Development of Root Caries Prevention by Nano-Hydroxyapatite Coating and Improvement of Dentin Acid Resistance
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Bovine Tooth Dentin Block Samples
2.2. Acid Challenge Experiment
2.3. 3D Measurement Laser Microscopic Observation
2.4. Micro Vickers Hardness Test
2.5. Scanning Electron Microscope Observation
2.6. Contact Microradiography
2.7. Statistical Analysis
3. Results
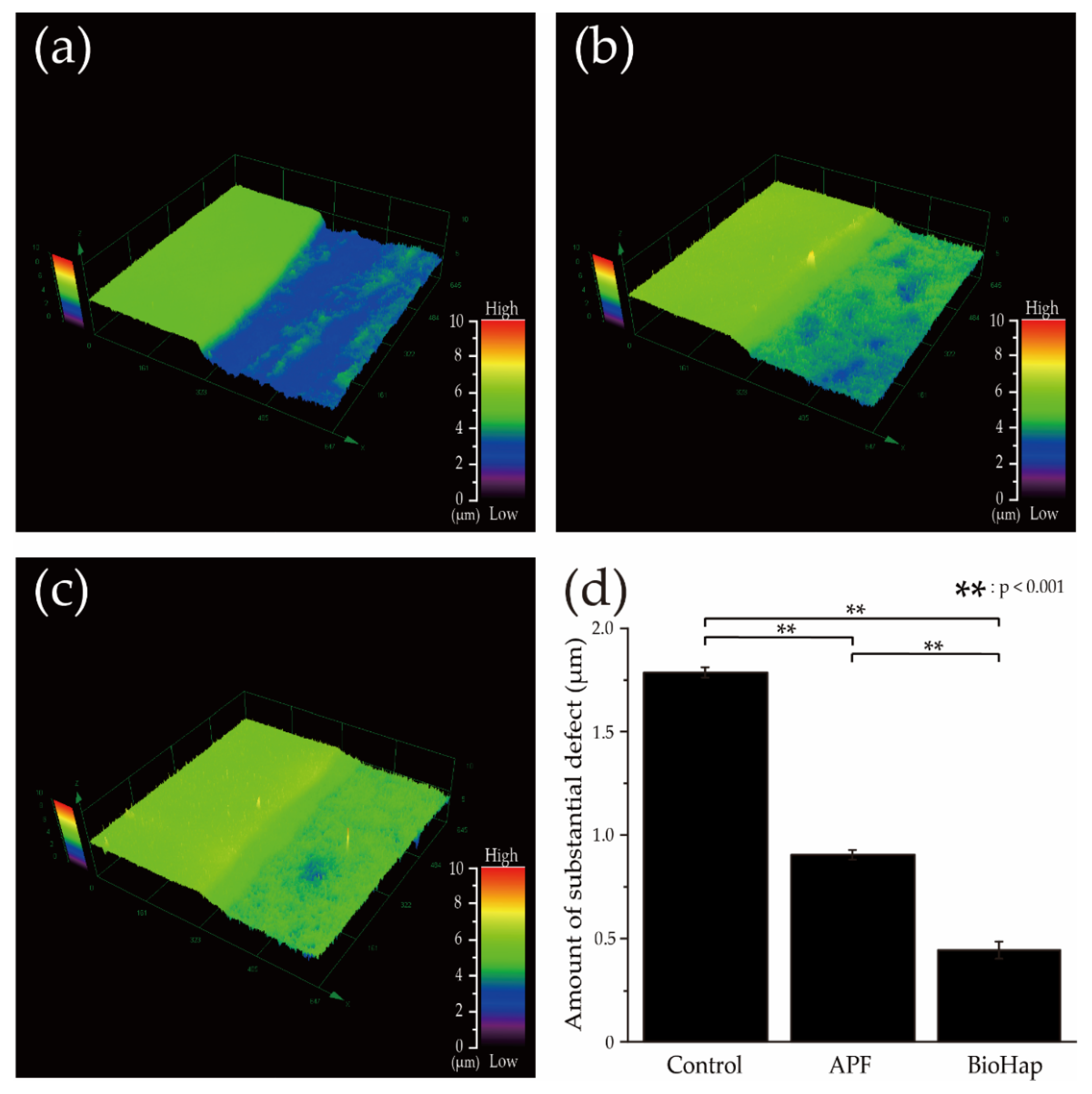
3.1. Amount of Substantial Defect after an Acid Challenge by the 3D Measurement Laser Microscope
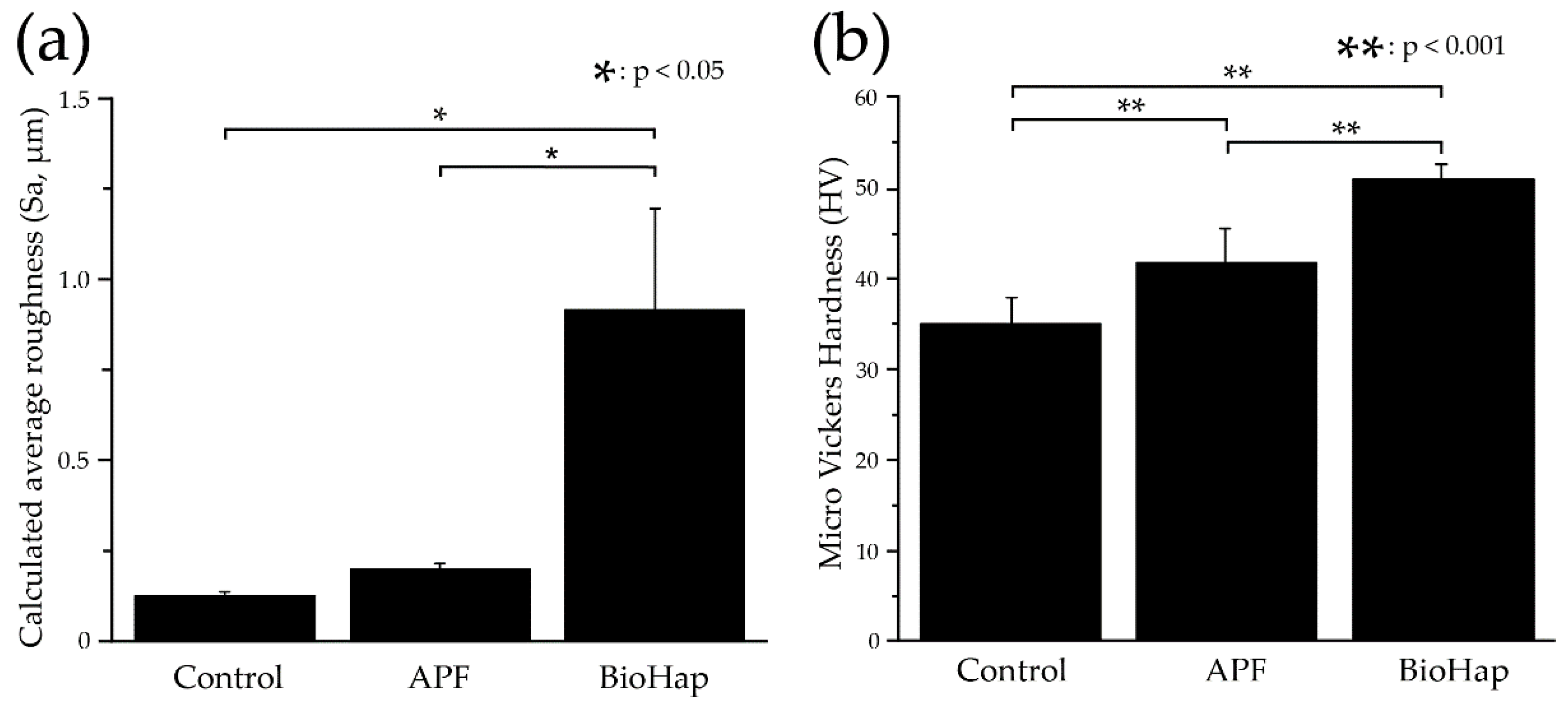
3.2. Calculated Average Roughness after an Acid Challenge by the 3D Measurement Laser Microscope and Micro Vickers Hardness Measurements by Micro Vickers Hardness Tester
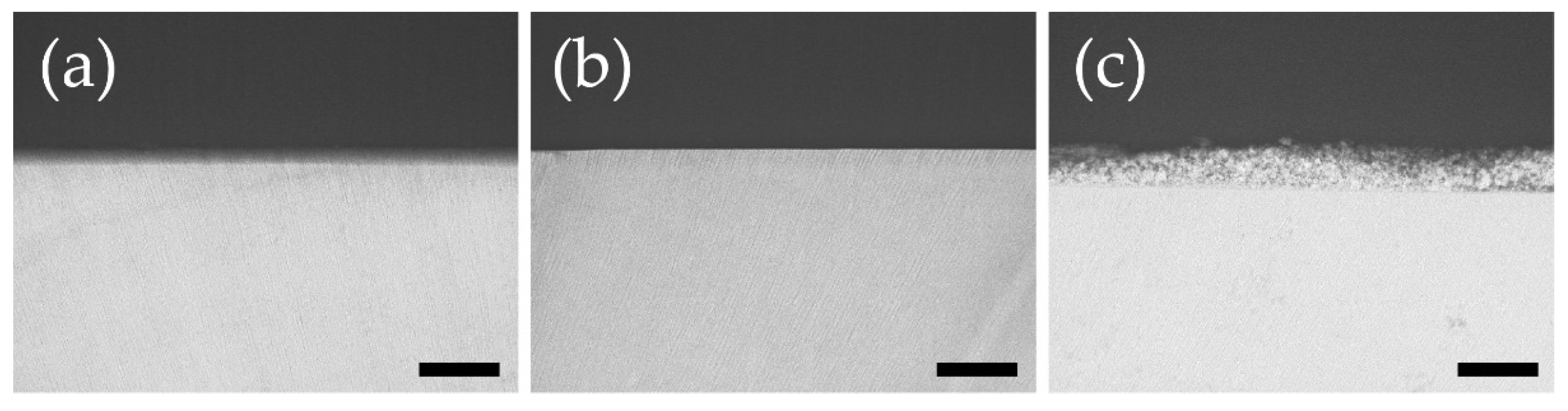
3.3. Dentin Surface and Cross-Section Scanning Electron Microscope Observations after Acid Challenge
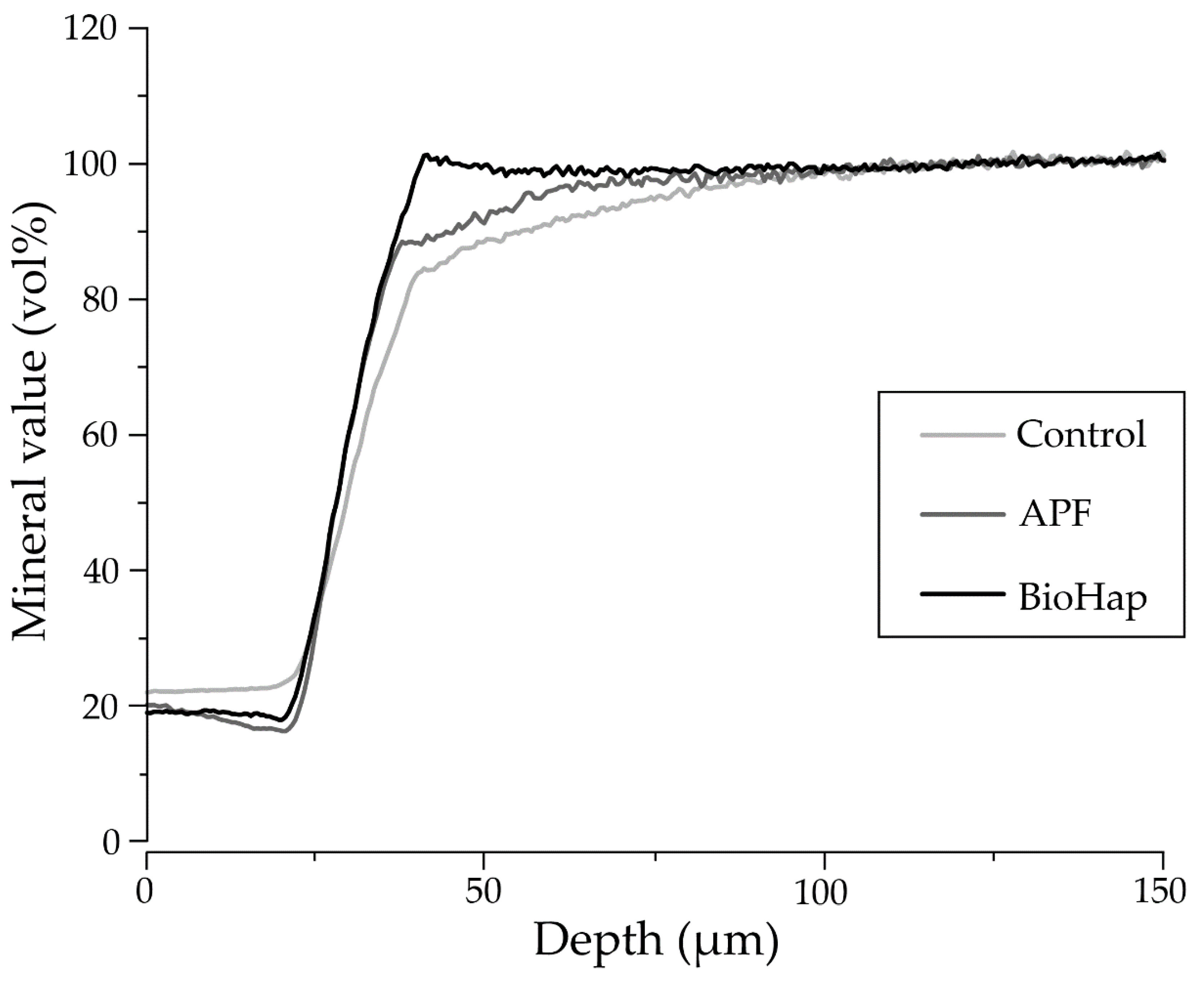
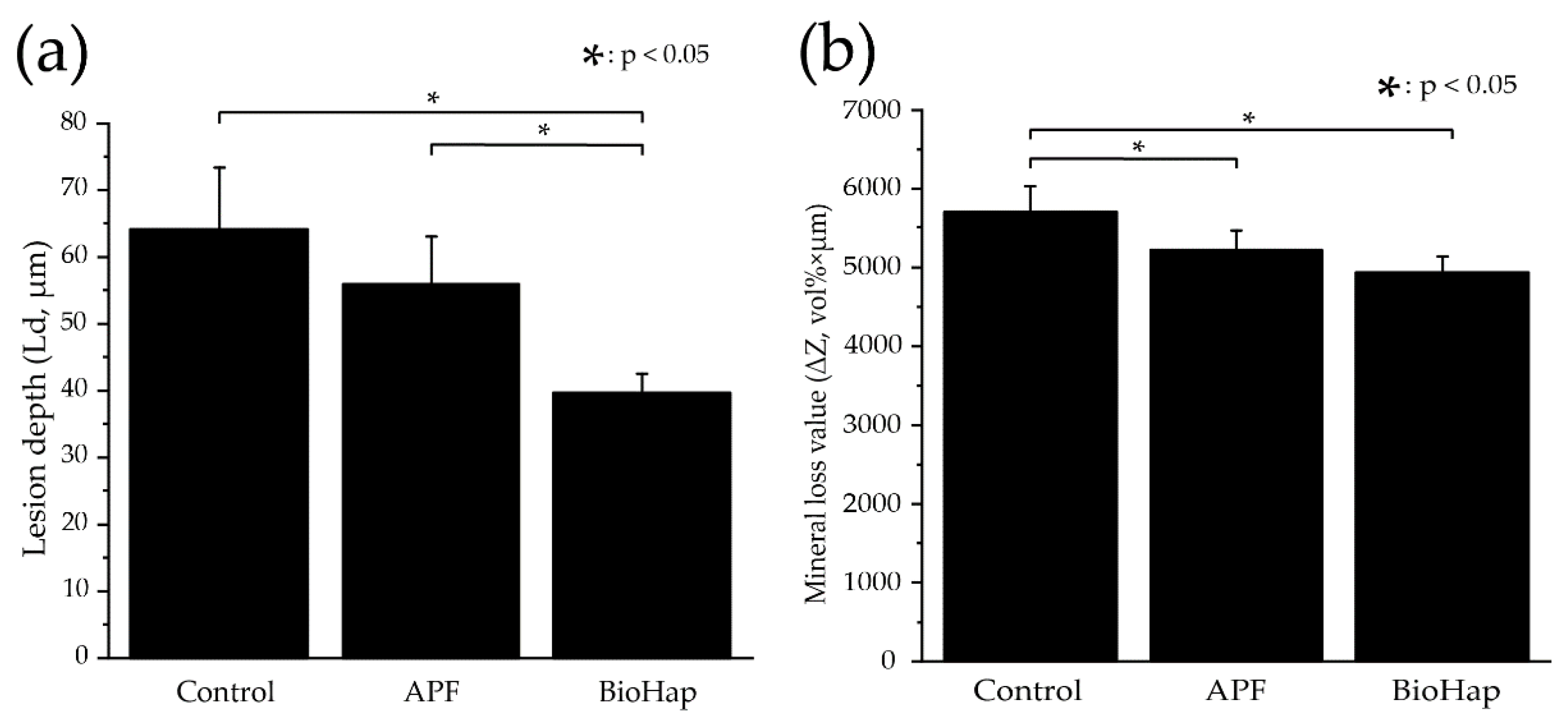
3.4. Measurement of Lesion Depth and Mineral Loss Value by Contact Microradiography Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Sardana, D.; Li, K.Y.; Leung, K.C.M.; Lo, E.C.M. Topical fluoride to prevent root caries: Systematic review with network meta-analysis. J. Dent. Res. 2020, 99, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Walsh, L.J. Minimal intervention management of the older patient. Br. Dent. J. 2017, 223, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M.; Brown, W. Dentin erosion: Method validation and efficacy of fluoride protection. Dent. J. 2017, 5, 27. [Google Scholar] [CrossRef]
