Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Cell Culture
2.3. Cell Proliferation Assays and Statistical Analysis
2.4. Morphological Analysis
2.4.1. Morphological Analysis—LM
2.4.2. Morphological Analysis—SEM
3. Results
3.1. Cell Proliferation Assays and Statistical Analysis
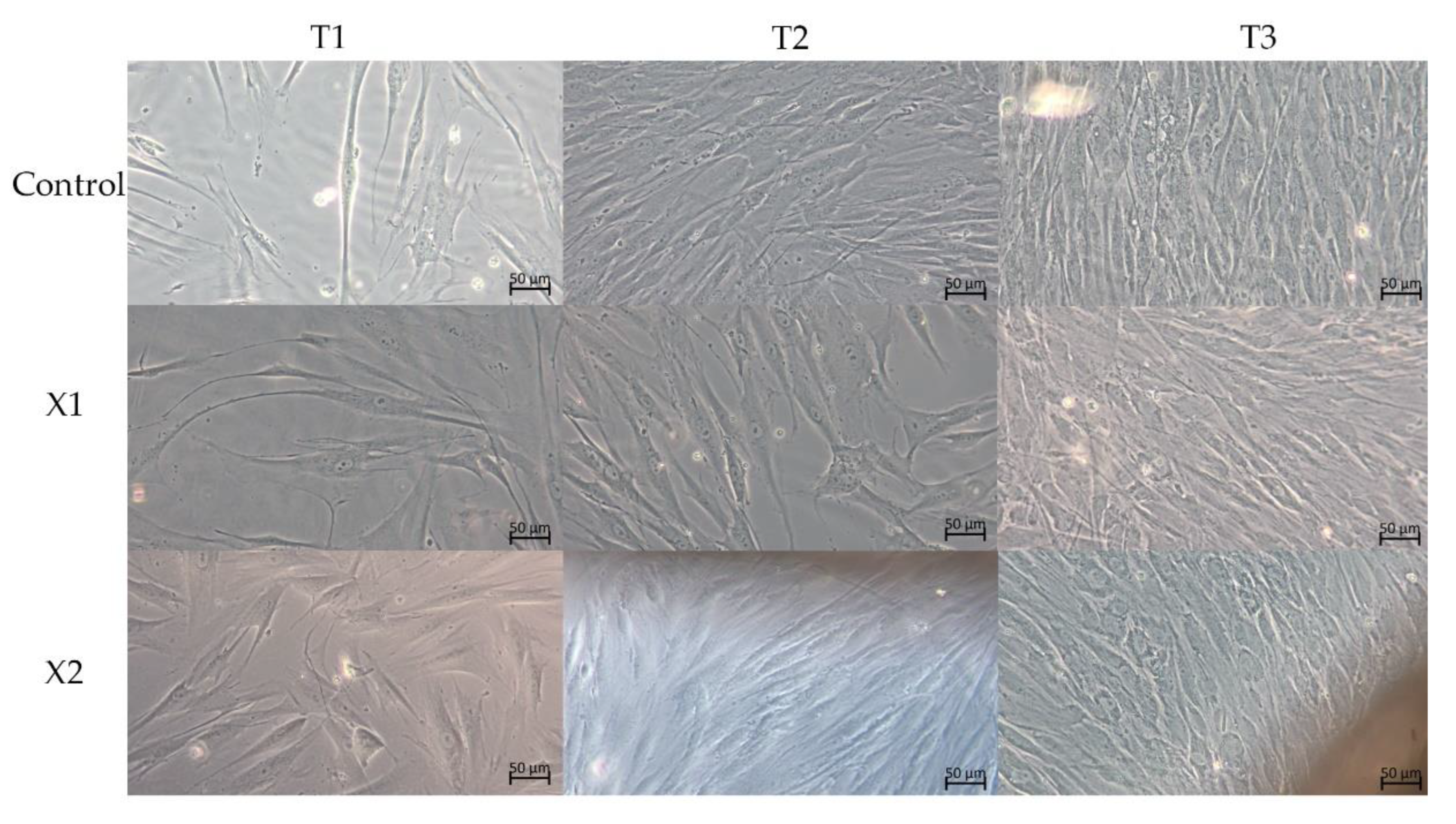
3.2. Morphological Analysis—LM
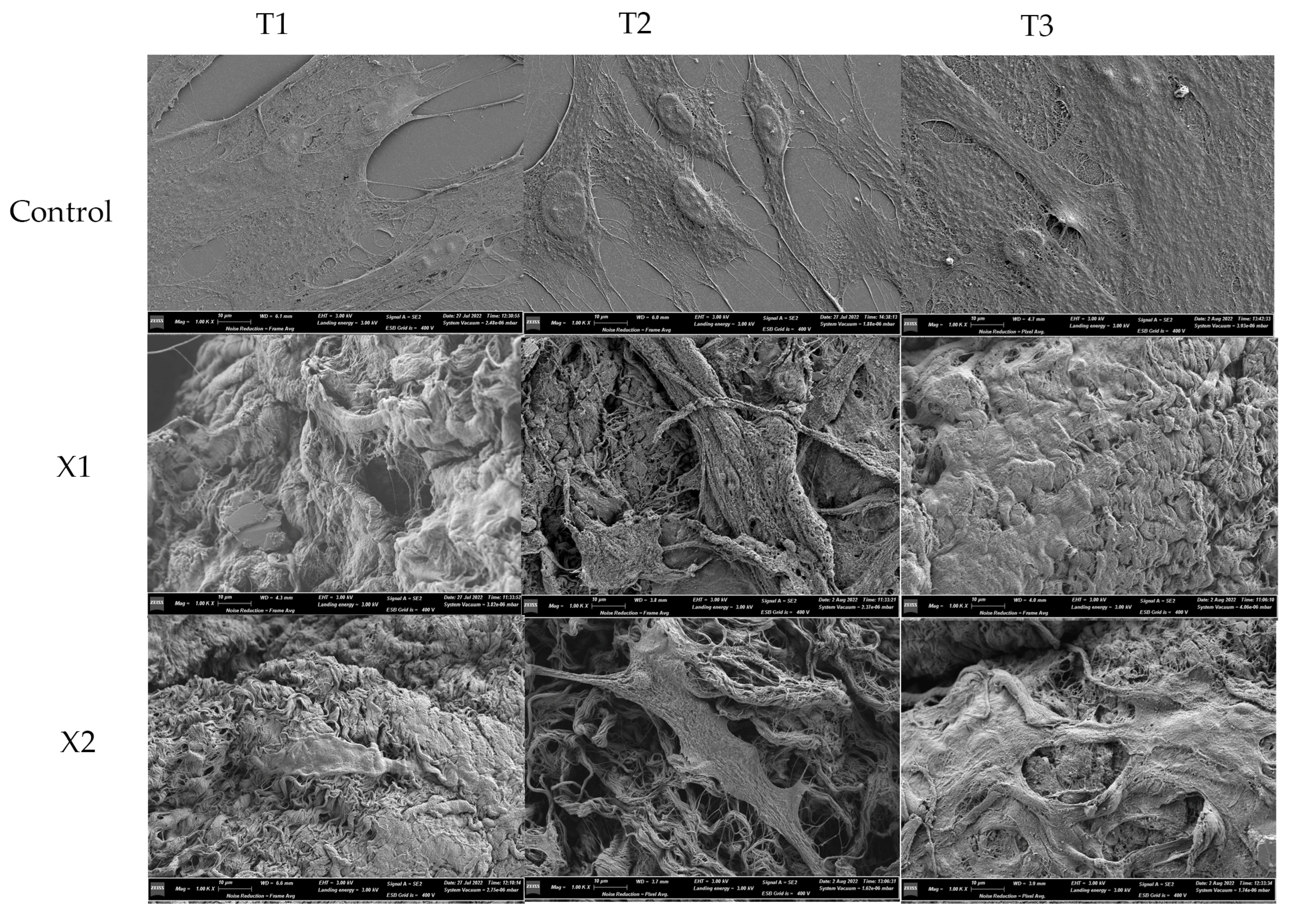
3.3. Morphological Analysis—SEM
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mehta, D.B.; Deshpande, N.C.; Dandekar, S.A. Comparative Evaluation of Platelet-Rich Fibrin Membrane and Collagen Membrane along with Demineralized Freeze-Dried Bone Allograft in Grade II Furcation Defects: A Randomized Controlled Study. J. Indian Soc. Periodontol. 2018, 22, 322–327. [Google Scholar] [CrossRef]
- Bernardi, S.; Macchiarelli, G.; Bianchi, S. Autologous Materials in Regenerative Dentistry: Harvested Bone, Platelet Concentrates and Dentin Derivates. Molecules 2020, 25, 5330. [Google Scholar] [CrossRef]
- Arjunan, A.; Baroutaji, A.; Robinson, J.; Praveen, A.S.; Pollard, A.; Wang, C. Future Directions and Requirements for Tissue Engineering Biomaterials. In Encyclopedia of Smart Materials; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Liu, Z.Z.; Wong, M.L.; Griffiths, L.G. Effect of Bovine Pericardial Extracellular Matrix Scaffold Niche on Seeded Human Mesenchymal Stem Cell Function. Sci. Rep. 2016, 6, 37089. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and Options Using Resorbable versus Nonresorbable Membranes for Successful Guided Bone Regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Hämmerle, C.H.F.; Jung, R.E. Bone Augmentation by Means of Barrier Membranes. Periodontol. 2000 2003, 33, 36–53. [Google Scholar] [CrossRef]
