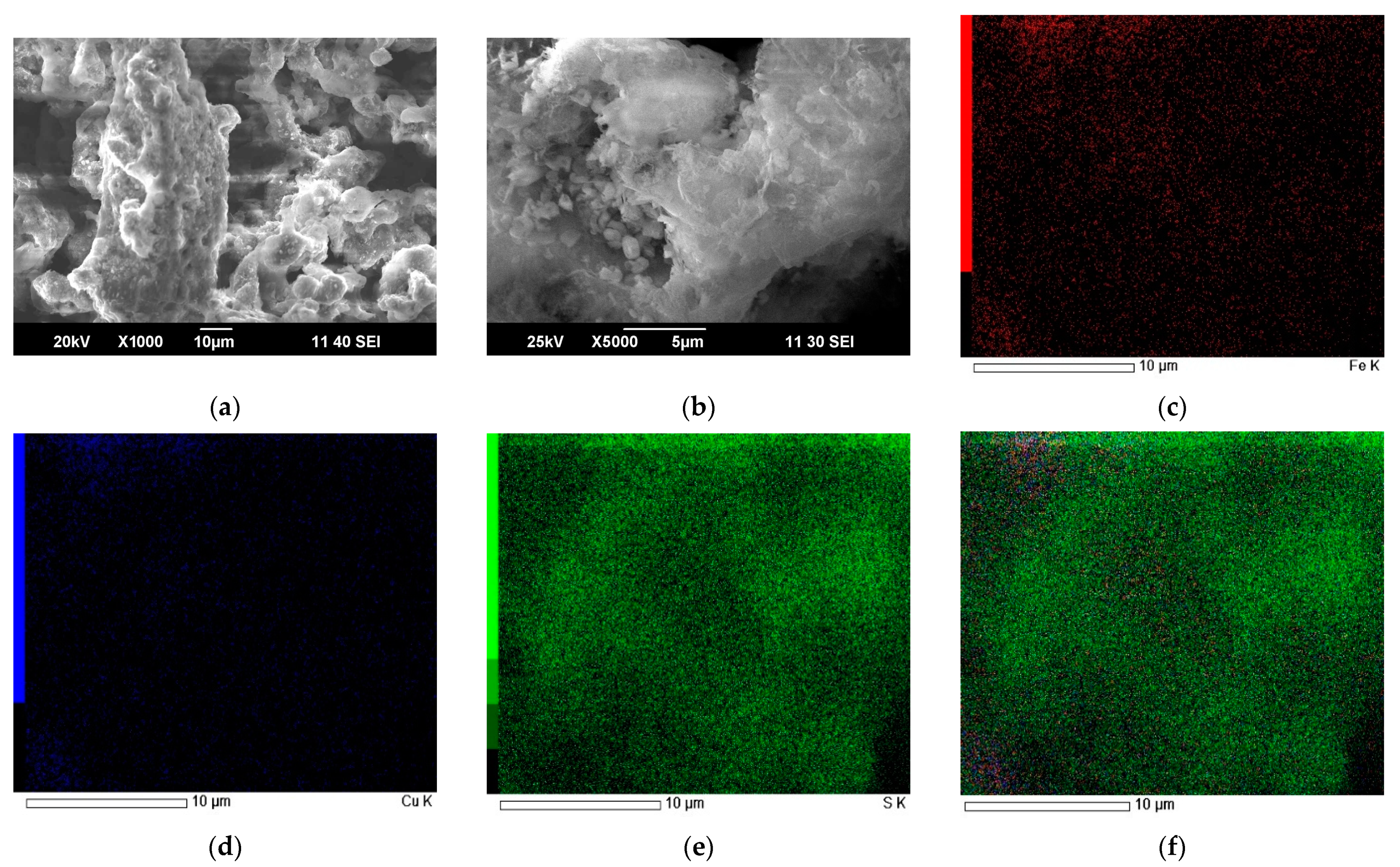
4. Characterization of Residues
Characteristics of the Received Cakes
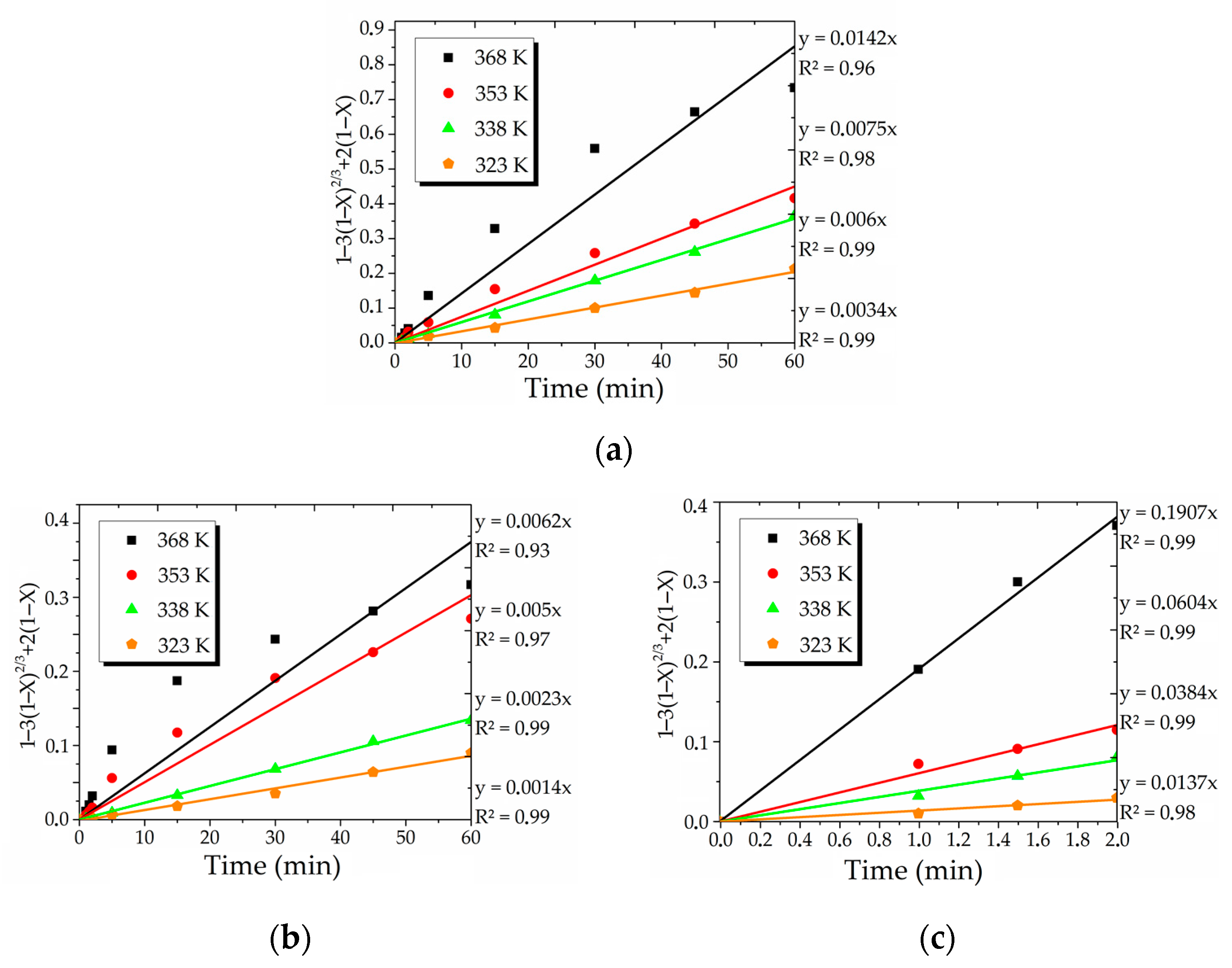
5. Kinetics Analysis
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| № | Abbreviation/Symbol | Description |
| 1 | Ea | Activation energy |
| 2 | SCM | Shrinking core model |
| 3 | XRD | X-ray diffraction |
| 4 | SEM-EDS (or EDX) | Scanning electron microscopy/energy dispersive X-ray spectrometry |
| 5 | EDS-mapping (or EDX-mapping) | X-ray characterization technique that allows extremely rapid elemental concentrations to be gathered and collected as map |
| 6 | ICP-MS | Inductively coupled plasma mass spectrometry |
| 7 | X | Fraction reacted (decimal share) |
| 8 | E | Conversion (%) |
| 9 | Synt. conc | Synthetic concentrate |
| 10 | FeS2/synt. conc | Mass ratio of pyrite to synthetic concentrate in a sample of material before the start of the experiment run |
| 11 | HNO3 | Nitric acid |
| 12 | Cu12As4S13 | Tennantite |
| 13 | CuFeS2 | Chalcopyrite |
| 14 | ZnS | Sphalerite |
| 15 | FeS2 | Pyrite |
| 16 | FeAsS | Arsenopyrite |
| 17 | S° | Elemental sulphur |
References
- Alguacil, F. The Chemistry of Gold Extraction, 2nd ed.; Marsden, J.O., House, C.I., Eds.; Gold Bulletin; SME: Littleton, CO, USA, 2006; Volume 39, p. 138. [Google Scholar] [CrossRef]
- Vikentev, A. Selenium, tellurium and precious metal mineralogy in Uchalinsk copper-zinc-pyritic district, the Urals. IOP Conf. Ser. Mater. Sci. Eng. 2016, 123, 012027. [Google Scholar] [CrossRef]
- Shibayama, A.; Takasaki, Y.; William, T.; Yamatodani, A.; Higuchi, Y.; Sunagawa, S.; Ono, E. Treatment of smelting residue for arsenic removal and recovery of copper using pyro–hydrometallurgical process. J. Hazard. Mater. 2010, 181, 1016–1023. [Google Scholar] [CrossRef]
