Impact-Resistant Poly(3-Hydroxybutyrate)/Poly(ε-Caprolactone)-Based Materials, through Reactive Melt Processing, for Compression-Molding and 3D-Printing Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. Characterization
2.3.1. Mechanical Characterization
2.3.2. Thermal Characterization
2.3.3. Structural Characterization
3. Results
3.1. Properties of Non-Compatibilized PHB/PCL Blends
3.2. Effect of Reactive Melt Processing
3.3. Effect of Combining Reactive Melt Processing and Plasticization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, L.; Liang, S.; McDonald, A.G. Thermophysical properties and biodegradation behavior of green composites made from polyhydroxybutyrate and potato peel waste fermentation residue. Ind. Crops Prod. 2015, 69, 91–103. [Google Scholar] [CrossRef]
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khalil, S.; Abid, S. Recent progress in development and chemical modification of poly(hydroxybutyrate)-based blends for potential medical applications. Int. J. Biol. Macromol. 2020, 160, 77–100. [Google Scholar] [CrossRef] [PubMed]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V.A. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Auriemma, M.; Piscitelli, A.; Pasquino, R.; Cerruti, P.; Malinconico, M.; Grizzuti, N. Blending poly(3-hydroxybutyrate) with tannic acid: Influence of a polyphenolic natural additive on the rheological and thermal behavior. Eur. Polym. J. 2015, 63, 123–131. [Google Scholar] [CrossRef]
- Hong, S.G.; Hsu, H.W.; Ye, M.T. Thermal properties and applications of low molecular weight polyhydroxybutyrate. J. Therm. Anal. Calorim. 2013, 111, 1243–1250. [Google Scholar] [CrossRef]
- De Koning, G.J.M.; Lemstra, P.J.; Hill, D.J.T.; Carswell, T.G.; O’Donnell, J.H. Ageing phenomena in bacterial poly[(R)-3-hydroxybutyrate]: 1. A study on the mobility in poly[(R)-3-hydroxybutyrate] powders by monitoring the radical decay with temperature after γ-radiolysis at 77 K. Polymer 1992, 33, 3295–3297. [Google Scholar] [CrossRef] [Green Version]
- Gazzano, M.; Focarete, M.L.; Riekel, C.; Ripamonti, A.; Scandola, M. Structural Investigation of Poly(3-hydroxybutyrate) Spherulites by Microfocus X-Ray Diffraction. Macromol. Chem. Phys. 2001, 202, 1405–1409. [Google Scholar] [CrossRef]
- Poirier, Y.; Dennis, D.E.; Nawrath, C.; Somerville, C. Progress toward biologically produced Biodegradable Thermoplastics. Adv. Mater. 1993, 5, 30–37. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.; George, G. Thermal stability and miscibility of poly(hydroxybutyrate) and soda lignin blends. Ind. Crops Prod. 2010, 32, 656–661. [Google Scholar] [CrossRef]
- Sharma, V.; Sehgal, R.; Gupta, R. Polyhydroxyalkanoate (PHA): Properties and Modifications. Polymer 2020, 212, 123161. [Google Scholar] [CrossRef]
- Blends and Composites of Polyhydroxyalkanoates (PHAs) and Their Applications—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0014305721005589 (accessed on 22 June 2022).