- Carpio, L.; Loza, J.; Lynch, S.; Genco, R. Guided Bone Regeneration around Endosseous Implants with Anorganic Bovine Bone Mineral. A Randomized Controlled Trial Comparing Bioabsorbable versus Non-Resorbable Barriers. J. Periodontol. 2000, 71, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Gutta, R.; Baker, R.A.; Bartolucci, A.A.; Louis, P.J. Barrier Membranes Used for Ridge Augmentation: Is There an Optimal Pore Size? J. Oral Maxillofac. Surg. 2009, 67, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Ronda, M.; Rebaudi, A.; Torelli, L.; Stacchi, C. Expanded vs. Dense Polytetrafluoroethylene Membranes in Vertical Ridge Augmentation around Dental Implants: A Prospective Randomized Controlled Clinical Trial. Clin. Oral Implant. Res. 2014, 25, 859–866. [Google Scholar] [CrossRef]
- Lee1, J.-Y.; Kim1, Y.-K.; Yun1, P.-Y.; Oh2, J.-S.; Kim2, S.-G. Guided Bone Regeneration Using Two Types of Non-Resorbable Barrier Membranes. J. Korean Assoc. Oral Maxillofac. Surg. 2010, 36, 275–279. [Google Scholar] [CrossRef]
- Pitaru, S.; Tal, H.; Soldinger, M.; Noff, M. Collagen Membranes Prevent Apical Migration of Epithelium and Support New Connective Tissue Attachment during Periodontal Wound Healing in Dogs. J. Periodontal. Res. 1989, 24, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, E.M.; Klana, N.A.P. Concise Review: Periodontal Tissue Regeneration Using Pericardium Membrane as Guided Bone Regeneration. AIP Conf. Proc. 2020, 2314, 050015. [Google Scholar]
- Athar, Y.; Zainuddin, S.L.A.; Berahim, Z.; Hassan, A.; Sagheer, A.; Alam, M.K. Bovine Pericardium Membrane and Periodontal Guided Tissue Regeneration: A Sem Study. Int. Med. J. 2014, 21, 325–327. [Google Scholar]
- Pizzicannella, J.; Marconi, G.D.; Pierdomenico, S.D.; Cavalcanti, M.F.X.B.; Diomede, F.; Trubiani, O. Bovine Pericardium Membrane, Gingival Stem Cells, and Ascorbic Acid: A Novel Team in Regenerative Medicine. Eur. J. Histochem. 2019, 63, 3064. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, V.; Kazakos, K.; Lyras, D.N.; Dimitrakopoulos, I.; Lazaridis, N.; Karakasis, D.; Botaitis, S.; Agrogiannis, G. Comparative Study of 5 Different Membranes for Guided Bone Regeneration of Rabbit Mandibular Defects beyond Critical Size. Med. Sci. Monit. 2008, 14, BR67–BR73. [Google Scholar] [PubMed]