- Jarošíková, A.; Ettler, V.; Mihaljevič, M.; Penížek, V.; Matoušek, T.; Culka, A.; Drahota, P. Transformation of arsenic-rich copper smelter flue dust in contrasting soils: A 2-year field experiment. Environ. Pollut. 2018, 237, 83–92. [Google Scholar] [CrossRef]
- Lv, X.-D.; Li, G.; Xin, Y.-T.; Yan, K.; Yi, Y. Selective Leaching of Arsenic from High-Arsenic Dust in the Alkaline System and its Prediction Model Using Artificial Neural Network. Min. Metall. Explor. 2021, 28, 2133–2144. [Google Scholar] [CrossRef]
- Guo, X.-Y.; Yi, Y.; Shi, J.; Tian, Q.-H. Leaching behavior of metals from high-arsenic dust by NaOH-Na2S alkaline leaching. Trans. Nonferrous Met. Soc. China 2016, 26, 575–580. [Google Scholar] [CrossRef]
- Isabaev, S.; Kuzgibekova, K.; Zikanova, T.; Zhinova, E. Complex hydrometallurgical processing of lead arsenic-containing dust from copper production. Tsvetnye Met. 2017, 8, 33–38. [Google Scholar] [CrossRef]
- Mamyachenkov, S.V.; Anisimova, O.S.; Kostina, D.A. Improving the precipitation of arsenic trisulfide from washing waters of sulfuric-acid production of copper smelteries. Russ. J. Non-Ferr. Met. 2017, 58, 212–217. [Google Scholar] [CrossRef]
- Montenegro, V.; Sano, H.; Fujisawa, T. Recirculation of high arsenic content copper smelting dust to smelting and converting processes. Miner. Eng. 2013, 49, 184–189. [Google Scholar] [CrossRef]
- Montenegro, V.; Sano, H.; Fujisawa, T. Recirculation of chilean copper smelting dust with high arsenic content to the smelting process. Mater. Trans. 2008, 49, 2112–2118. [Google Scholar] [CrossRef]
- Xue, J.; Long, D.; Zhong, H.; Wang, S.; Liu, L. Comprehensive recovery of arsenic and antimony from arsenic-rich copper smelter dust. J. Hazard. Mater. 2021, 413, 125365. [Google Scholar] [CrossRef]