- Zhang, M.; Thomas, N.L. Preparation and properties of polyhydroxybutyrate blended with different types of starch. J. Appl. Polym. Sci. 2010, 116, 688–694. [Google Scholar] [CrossRef]
- Zhang, L.; Deng, X.; Zhao, S.; Huang, Z. Biodegradable polymer blends of poly(3-hydroxybutyrate) and starch acetate. Polym. Int. 1997, 44, 104–110. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Willett, J.L.; Kotnis, M.A.; O’Brien, G.S.; Fanta, G.F.; Gordon, S.H. Properties of starch-graft-poly(glycidyl methacrylate)–PHBV composites. J. Appl. Polym. Sci. 1998, 70, 1121–1127. [Google Scholar] [CrossRef]
- Lai, S.-M.; Sun, W.-W.; Don, T.-M. Preparation and characterization of biodegradable polymer blends from poly(3-hydroxybutyrate)/poly(vinyl acetate)-modified corn starch. Polym. Eng. Sci. 2015, 55, 1321–1329. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; López-Martínez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Dong, Q.; Han, C.; Bian, Y.; Zhang, X.; Dong, L. Toward environment-friendly composites of poly(ε-caprolactone) reinforced with stereocomplex-type poly(l -lactide)/poly(d -lactide). J. Appl. Polym. Sci. 2014, 131, 31–34. [Google Scholar] [CrossRef]
- Fernandez, J.M.; Molinuevo, M.S.; Cortizo, M.S.; Cortizo, A.M. Development of an osteoconductive PCL–PDIPF–hydroxyapatite composite scaffold for bone tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, e126–e135. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Cheng, G.; Cai, Z.; Wang, L. Biocompatibility and biodegradation of poly(hydroxybutyrate)/poly(ethylene glycol) blend films. J. Mater. Sci. Mater. Med. 2003, 14, 1073–1078. [Google Scholar] [CrossRef]