- Miller, N.; Penaud, J.; Foliguet, B.; Membre, H.; Ambrosini, P.; Plombas, M. Resorption Rates of 2 Commercially Available Bioresorbable Membranes. A Histomorphometric Study in a Rabbit Model. J. Clin. Periodontol. 1996, 23, 1051–1059. [Google Scholar] [CrossRef]
- Juodzbalys, G.; Raustia, A.M.; Kubilius, R. A 5-Year Follow-up Study on One-Stage Implants Inserted Concomitantly with Localized Alveolar Ridge Augmentation. J. Oral. Rehabil. 2007, 34, 781–789. [Google Scholar] [CrossRef]
- Duskova, M.; Leamerova, E.; Sosna, B.; Gojis, O. Guided Tissue Regeneration, Barrier Membranes and Reconstruction of the Cleft Maxillary Alveolus. J. Craniofac. Surg. 2006, 17, 1153–1160. [Google Scholar] [CrossRef]
- Lundgren, D.; Laurell, L.; Gottlow, J.; Rylander, H.; Mathisen, T.; Nyman, S.; Rask, M. The Influence of the Design of Two Different Bioresorbable Barriers on the Results of Guided Tissue Regeneration Therapy. An Intra-Individual Comparative Study in the Monkey. J. Periodontol. 1995, 66, 605–612. [Google Scholar] [CrossRef]
- Shulman, J. Clinical Evaluation of an Acellular Dermal Allograft for Increasing the Zone of Attached Gingiva. Pract. Periodontics Aesthet. Dent. 1996, 8, 201–208. [Google Scholar]
- Wang, H.-L.; Boyapati, L. “PASS” Principles for Predictable Bone Regeneration. Implant Dent 2006, 15, 8–17. [Google Scholar] [CrossRef]
- Oswal, D.; Korossis, S.; Mirsadraee, S.; Wilcox, H.; Watterson, K.; Fisher, J.; Ingham, E. Biomechanical Characterization of Decellularized and Cross-Linked Bovine Pericardium. J. Heart Valve Dis. 2007, 16, 165–174. [Google Scholar] [PubMed]
- Gonçalves, A.C.; Griffiths, L.G.; Anthony, R.V.; Orton, E.C. Decellularization of Bovine Pericardium for Tissue-Engineering by Targeted Removal of Xenoantigens. J. Heart Valve Dis. 2005, 14, 212–217. [Google Scholar] [PubMed]