- Karimov, K.; Naboichenko, S.; Kritskii, A.; Tret’yak, M.; Kovyazin, A. Oxidation Sulfuric Acid Autoclave Leaching of Copper Smelting Production Fine Dust. Metallurgist 2019, 62, 1244–1249. [Google Scholar] [CrossRef]
- Riveros, P.; Dutrizac, J. The leaching of tennantite, tetrahedrite and enargite in acidic sulfate and chloride media. Can. Metall. Q. 2008, 47, 235–244. [Google Scholar] [CrossRef]
- Correia, M.; Carvalho, J.; Monhemius, J. The Effect of Tetrahedrite Composition on Its Leaching Behaviour in FeCl3-NaCl-HCl Solutions. Miner. Eng. 2001, 14, 185–195. [Google Scholar] [CrossRef]
- Lin, H. Electrochemical Behavior of Tennantite in Chloride Solutions. J. Electrochem. Soc. 2006, 153, 74–79. [Google Scholar] [CrossRef]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of Arsenic Metallurgy: Treatment of Arsenical Minerals and the Immobilization of Arsenic. Hydrometallurgy 2017, 174, 258–281. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Abdollahi, H.; Gharabaghi, M.; Mirmohammadi, M. Selective leaching of antimony from tetrahedrite rich concentrate using alkaline sulfide solution with experimental design: Optimization and kinetic studies. J. Taiwan Inst. Chem. Eng. 2021, 119, 298–312. [Google Scholar] [CrossRef]
- Awe, S.; Sandström, A. Selective leaching of arsenic and antimony from a tetrahedrite rich complex sulphide concentrate using alkaline sulphide solution. Miner. Eng. 2010, 23, 1227–1236. [Google Scholar] [CrossRef]
- Awe, S.; Samuelsson, C.; Sandström, A. Dissolution kinetics of tetrahedrite mineral in alkaline sulphide media. Hydrometallurgy 2010, 103, 167–172. [Google Scholar] [CrossRef]
- Neiva Correia, M.; Carvalho, J.; Monhemius, A. Study of the autoclave leaching of a tetrahedrite concentrate. Miner. Eng. 1993, 11, 1117–1125. [Google Scholar] [CrossRef]