- Zhang, J.; Leung, K.C.M.; Sardana, D.; Wong, M.C.M.; Lo, E.C.M. Risk predictors of dental root caries: A systematic review. J. Dent. 2019, 89, 103166. [Google Scholar] [CrossRef]
- AlQranei, M.S.; Balhaddad, A.A.; Melo, M.A.S. The burden of root caries: Updated perspectives and advances on management strategies. Gerodontology 2021, 38, 136–153. [Google Scholar] [CrossRef]
- Girestam Croonquist, C.; Dalum, J.; Skott, P.; Sjögren, P.; Wårdh, I.; Morén, E. Effects of domiciliary professional oral care for care-dependent elderly in nursing homes—Oral hygiene, gingival bleeding, root caries and nursing Staff’s Oral Health knowledge and attitudes. Clin. Interv. Aging 2020, 15, 1305–1315. [Google Scholar] [CrossRef]
- Gavriilidou, N.N.; Belibasakis, G.N. Root caries: The intersection between periodontal disease and dental caries in the course of ageing. Br. Dent. J. 2019, 227, 1063–1067. [Google Scholar] [CrossRef]
- Aoyagi, Y. In vitro histological studies on the caries-like lesion of the tooth root surface. J. Dent. Health 1991, 41, 693–715. [Google Scholar] [CrossRef][Green Version]
- Castelo, R.; Attik, N.; Catirse, A.B.C.E.B.; Pradelle-Plasse, N.; Tirapelli, C.; Grosgogeat, B. Is there a preferable management for root caries in middle-aged and older adults? A systematic review. Br. Dent. J. 2021, 1–7. [Google Scholar] [CrossRef]