- Li, R.; Guo, W.; Yang, B.; Guo, L.; Sheng, L.; Chen, G.; Li, Y.; Zou, Q.; Xie, D.; An, X.; et al. Human Treated Dentin Matrix as a Natural Scaffold for Complete Human Dentin Tissue Regeneration. Biomaterials 2011, 32, 4525–4538. [Google Scholar] [CrossRef] [PubMed]
- de Santana, R.B.; de Mattos, C.M.L.; Francischone, C.E.; Van Dyke, T. Superficial Topography and Porosity of an Absorbable Barrier Membrane Impacts Soft Tissue Response in Guided Bone Regeneration. J. Periodontol. 2010, 81, 926–933. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Rodell, C.B.; Burdick, J.A.; Anseth, K.S. Progress in Material Design for Biomedical Applications. Proc. Natl. Acad. Sci. USA 2015, 112, 14444–14451. [Google Scholar] [CrossRef]
- Kim, A.; Yu, H.Y.; Lim, J.; Ryu, C.M.; Kim, Y.H.; Heo, J.; Han, J.Y.; Lee, S.; Bae, Y.S.; Kim, J.Y.; et al. Improved Efficacy and in Vivo Cellular Properties of Human Embryonic Stem Cell Derivative in a Preclinical Model of Bladder Pain Syndrome. Sci. Rep. 2017, 7, 8872. [Google Scholar] [CrossRef]
- Urciuolo, A.; de Coppi, P. Decellularized Tissue for Muscle Regeneration. Int. J. Mol. Sci. 2018, 19, 2392. [Google Scholar] [CrossRef]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical Qualification of Collagen Membranes Used in Dentistry. Ann. Ist. Super Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef]
- Sheikh, Z.; Qureshi, J.; Alshahrani, A.M.; Nassar, H.; Ikeda, Y.; Glogauer, M.; Ganss, B. Collagen Based Barrier Membranes for Periodontal Guided Bone Regeneration Applications. Odontology 2017, 105, 1–12. [Google Scholar] [CrossRef]
- Mahajan, R.; Khinda, P.; Shewale, A.; Ghotra, K.; Bhasin, M.T.; Bhasin, P. Comparative Efficacy of Placental Membrane and HealiguideTM in Treatment of Gingival Recession Using Guided Tissue Regeneration. J. Indian Soc. Periodontol. 2018, 22, 513–522. [Google Scholar] [CrossRef]