- Kritskii, A.; Naboichenko, S.; Karimov, K.; Agarwal, V.; Lundström, M. Hydrothermal Pretreatment of Chalcopyrite Concentrate with Copper Sulfate Solution. Hydrometallurgy 2020, 195, 105359. [Google Scholar] [CrossRef]
- Kritskii, A.; Naboichenko, S. Hydrothermal Treatment of Arsenopyrite Particles with CuSO4 Solution. Materials 2021, 23, 7472. [Google Scholar] [CrossRef] [PubMed]
- Corkhill, C.; Wincott, P.; Lloyd, J.; Vaughan, D. The oxidative dissolution of arsenopyrite (FeAsS) and enargite (Cu3AsS4) by Leptospirillum ferrooxidans. Geochim. Cosmochim. Acta 2008, 72, 5616–5633. [Google Scholar] [CrossRef]
- Xu, R.; Li, Q.; Meng, F.; Yang, Y.; Xu, B.; Yin, H.; Jiang, T. Bio-Oxidation of a Double Refractory Gold Ore and Investigation of Preg-Robbing of Gold from Thiourea Solution. Metals 2020, 10, 1216. [Google Scholar] [CrossRef]
- Kritskii, A.; Naboichenko, S.; Klyshnikov, A.; Musaev, V. Pressure Oxidative Leaching of Chalcopyrite Concentrates: Influence of the Process Temperature on Cakes Cyanidation Efficiency. Tsvetnye Met. 2020, 4, 25–29. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Moats, M.; Miller, J. Recent trends in the processing of enargite concentrates. Miner. Process. Extr. Metall. Rev. 2014, 35, 283–367. [Google Scholar] [CrossRef]
- Anderson, C.; Twidwell, L. Hydrometallurgical processing of gold-bearing copper enargite concentrates. Can. Metall. Q. 2008, 47, 337–346. [Google Scholar] [CrossRef]
- Rogozhnikov, D.; Shoppert, A.; Karimova, L. Investigating of nitric acid leaching of high-sulfur copper concentrate. Solid State Phenom. 2020, 229, 968–973. [Google Scholar] [CrossRef]
- Shinoda, K.; Tanno, T.; Fujita, T.; Suzuki, S. Coprecipitation of Large Scorodite Particles from Aqueous Fe(II) and As(V) Solution by Oxygen Injection. Mater. Trans. 2009, 50, 1196–1201. [Google Scholar] [CrossRef]