- Velázquez-Olmedo, L.B.; Borges-Yáñez, S.A.; Andrade Palos, P.A.; García-Peña, C.; Gutiérrez-Robledo, L.M.; Sánchez-García, S. Oral Health condition and development of frailty over a 12-month period in community-dwelling older adults. BMC Oral Health 2021, 21, 355. [Google Scholar] [CrossRef]
- Khamverdi, Z.; Kordestani, M.; Soltanian, A.R. Effect of proanthocyanidin, fluoride and casein phosphopeptide amorphous calcium phosphate remineralizing agents on microhardness of demineralized dentin. J. Dent. 2017, 14, 76–83. [Google Scholar]
- Wadia, R. Topical fluoride to prevent root caries. Br. Dent. J. 2020, 228, 761. [Google Scholar] [CrossRef] [PubMed]
- Petersson, L.G. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin. Oral Investig. 2013, 17 (Suppl. S1), S63–S71. [Google Scholar] [CrossRef] [PubMed]
- Souza, B.M.; Comar, L.P.; Vertuan, M.; Fernandes Neto, C.F.; Buzalaf, M.A.R.; Magalhães, A.C. Effect of an experimental paste with hydroxyapatite nanoparticles and fluoride on dental demineralisation and remineralisation in situ. Caries Res. 2015, 49, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Burrow, M.F.; Manton, D.J.; Hardiman, R.; Palamara, J.E.A. Remineralising effects of fluoride varnishes containing calcium phosphate on artificial root caries lesions with adjunctive application of proanthocyanidin. Dent. Mater. 2021, 37, 143–157. [Google Scholar] [CrossRef]
- Leal, A.M.C.; Beserra Dos Santos, M.V.; da Silva Filho, E.C.; Menezes de Carvalho, A.L.; Tabchoury, C.P.M.; Vale, G.C. Development of an experimental dentifrice with hydroxyapatite nanoparticles and high fluoride concentration to manage root dentin demineralization. Int. J. Nanomed. 2020, 15, 7469–7479. [Google Scholar] [CrossRef]