- Nisbet, D.R.; Forsythe, J.S.; Shen, W.; Finkelstein, D.I.; Horne, M.K. Review Paper: A Review of the Cellular Response on Electrospun Nanofibers for Tissue Engineering. J. Biomater. Appl. 2009, 24, 7–29. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The Extracellular Matrix at a Glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.N.; Tran, H.L.B. Effect of Modified Bovine Pericardium on Human Gingival Fibroblasts in Vitro. Cells Tissues Organs 2019, 206, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi Ngoc, M. ID:2052 manufacturing biological membrane from bovine pericardium extracellular matrix toward the periodontal application. Biomed. Res. Ther. 2017, 4, S82. [Google Scholar] [CrossRef][Green Version]
- Giragosyan, K.; Chenchev, I.; Ivanova, V.; Zlatev, S. Immunological Response to Nonresorbable Barrier Membranes Used for Guided Bone Regeneration and Formation of Pseudo Periosteum: A Narrative Review. Folia Med. 2022, 64, 13–20. [Google Scholar] [CrossRef]
- Berahim, Z.; Moharamzadeh, K.; Rawlinson, A.; Jowett, A.K. Biologic Interaction of Three-Dimensional Periodontal Fibroblast Spheroids with Collagen-Based and Synthetic Membranes. J. Periodontol. 2011, 82, 790–797. [Google Scholar] [CrossRef]
- Hamilton, D.W.; Oates, C.J.; Hasanzadeh, A.; Mittler, S. Migration of Periodontal Ligament Fibroblasts on Nanometric Topographical Patterns: Influence of Filopodia and Focal Adhesions on Contact Guidance. PLoS ONE 2010, 5, e15129. [Google Scholar] [CrossRef][Green Version]
- da Silva Brum, I.; Frigo, L.; Lana Devita, R.; da Silva Pires, J.L.; Hugo Vieira de Oliveira, V.; Rosa Nascimento, A.L.; de Carvalho, J.J. Histomorphometric, Immunohistochemical, Ultrastructural Characterization of a Nano-Hydroxyapatite/Beta-Tricalcium Phosphate Composite and a Bone Xenograft in Sub-Critical Size Bone Defect in Rat Calvaria. Materials 2020, 13, 4594. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, C.N.E.E.-I. Factors Affecting the Success of Dental Implants. In Implant Dentistry: A Rapidly Evolving Practice; IntechOpen: Rijeka, Croatia, 2011; p. 14. [Google Scholar]