- Karimov, K.; Rogozhnikov, D.; Kuzas, E.; Dizer, O.; Golovkin, D.; Tretiak, M. Deposition of Arsenic from Nitric Acid Leaching Solutions of Gold—Arsenic Sulphide Concentrates. Metals 2021, 11, 889. [Google Scholar] [CrossRef]
- Tang, Z.; Tang, X.; Liu, H.; Xiao, Z. A Clean Process for Recovering Antimony from Arsenic-Bearing Crystals and Immobilizing Arsenic as Scorodite. Sep. Purif. Technol. 2022, 295, 121276. [Google Scholar] [CrossRef]
- Langmuir, D.; Mahoney, J.; Rowson, J. Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4⋯2H2O) and their application to arsenic behavior in buried mine tailings. Geochim. Cosmochim. Acta 2006, 70, 2942–2956. [Google Scholar] [CrossRef]
- Paktunc, D.; Dutrizac, J.; Gertsman, V. Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: Implications for arsenic mobility. Geochim. Cosmochim. Acta 2008, 75, 2649–2672. [Google Scholar] [CrossRef]
- Dixon, D.; Mayne, D.; Baxter, K. Galvanox™—A novel galvanically-assisted atmospheric leaching technology for copper concentrates. Can. Metall. Q. 2008, 47, 327–336. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.; Dreisinger, D. Enhancing the kinetics of chalcopyrite leaching in the GalvanoxTM process. Hydrometallurgy 2011, 105, 251–258. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.; Dreisinger, D. The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the GalvanoxTM process. Hydrometallurgy 2012, 113–114, 122–130. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.; Dreisinger, D. The role of silver-enhanced pyrite in enhancing the electrical conductivity of sulfur product layer during chalcopyrite leaching in the GalvanoxTM process. Hydrometallurgy 2012, 113–114, 177–184. [Google Scholar] [CrossRef]