- Pivsa-Art, W.; Fujii, K.; Nomura, K.; Aso, Y.; Ohara, H.; Yamane, H. The effect of poly(ethylene glycol) as plasticizer in blends of poly(lactic acid) and poly(butylene succinate). J. Appl. Polym. Sci. 2016, 133, 43044. [Google Scholar] [CrossRef]
- Zytner, P.; Wu, F.; Misra, M.; Mohanty, A.K. Toughening of Biodegradable Poly(3-hydroxybutyrate- co -3-hydroxyvalerate)/Poly(ε-caprolactone) Blends by In Situ Reactive Compatibilization. ACS Omega 2020, 5, 14900–14910. [Google Scholar] [CrossRef]
- Ivorra-Martinez, J.; Verdu, I.; Fenollar, O.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Manufacturing and Properties of Binary Blend from Bacterial Polyester Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) and Poly(caprolactone) with Improved Toughness. Polymers 2020, 12, 1118. [Google Scholar] [CrossRef] [PubMed]
- Hinuber, C.; Haussler, L.; Vogel, R.; Brunig, H.; Heinrich, G.; Werner, C. Hollow fibers made from a poly(3-hydroxybutyrate)/poly-ε-caprolactone blend. Express Polym. Lett. 2011, 5, 643–652. [Google Scholar] [CrossRef]
- Lovera, D.; Márquez, L.; Balsamo, V.; Taddei, A.; Castelli, C.; Müller, A.J. Crystallization, Morphology, and Enzymatic Degradation of Polyhydroxybutyrate/Polycaprolactone (PHB/PCL) Blends. Macromol. Chem. Phys. 2007, 208, 924–937. [Google Scholar] [CrossRef]