- Yoshino, F.; Sasaki, R.; Asada, Y.; Shiozaki, K.; Shimoda, S.; Yamamoto, T. Studies on change in solubility over time of the bioactive material amorphous calcium phosphate and precipitation of hydroxyapatite. J. Hard Tissue Biol. 2022, 31, 1–8. [Google Scholar] [CrossRef]
- Eanes, E.D.; Meyer, J.L. The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif. Tissue Res. 1977, 23, 259–269. [Google Scholar] [CrossRef]
- Elkassas, D.; Arafa, A. Remineralizing efficacy of different calcium-phosphate and fluoride based delivery vehicles on artificial caries like enamel lesions. J. Dent. 2014, 42, 466–474. [Google Scholar] [CrossRef]
- Alencar, C.R.B.; Oliveira, G.C.; Magalhães, A.C.; Buzalaf, M.A.R.; Machado, M.A.A.M.; Honório, H.M.; Rios, D. In situ effect of CPP-ACP chewing gum upon erosive enamel loss. J. Appl. Oral Sci. 2017, 25, 258–264. [Google Scholar] [CrossRef]
- Huysmans, M.C.; Young, A.; Ganss, C. The role of fluoride in erosion therapy. Monogr. Oral Sci. 2014, 25, 230–243. [Google Scholar] [CrossRef]
- Balasooriya, I.L.; Chen, J.; Korale Gedara, S.M.; Han, Y.; Wickramaratne, M.N. Applications of Nano Hydroxyapatite as Adsorbents: A Review. Nanomaterials 2022, 12, 2324. [Google Scholar] [CrossRef] [PubMed]
- Juntavee, A.; Juntavee, N.; Sinagpulo, A.N. Nano-Hydroxyapatite Gel and Its Effects on Remineralization of Artificial Carious Lesions. Int. J. Dent. 2021, 2021, 7256056. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Gao, S.; Cheng, L.