- Córdoba, E.; Muñoz, J.; Blázquez, M.; González, F.; Ballester, A. Leaching of chalcopyrite with ferric ion. Part I: General aspects. Hydrometallurgy 2008, 93, 81–87. [Google Scholar] [CrossRef]
- Yu, S.; Yang, B.; Fang, C.; Zhang, Y.; Liu, S.; Zhang, Y.; Shen, L.; Xie, J.; Wang, J. Dissolution Mechanism of the Oxidation Process of Covellite by Ferric and Ferrous Ions. Hydrometallurgy 2021, 201, 105585. [Google Scholar] [CrossRef]
- Hidalgo, T.; Kuhar, L.; Beinlich, A.; Putnis, A. Kinetics and mineralogical analysis of copper dissolution from a bornite/chalcopyrite composite sample in ferric chloride and methanesulfonic-acid solutions. Hydrometallurgy 2019, 188, 140–156. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Iglesias-Gonzalez, N.; Romero, R.; Mazuelos, A.; Carranza, F. Ferric leaching of the sphalerite contained in a bulk concentrate: Kinetic study. Miner. Eng. 2018, 125, 50–59. [Google Scholar] [CrossRef]
- Neil, C.; Jun, Y. 2016. Fe3+ addition promotes arsenopyrite dissolution and iron (III) (Hydr)oxide formation and phase transformation. Environ. Sci. Technol. Lett 2016, 3, 30–35. [Google Scholar] [CrossRef]
- Lorenzo-Tallafigo, J.; Romero-García, A.; Iglesias-González, N.; Mazuelos, A.; Romero, R.; Carranza, F. A Novel Hydrometallurgical Treatment for the Recovery of Copper, Zinc, Lead and Silver from Bulk Concentrates. Hydrometallurgy 2021, 200, 105548. [Google Scholar] [CrossRef]
- Dizer, O.; Rogozhnikov, D.; Karimov, K.; Kuzas, E.; Suntsov, A. Nitric Acid Dissolution of Tennantite, Chalcopyrite and Sphalerite in the Presence of Fe (III) Ions and FeS2. Materials 2022, 15, 1545. [Google Scholar] [CrossRef] [PubMed]
- Rogozhnikov, D.; Karimov, K.; Shoppert, A.; Dizer, O.; Naboichenko, S. Kinetics and mechanism of arsenopyrite leaching in nitric acid solutions in the presence of pyrite and Fe(III) ions. Hydrometallurgy 2021, 199, 105525. [Google Scholar] [CrossRef]