- Laoutid, F.; François, D.; Paint, Y.; Bonnaud, L.; Dubois, P. Using Nanosilica to Fine-Tune Morphology and Properties of Polyamide 6/Poly(propylene) Blends. Macromol. Mater. Eng. 2013, 298, 328–338. [Google Scholar] [CrossRef]
- Díaz, M.F.; Barbosa, S.E.; Capiati, N.J. Reactive compatibilization of PE/PS blends. Effect of copolymer chain length on interfacial adhesion and mechanical behavior. Polymer 2007, 48, 1058–1065. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The effect of crosslinking on the mechanical properties of polylactic acid/polycaprolactone blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Kotaki, M.; Hamada, H. Effect of compounding procedure on mechanical properties and dispersed phase morphology of poly(lactic acid)/polycaprolactone blends containing peroxide. J. Appl. Polym. Sci. 2007, 103, 1066–1074. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Zhang, Y.; Wan, C.; Ma, P. Toughening modification of PLLA/PBS blends via in situ compatibilization. Polym. Eng. Sci. 2009, 49, 26–33. [Google Scholar] [CrossRef]
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of partial crosslinking on morphology and properties of the poly(β-hydroxybutyrate)/poly(d,l-lactic acid) blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via In situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Garcia, D.; Rayón, E.; Carbonell-Verdu, A.; Lopez-Martinez, J.; Balart, R. Improvement of the compatibility between poly(3-hydroxybutyrate) and poly(ε-caprolactone) by reactive extrusion with dicumyl peroxide. Eur. Polym. J. 2017, 86, 41–57. [Google Scholar] [CrossRef] [Green Version]