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Orsini, G.; Procaccini, M.; Manzoli, L.; Sparabombe, S.; Tiriduzzi, P.; Bambini, F.; Putignano, A. A 3-Day Randomized Clinical Trial to Investigate the Desensitizing Properties of Three Dentifrices. J. Periodontol. 2013, 84, e65–e73. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Gómez, R.P.; Rivera-Muñoz, E.M.; Luna-Barcenas, G.; Alanis-Gómez, J.R.; Velázquez-Castillo, R. Improving the Mechanical Resistance of Hydroxyapatite/Chitosan Composite Materials Made of Nanofibers with Crystalline Preferential Orientation. Materials 2022, 15, 4718. [Google Scholar] [CrossRef] [PubMed]
- Amaechi, B.T.; Alshareif, D.O.; Azees, P.A.A.; Shehata, M.A.; Lima, P.P.; Abdollahi, A.; Kalkhorani, P.S.; Evans, V.; Bagheri, A.; Okoye, L.O. Anti-caries evaluation of a nano-hydroxyapatite dental lotion for use after toothbrushing: An in situ study. J. Dent. 2021, 115, 103863. [Google Scholar] [CrossRef]
- Bystrova, A.; Dekhtyar, Y.D.; Popov, A.; Coutinho, J.; Bystrov, V. Modified hydroxyapatite structure and properties: Modeling and synchrotron data analysis of modified hydroxyapatite structure. Ferroelectrics 2015, 475, 135–147. [Google Scholar] [CrossRef]
- Hübner, W.; Blume, A.; Pushnjakova, R.; Dekhtyar, Y.; Hein, H.-J. The influence of X-ray radiation on the mineral/organic matrix interaction of bone tissue: An FT-IR microscopic investigation. Int. J. Artif. Organs 2005, 28, 66–73. [Google Scholar] [CrossRef]