- Zelzer, M.; Albutt, D.; Alexander, M.R.; Russell, N.A. The Role of Albumin and Fibronectin in the Adhesion of Fibroblasts to Plasma Polymer Surfaces. Plasma Process. Polym. 2012, 9, 149–156. [Google Scholar] [CrossRef]
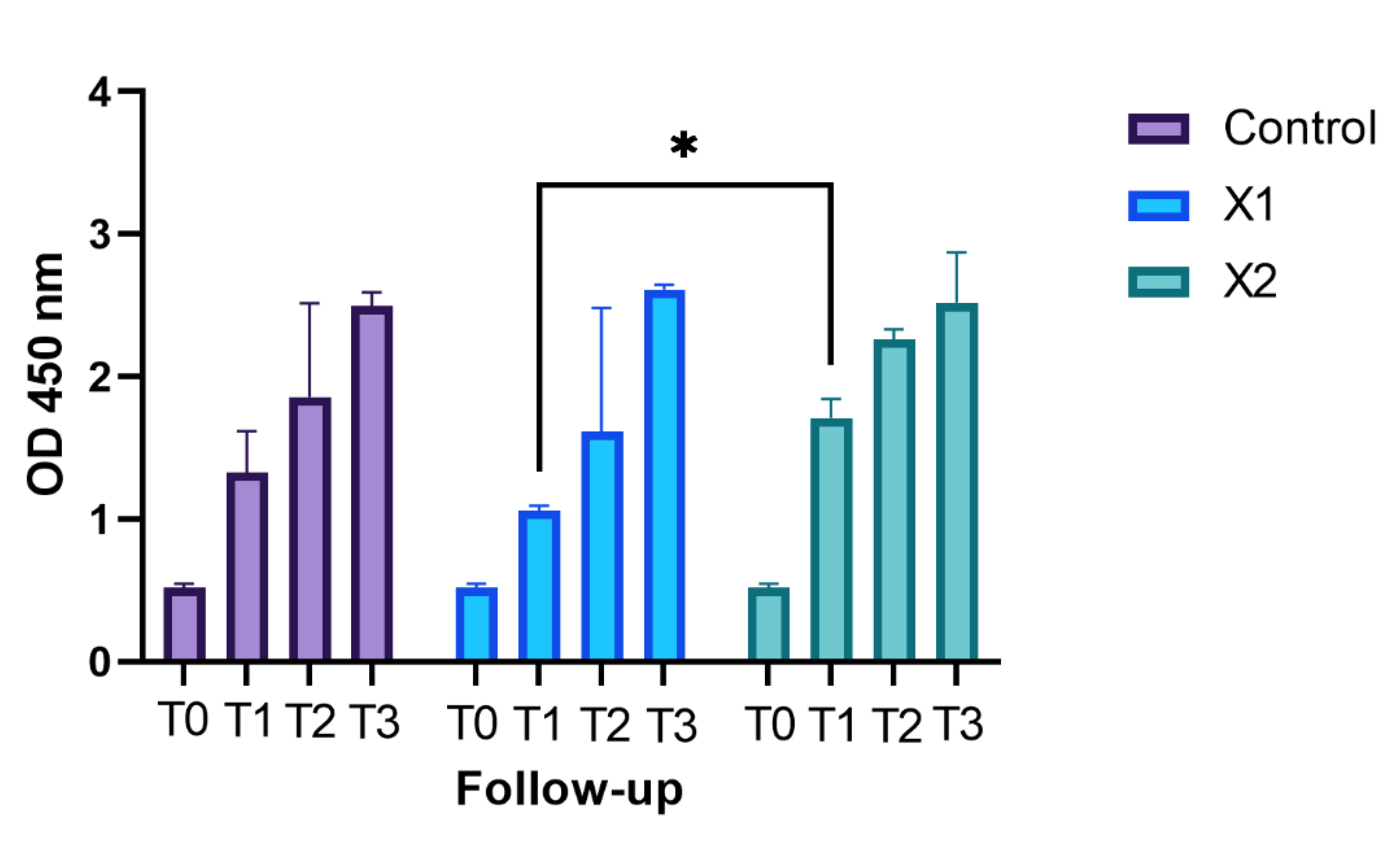
| Bonferroni’s Multiple Comparisons Test | |||
|---|---|---|---|
| T0 | Mean Diff. | 95.00% CI of Diff. | p Value |
| Control vs. X1 | 0 | −0.09880 to 0.09880 | N.S. |
| Control vs. X2 | 0 | −0.09880 to 0.09880 | N.S. |
| X1 vs. X2 | 0 | −0.09880 to 0.09880 | N.S. |
| T1 | |||
| Control vs. X1 | 0.2643 | −0.9920 to 1.521 | N.S. |
| Control vs. X2 | −0.3857 | −1.347 to 0.5760 | N.S. |
| X1 vs. X2 | −0.65 | −1.172 to −0.1279 | <0.05 |
| T2 | |||
| Control vs. X1 | 0.248 | −2.358 to 2.854 | N.S. |
| Control vs. X2 | −0.4037 | −3.223 to 2.416 | N.S. |
| X1 vs. X2 | −0.6517 | −4.440 to 3.136 | N.S. |
| T3 | |||
| Control vs. X1 | −0.1167 | −0.4698 to 0.2365 | N.S. |
| Control vs. X2 | −0.025 | −1.353 to 1.303 | N.S. |
| X1 vs. X2 | 0.09167 | −1.424 to 1.607 | N.S. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, S.; Bernardi, S.; Simeone, D.; Torge, D.; Macchiarelli, G.; Marchetti, E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials 2022, 15, 8284. https://doi.org/10.3390/ma15238284
Bianchi S, Bernardi S, Simeone D, Torge D, Macchiarelli G, Marchetti E. Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials. 2022; 15(23):8284. https://doi.org/10.3390/ma15238284
Chicago/Turabian StyleBianchi, Serena, Sara Bernardi, Davide Simeone, Diana Torge, Guido Macchiarelli, and Enrico Marchetti. 2022. "Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study" Materials 15, no. 23: 8284. https://doi.org/10.3390/ma15238284
APA StyleBianchi, S., Bernardi, S., Simeone, D., Torge, D., Macchiarelli, G., & Marchetti, E. (2022). Proliferation and Morphological Assessment of Human Periodontal Ligament Fibroblast towards Bovine Pericardium Membranes: An In Vitro Study. Materials, 15(23), 8284. https://doi.org/10.3390/ma15238284