- Sauer, B.B.; Kampert, W.G.; Neal Blanchard, E.; Threefoot, S.A.; Hsiao, B.S. Temperature modulated DSC studies of melting and recrystallization in polymers exhibiting multiple endotherms. Polymer 2000, 41, 1099–1108. [Google Scholar] [CrossRef]
- Hsiao, B.S.; Zuo, F.; Mao, Y.; Schick, C. Experimental Techniques. In Handbook of Polymer Crystallization; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–30. ISBN 978-1-118-54183-8. [Google Scholar]
- Menczel, J.D.; Judovits, L.H.; Prime, R.B.; Bair, H.E.; Reading, M.; Swier, S. Differential scanning calorimetry (DSC). In Thermal Analysis of Polymers: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 2, pp. 7–240. [Google Scholar]
- Gunaratne, L.M.W.K.; Shanks, R.A.; Amarasinghe, G. Thermal history effects on crystallisation and melting of poly(3-hydroxybutyrate). Thermochim. Acta 2004, 423, 127–135. [Google Scholar] [CrossRef]
- Gunaratne, L.M.W.K.; Shanks, R.A. Multiple melting behaviour of poly(3-hydroxybutyrate-co-hydroxyvalerate) using step-scan DSC. Eur. Polym. J. 2005, 41, 2980–2988. [Google Scholar] [CrossRef]
- Anbukarasu, P.; Sauvageau, D.; Elias, A. Tuning the properties of polyhydroxybutyrate films using acetic acid via solvent casting. Sci. Rep. 2015, 5, 17884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laoutid, F.; Persenaire, O.; Bonnaud, L.; Dubois, P. Flame retardant polypropylene through the joint action of sepiolite and polyamide 6. Polym. Degrad. Stab. 2013, 98, 1972–1980. [Google Scholar] [CrossRef]