- Levenspiel, O. Chemical Reaction Engineering. Ind. Eng. Chem. Res. 1999, 38, 4140–4143. [Google Scholar] [CrossRef]
- Baba, A.; Adekola, F. Hydrometallurgical Processing of a Nigerian Sphalerite in Hydrochloric Acid: Characterization and Dissolution Kinetics. Hydrometallurgy 2010, 101, 69–75. [Google Scholar] [CrossRef]
- Gok, O.; Anderson, C.; Cicekli, G.; Cöcen, I. Leaching Kinetics of Copper from Chalcopyrite Concentrate In Nitrous-Sulfuric Acid. Physicochem. Probl. Miner. Process. 2013, 50, 399. [Google Scholar]
- Barlett, R. Upgrading copper concentrate by hydrothermal converting chalcopyrite to digenite. Metall. Trans. B 1992, 23, 241–248. [Google Scholar] [CrossRef]
- Fuentes, G.; Viñals, J.; Herreros, O. Hydrothermal purification and enrichment of Chilean copper concentrates Part 1: The behavior of bornite, covellite and pyrite. Hydrometallurgy 2009, 95, 104–112. [Google Scholar] [CrossRef]
- Kritskii, A.; Celep, O.; Yazici, E.; Deveci, H.; Naboichenko, S. Hydrothermal treatment of sphalerite and pyrite particles with CuSO4 solution. Miner. Eng. 2022, 180, 107507. [Google Scholar] [CrossRef]
- Viñals, J.; Fuentes, G.; Hernández, M. Transformation of sphalerite particles into copper sulfide particles by hydrothermal treatment with Cu(II) ions. Hydrometallurgy 2004, 75, 177–187. [Google Scholar] [CrossRef]
- Dickinson, C.; Heal, G. Solid-Liquid Diffusion Controlled Rate Equations. Thermochim. Acta 1999, 340–341, 89–103. [Google Scholar] [CrossRef]
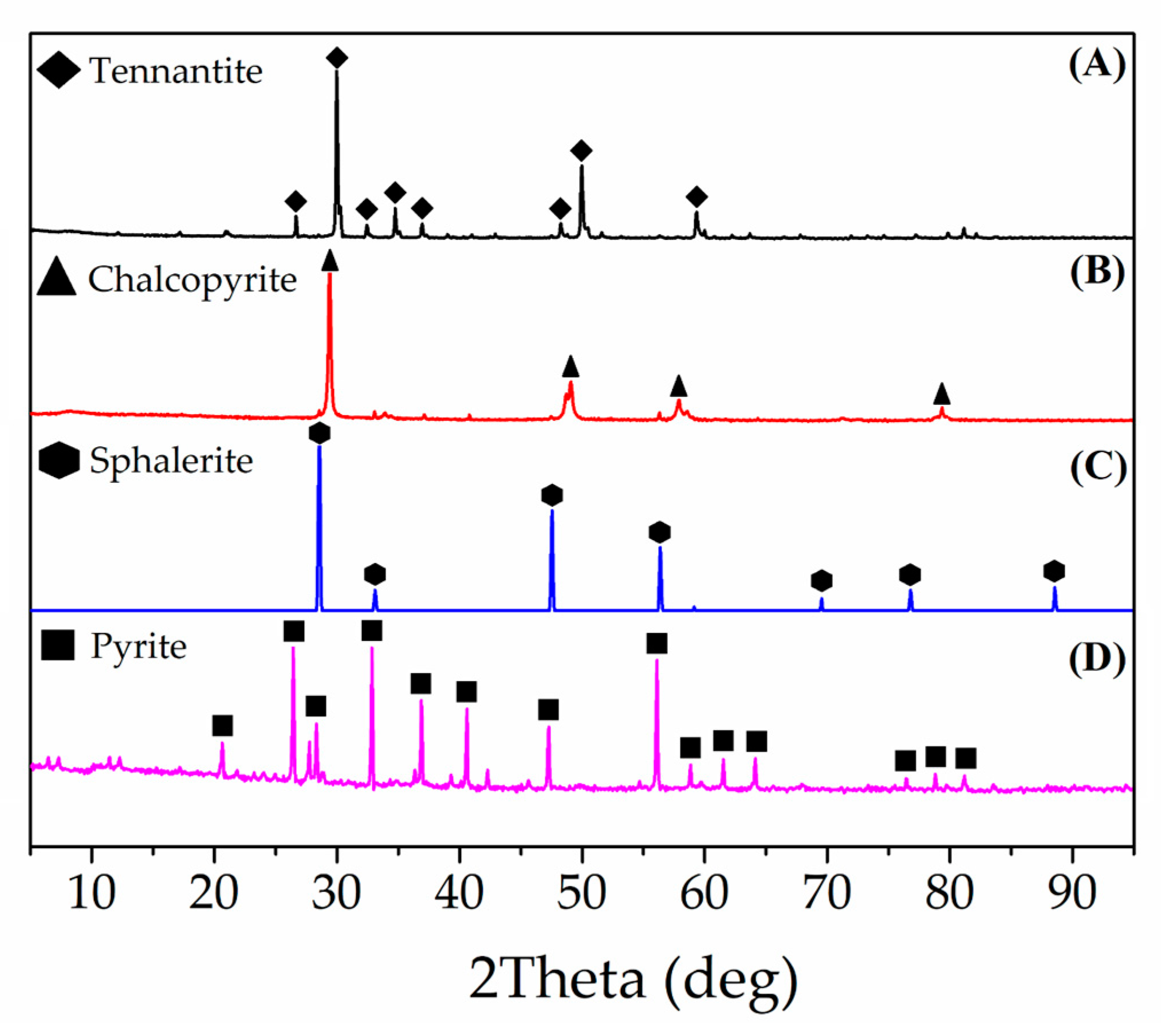


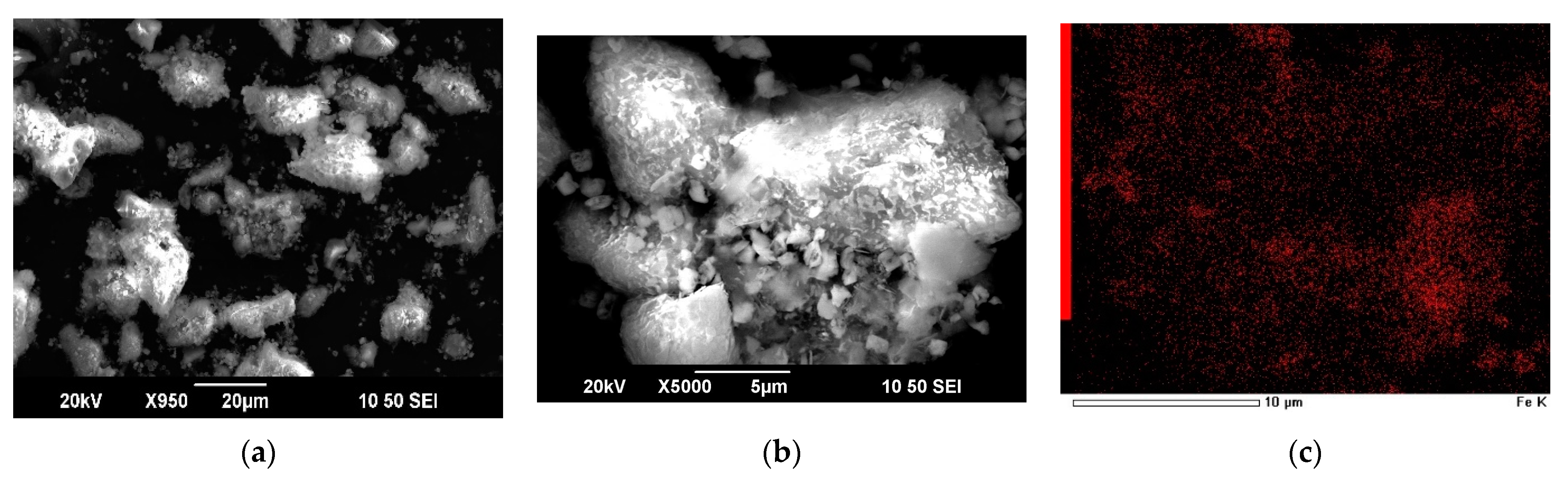


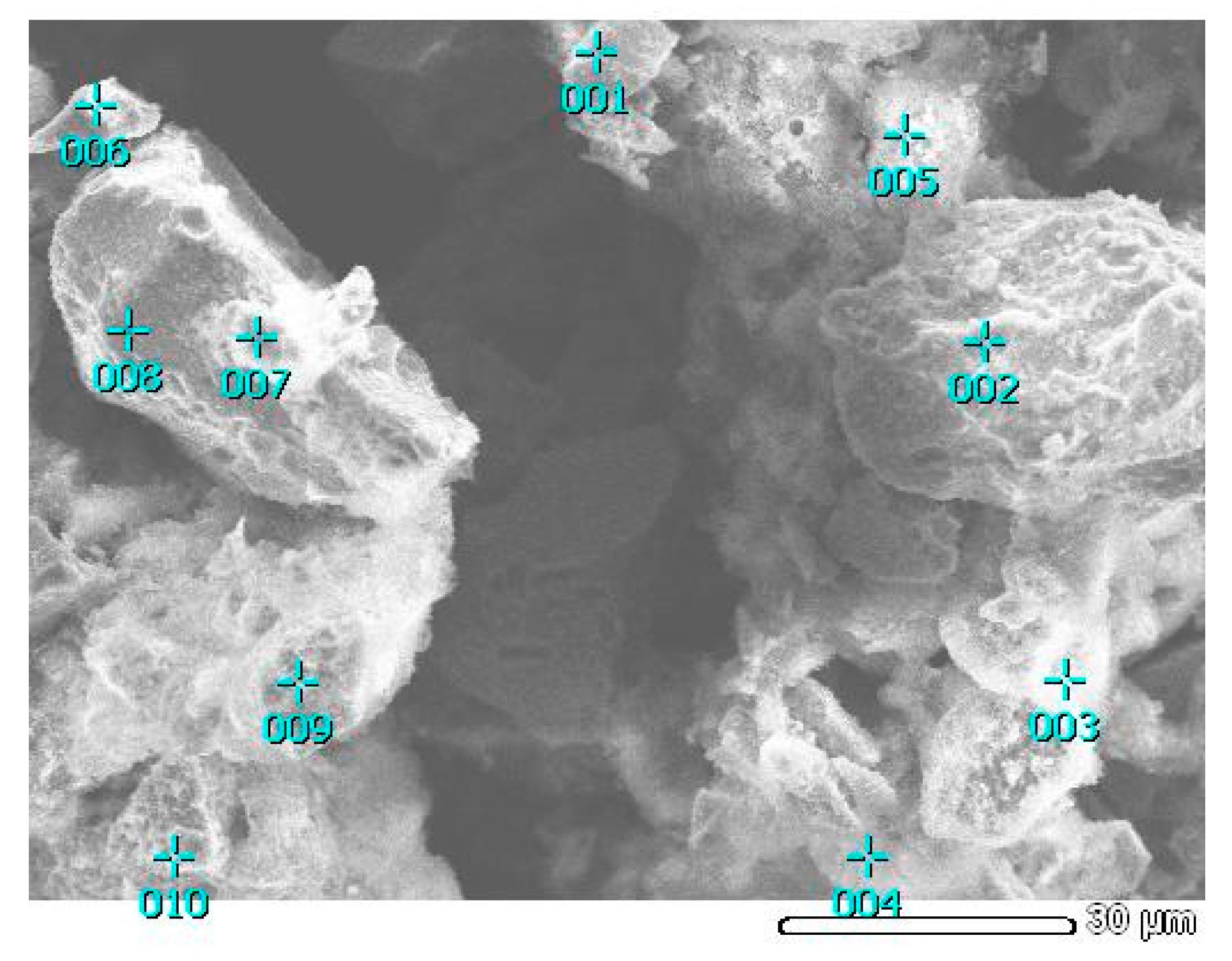


| Cu | Fe | S | As | Zn |
|---|---|---|---|---|
| 41.8 | 7.15 | 30.2 | 13.2 | 7.45 |
| Element | Fe | Cu | As | Ssulfide | S0 | Total |