- Dubey, A.; Jaiswal, S.; Garg, A.; Jain, V.; Lahiri, D. Synthesis and evaluation of magnesium/co-precipitated hydroxyapatite based composite for biomedical application. J. Mech. Behav. Biomed. Mater. 2021, 118, 104460. [Google Scholar] [CrossRef]
- Kodaka, T.; Debari, K.; Abe, M. Hexahedrally based crystals in human tooth enamel. Caries Res. 1992, 26, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.E. Formation and inhibition of dental calculus. J. Periodontol. 1969, 40, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Miake, Y.; Nozue, S.; Moriguchi, M.; Yamazaki, T.; Sawada, T.; Yanagisawa, T. The ability of xylitol containing gum with calcified seaweed in preventing demineralization of tooth surfaces. J. Hard Tissue Biol. 2011, 20, 87–92. [Google Scholar] [CrossRef]
- Angmar, B.; Carlstrom, D.; Glas, J.E. Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J. Ultrastruct. Res. 1963, 8, 12–23. [Google Scholar] [CrossRef]
- Ogaard, B. CaF2 formation: Cariostatic properties and factors of enhancing the effect. Caries Res. 2001, 35 (Suppl. S1), 40–44. [Google Scholar] [CrossRef]
- Petzold, M. The influence of different fluoride compounds and treatment conditions on dental enamel: A descriptive in vitro study of the CaF2 precipitation and microstructure. Caries Res. 2001, 35 (Suppl. S1), 45–51. [Google Scholar] [CrossRef]
- Ga, Y.; Okamoto, Y.; Baba, A.; Motokawa, W.; Miyazaki, K. Study of properties of APF for the acid resistance ability of enamel. Jpn. J. Pediatr. Dent. 2007, 45, 65–73. [Google Scholar]