- Jost, V.; Langowski, H.-C. Effect of different plasticisers on the mechanical and barrier properties of extruded cast PHBV films. Eur. Polym. J. 2015, 68, 302–312. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Z.; Hu, X.; Wang, Z.; Su, T.; Yang, L.; Yan, S. Blending Modification of PHBV/PCL and its Biodegradation by Pseudomonas mendocina. J. Polym. Environ. 2017, 25, 156–164. [Google Scholar] [CrossRef]
- Park, S.; Shou, W.; Makatura, L.; Matusik, W.; Fu, K. (Kelvin) 3D printing of polymer composites: Materials, processes, and applications. Matter 2022, 5, 43–76. [Google Scholar] [CrossRef]


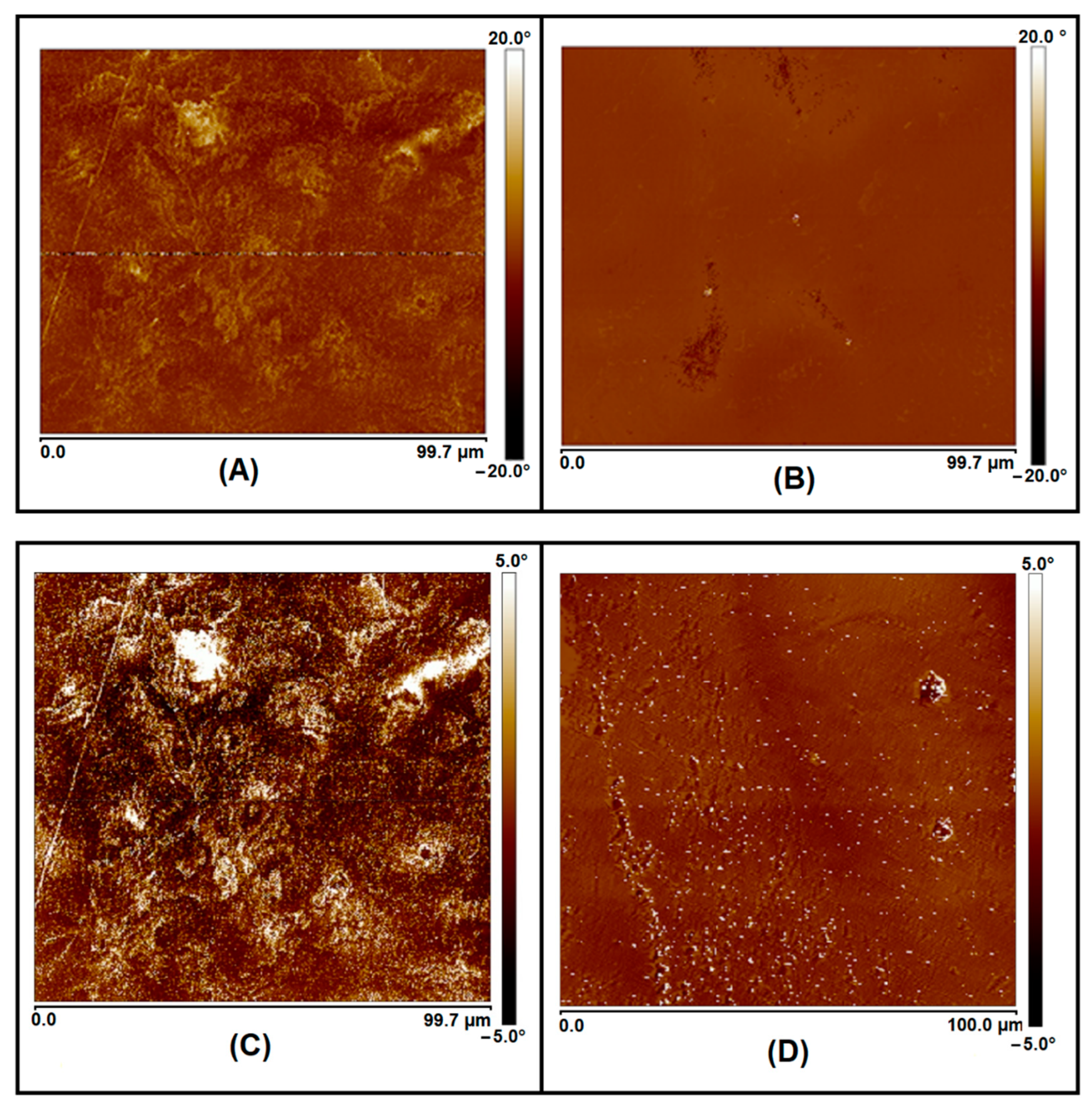
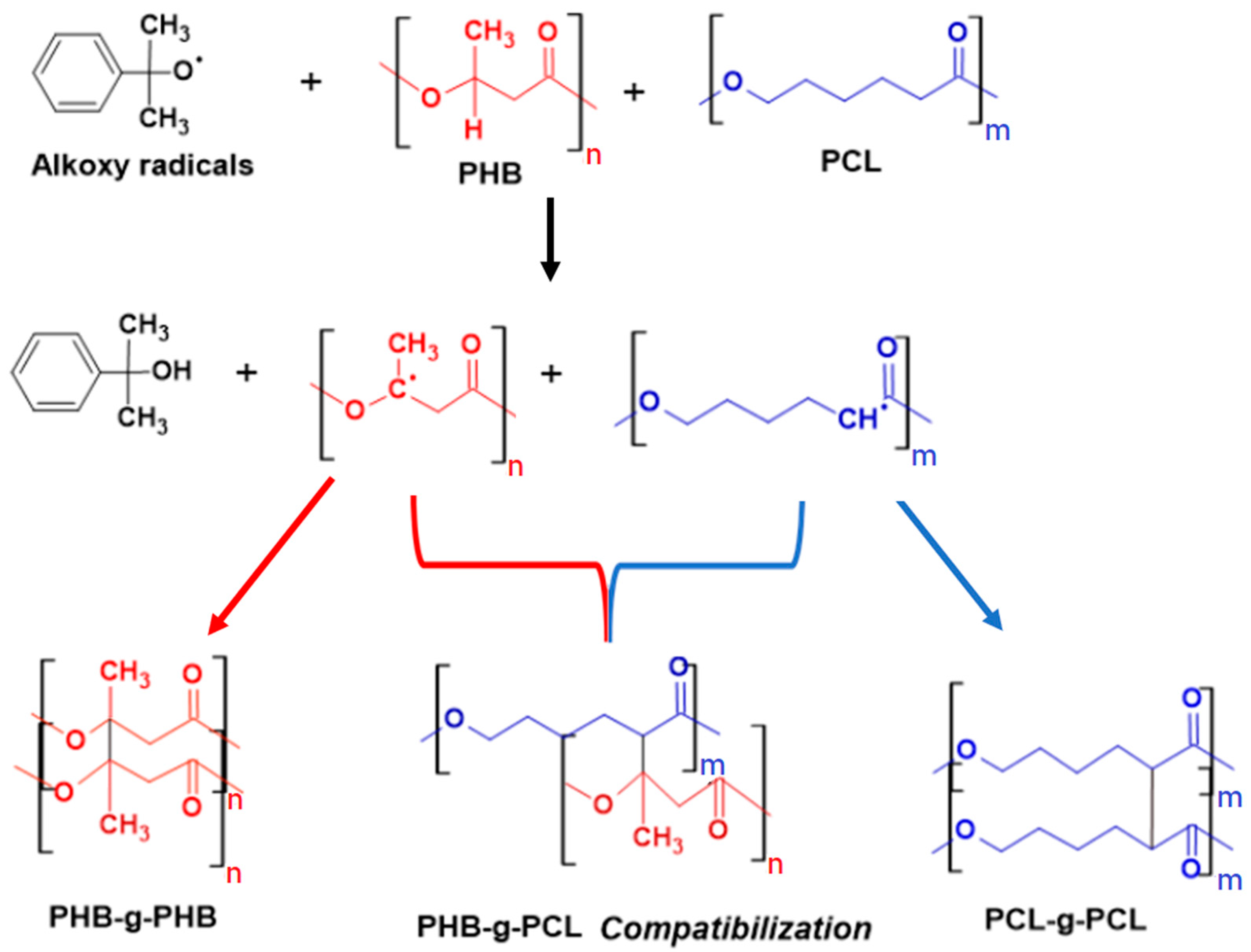
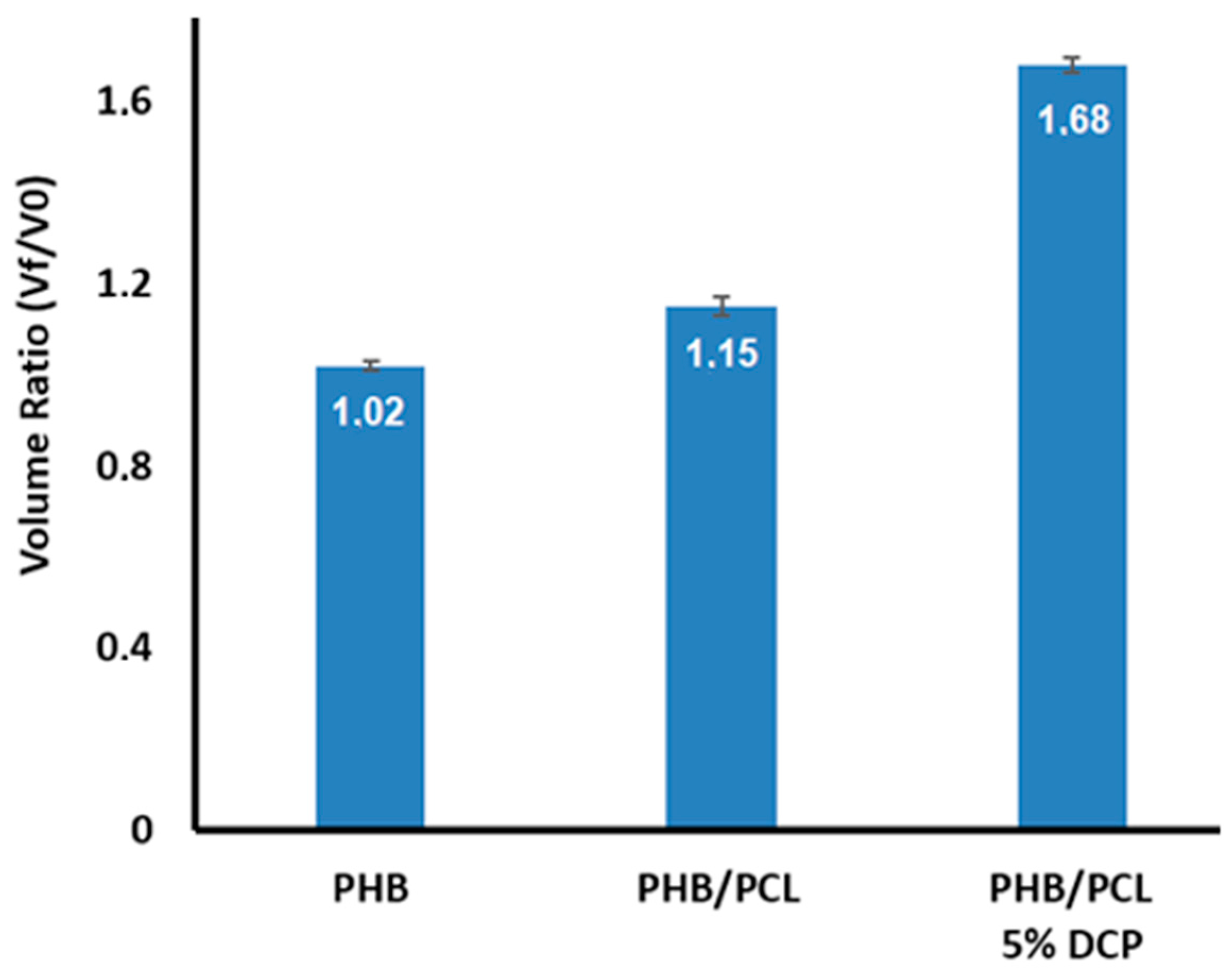
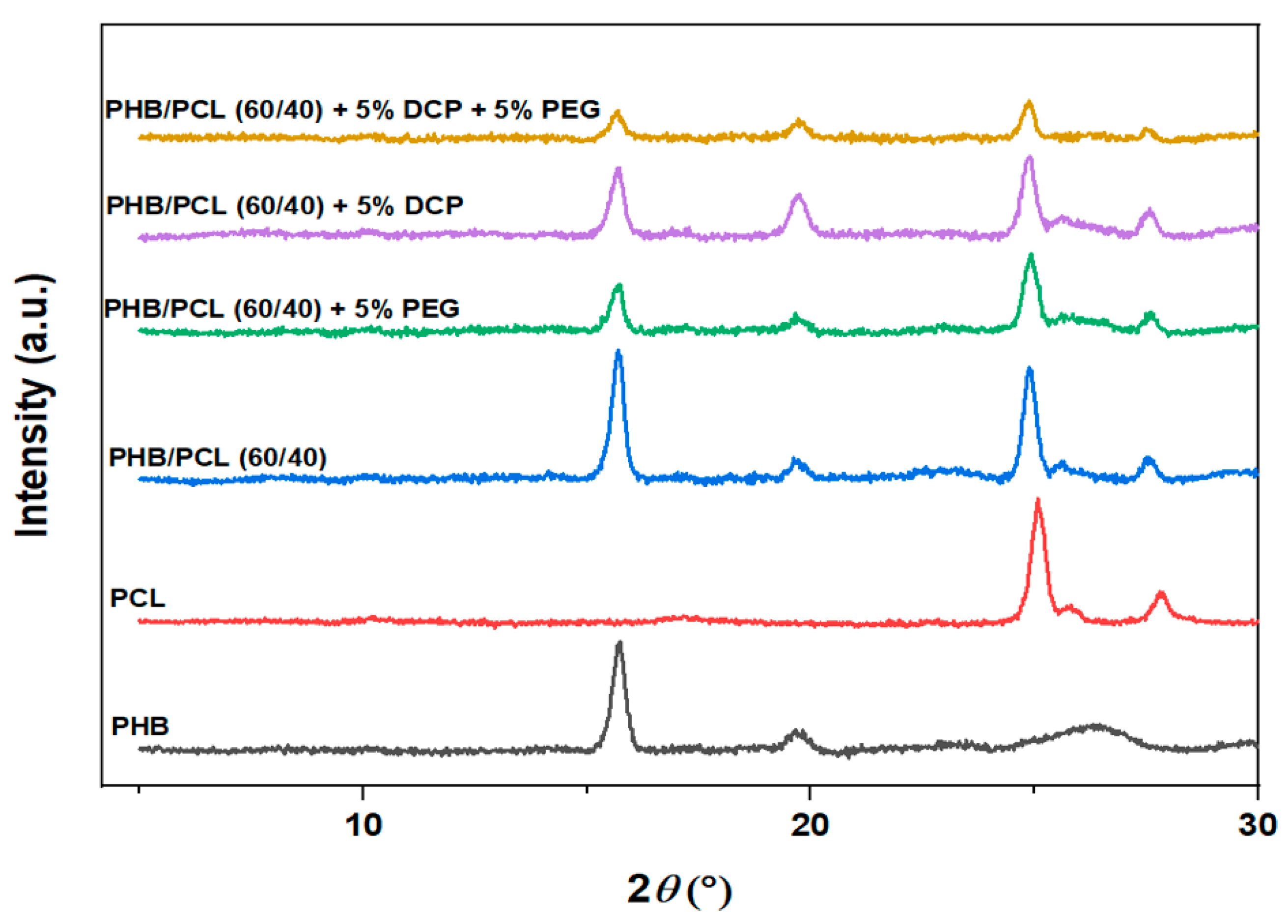


| Sample | PHB (wt.%) | PCL (wt.%) | DCP (wt.%) | PEG (wt.%) |
|---|---|---|---|---|
| PHB | 100 | 0 | 0 | 0 |
| PCL | 0 | 100 | 0 | 0 |
| PHB/PCL (80/20) | 80 | 20 | 0 | 0 |
| PHB/PCL (60/40) | 60 | 40 | 0 | 0 |
| PHB/PCL (60/40)-5DCP | 57 | 38 | 5 | 0 |
| PHB/PCL (60/40)-5PEG | 57 | 38 | 0 | 5 |
| PHB/PCL (60/40)-5DCP-5PEG | 54 | 36 | 5 | 5 |
| PHB/PCL (60/40)-1DCP-5PEG | 56.4 | 37.6 | 1 | 5 |
| Sample | Young’s Modulus (MPa) | Stress at Break (MPa) | Strain at Break (%) | Impact Resistance (kJ.m−2) |
|---|---|---|---|---|
| PHB | 1840 ± 115 | 42 ± 2.4 | 4.7 ± 0.3 | 1.3 ± 0.1 |
| PCL | 440 ± 34 | 33.0 ± 2 | 570 ± 30 | 4.6 ± 0.7 |