|---|---|---|---|---|---|---|
| Point 001 | 40.2 | 4.2 | 1.1 | 54.5 | 6.8 | 100.0 |
| Point 002 | 43.8 | 2.3 | 0.8 | 53.1 | 2.0 | 100.0 |
| Point 003 | 11.3 | 22.1 | 4.6 | 62.0 | 42.7 | 100.0 |
| Point 004 | 23.4 | 21.7 | 1.6 | 53.3 | 24.3 | 100.0 |
| Point 005 | 7.6 | 14.3 | 3.3 | 74.8 | 61.5 | 100.0 |
| Point 006 | 29.4 | 11.9 | 2.3 | 56.2 | 19.1 | 100.0 |
| Point 007 | 44.4 | 2.1 | 0.9 | 52.6 | 0.5 | 100.0 |
| Point 008 | 45.3 | 1.5 | 0.3 | 52.9 | 0.6 | 100.0 |
| Point 009 | 9.1 | 23.1 | 8.6 | 59.2 | 36.8 | 100.0 |
| Point 010 | 9.5 | 30.4 | 10.4 | 49.7 | 24.4 | 100.0 |
| № | Limiting Stage | Formula |
|---|---|---|
| 1 | Diffusion through the product layer (sp) | 1 − 3(1 − X) 2/3 + 2(1 − X) |
| 2 | Surface chemical reaction (sp) | 1 − (1 − X)1/3 |
| Cu12As4S13 | CuFeS2 | ZnS |
|---|---|---|
| HNO3 concentration | ||
| 1.2 | 1.4 | 1.6 |
| Fe(III) ions concentration | ||
| 0.34 | 0.82 | 0.62 |
| Amount of FeS2 | ||
| 0.47 | 0.69 | 0.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dizer, O.; Karimov, K.; Kritskii, A.; Rogozhnikov, D. Synthetic Sulfide Concentrate Dissolution Kinetics in HNO3 Media. Materials 2022, 15, 8149. https://doi.org/10.3390/ma15228149
Dizer O, Karimov K, Kritskii A, Rogozhnikov D. Synthetic Sulfide Concentrate Dissolution Kinetics in HNO3 Media. Materials. 2022; 15(22):8149. https://doi.org/10.3390/ma15228149
Chicago/Turabian StyleDizer, Oleg, Kirill Karimov, Aleksei Kritskii, and Denis Rogozhnikov. 2022. "Synthetic Sulfide Concentrate Dissolution Kinetics in HNO3 Media" Materials 15, no. 22: 8149. https://doi.org/10.3390/ma15228149
APA StyleDizer, O., Karimov, K., Kritskii, A., & Rogozhnikov, D. (2022). Synthetic Sulfide Concentrate Dissolution Kinetics in HNO3 Media. Materials, 15(22), 8149. https://doi.org/10.3390/ma15228149