- Fulmer, M.T.; Ison, I.C.; Hankermayer, C.R.; Constantz, B.R.; Ross, J. Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials 2002, 23, 751–755. [Google Scholar] [CrossRef]
- Saxegaard, E.; Lagerlöf, F.; Rølla, G. Dissolution of calcium fluoride in human saliva. Acta Odontol. Scand. 1988, 46, 355–359. [Google Scholar] [CrossRef]
- Miki, N.; Miake, Y.; Shimoda, S.; Mishima, H. Evaluation of Enamel Acid Resistance and Whitening Effect of the CAP System. Dent. J. 2022, 10, 161. [Google Scholar] [CrossRef]
- Chinelatti, M.A.; Tirapelli, C.; Corona, S.A.M.; Jasinevicius, R.G.; Peitl, O.; Zanotto, E.D.; Pires-de-Souza, F.C.P. Effect of a bioactive glass ceramic on the control of enamel and dentin erosion lesions. Braz. Dent. J. 2017, 28, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Chokshi, K.; Chokshi, A.; Konde, S.; Shetty, S.R.; Chandra, K.N.; Jana, S.; Mhambrey, S.; Thakur, S. An in vitro comparative evaluation of three remineralizing agents using confocal microscopy. J. Clin. Diagn. Res. 2016, 10, ZC39–ZC42. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Toyooka, H.; Ito, S.; Crenshaw, M.A. In vitro study of remineralization of dentin: Effects of ions on mineral induction by decalcified dentin matrix. Caries Res. 2003, 37, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Tung, M.S.; Tomazic, B.; Brown, W.E. The effects of magnesium and fluoride on the hydrolysis of octacalcium phosphate. Arch. Oral Biol. 1992, 37, 585–591. [Google Scholar] [CrossRef]