| PHB/PCL (80/20) | 1430 ± 110 | 34.5 ± 2.9 | 3.3 ± 0.2 | 1.3 ± 0.3 |
| PHB/PCL (60/40) | 1225 ± 40 | 19.3 ± 0.4 | 2.7 ± 0.5 | 1.7 ± 0.2 |
| PHB/PCL (60/40)-5DCP | 680 ± 70 | 19.1 ± 20 | 5.1 ± 0.4 | 7.2 ± 1.8 |
| PHB/PCL (60/40)-5PEG | 1060 ± 90 | 17.0 ± 1.6 | 1.8 ± 0.2 | 1.5 ± 0.4 |
| PHB/PCL (60/40)-5DCP-5PEG | 640 ± 5 | 18.6 ± 2 | 5.5 ± 1 | 15.4 ± 1.5 |
| PHB/PCL (60/40)-1DCP-5PEG | 890 ± 20 | 21.5 ± 0.1 | 4 ± 0.1 | 6 ± 0.5 |
| Sample | Tm (°C) (2nd Heating) | Tc (°C) | Degree of Crystallinity (%) | |||
|---|---|---|---|---|---|---|
| PHB | PCL | PHB | PCL | PHB | PCL | |
| PHB | 177.0 | n.a. | 124.0 | n.a. | 58.3 | n.a. |
| PCL | n.a. | 57.5 | n.a. | 20.2 | n.a. | 38.2 |
| PHB/PCL (60/40) | 171.0 | 57.3 | 112.0 | 32.4 | 57.0 | 33.0 |
| PHB/PCL (60-40)-5PEG | 172.0 | 63.0 | 115.0 | 34.0 | 64.0 | 33.0 |
| PHB/PCL (60/40)-5DCP | 153.5; 162.0 | 48 | 98.0 | 20.4 | 52.8 | 27.0 |
| PHB/PCL (60/40)-5DCP-5PEG | 153.6; 163.0 | 51.0 | 101.3 | 22.8 | 54.0 | 35.5 |
| Sample | Td-5% (°C) | Td PHB (°C) | Td PCL (°C) |
|---|---|---|---|
| PHB | 287 | 303 | --- |
| PCL | 395 | --- | 425 |
| PHB/PCL (60/40) | 287 | 300 | 420 |
| PHB/PCL (60/40)-5DCP | 290 | 313 | 422 |
| PHB/PCL (60/40)-5DCP-5PEG | 288 | 317 | 416 |
| PHB/PCL (60/40)-5PEG | 268 | 291 | 417 |
| Sample | Impact Resistance (kJ.m−2) | Fracture |
|---|---|---|
| 3D-printed samples | ||
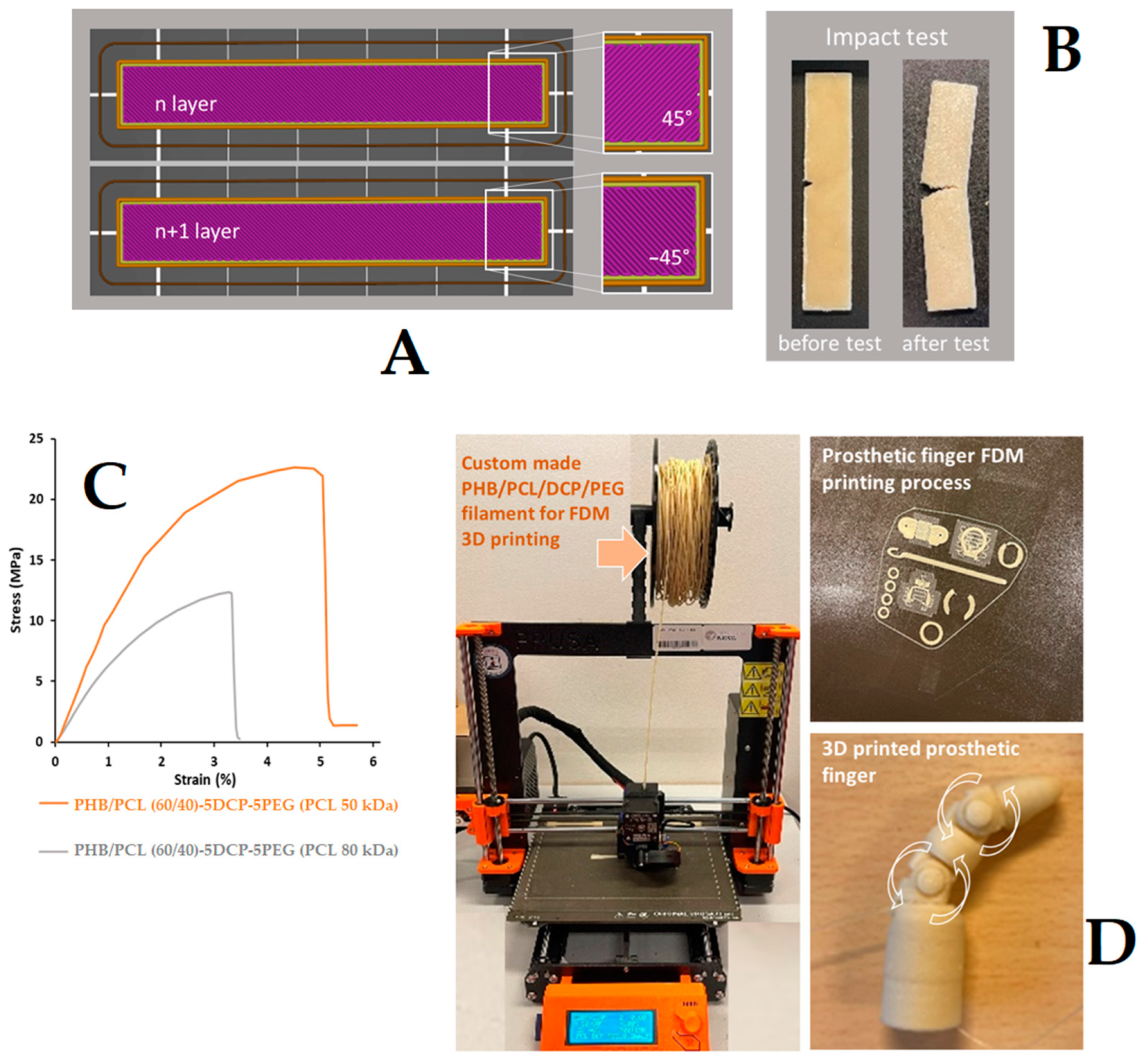
| PHB/PCL (60/40)-5DCP-5PEG (PCL 50 kDa) | 3.18 ± 0.3 | Total |
| PHB/PCL (60/40)-5DCP-5PEG (PCL 80 kDa) | 7.23 ± 1.5 | Partial |