- Cury, M.S.; Silva, C.B.; Nogueira, R.D.; Campos, M.G.D.; Palma-Dibb, R.G.; Geraldo-Martins, V.R. Surface roughness and bacterial adhesion on root dentin treated with diode laser and conventional desensitizing agents. Lasers Med. Sci. 2018, 33, 257–262. [Google Scholar] [CrossRef]
- Bollen, C.M.L.; Lambrechts, P.; Quirynen, M. Comparison of surface roughness of oral hard materials to the threshold surface roughness for bacterial plaque retention: A review of the literature. Dent. Mater. 1997, 13, 258–269. [Google Scholar] [CrossRef]
- Özdemir, O.; Kopac, T. Recent Progress on the Applications of Nanomaterials and Nano-Characterization Techniques in Endodontics: A Review. Materials 2022, 15, 5190. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, M.; Satou, R.; Sugihara, N. Development of Root Caries Prevention by Nano-Hydroxyapatite Coating and Improvement of Dentin Acid Resistance. Materials 2022, 15, 8263. https://doi.org/10.3390/ma15228263
Iwasaki M, Satou R, Sugihara N. Development of Root Caries Prevention by Nano-Hydroxyapatite Coating and Improvement of Dentin Acid Resistance. Materials. 2022; 15(22):8263. https://doi.org/10.3390/ma15228263
Chicago/Turabian StyleIwasaki, Miyu, Ryouichi Satou, and Naoki Sugihara. 2022. "Development of Root Caries Prevention by Nano-Hydroxyapatite Coating and Improvement of Dentin Acid Resistance" Materials 15, no. 22: 8263. https://doi.org/10.3390/ma15228263
APA StyleIwasaki, M., Satou, R., & Sugihara, N. (2022). Development of Root Caries Prevention by Nano-Hydroxyapatite Coating and Improvement of Dentin Acid Resistance. Materials, 15(22), 8263. https://doi.org/10.3390/ma15228263