| Compression-molded samples | ||
| PHB/PCL (60/40)-5DCP-5PEG (PCL 50 kDa) | 15.4 ± 1.5 | Partial |
| PHB/PCL (60/40)-5DCP-5PEG (PCL 80 kDa) | 14.2 ± 1.2 | Partial |
| Sample | Young’s Modulus (MPa) | Stress at Break (MPa) | Strain at Break (%) | χc PHB/χc PCL (%) |
|---|---|---|---|---|
| PHB/PCL (60/40)-5DCP-5PEG (PCL 50 kDa) | 1046.6 ± 65.6 | 21.3 ± 0.7 | 5.1 ± 0.1 | 32/14 |
| PHB/PCL (60/40)-5DCP-5PEG (PCL 80 kDa) | 642.5 ± 85.4 | 12.1 ± 0.9 | 3.5 ± 0.4 | 33/18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laoutid, F.; Lenoir, H.; Molins Santaeularia, A.; Toncheva, A.; Schouw, T.; Dubois, P. Impact-Resistant Poly(3-Hydroxybutyrate)/Poly(ε-Caprolactone)-Based Materials, through Reactive Melt Processing, for Compression-Molding and 3D-Printing Applications. Materials 2022, 15, 8233. https://doi.org/10.3390/ma15228233
Laoutid F, Lenoir H, Molins Santaeularia A, Toncheva A, Schouw T, Dubois P. Impact-Resistant Poly(3-Hydroxybutyrate)/Poly(ε-Caprolactone)-Based Materials, through Reactive Melt Processing, for Compression-Molding and 3D-Printing Applications. Materials. 2022; 15(22):8233. https://doi.org/10.3390/ma15228233
Chicago/Turabian StyleLaoutid, Fouad, Hadrien Lenoir, Adriana Molins Santaeularia, Antoniya Toncheva, Tim Schouw, and Philippe Dubois. 2022. "Impact-Resistant Poly(3-Hydroxybutyrate)/Poly(ε-Caprolactone)-Based Materials, through Reactive Melt Processing, for Compression-Molding and 3D-Printing Applications" Materials 15, no. 22: 8233. https://doi.org/10.3390/ma15228233





