Surface Modification of 3D-Printed PCL/BG Composite Scaffolds via Mussel-Inspired Polydopamine and Effective Antibacterial Coatings for Biomedical Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PCL-Bioactive Glass (BG) Filaments
2.3. Fabrication of Scaffolds
2.4. Sr-MBGNs/Gelatin Coating onto Mussel-Inspired PDA-Modified Scaffolds
2.5. Characterization
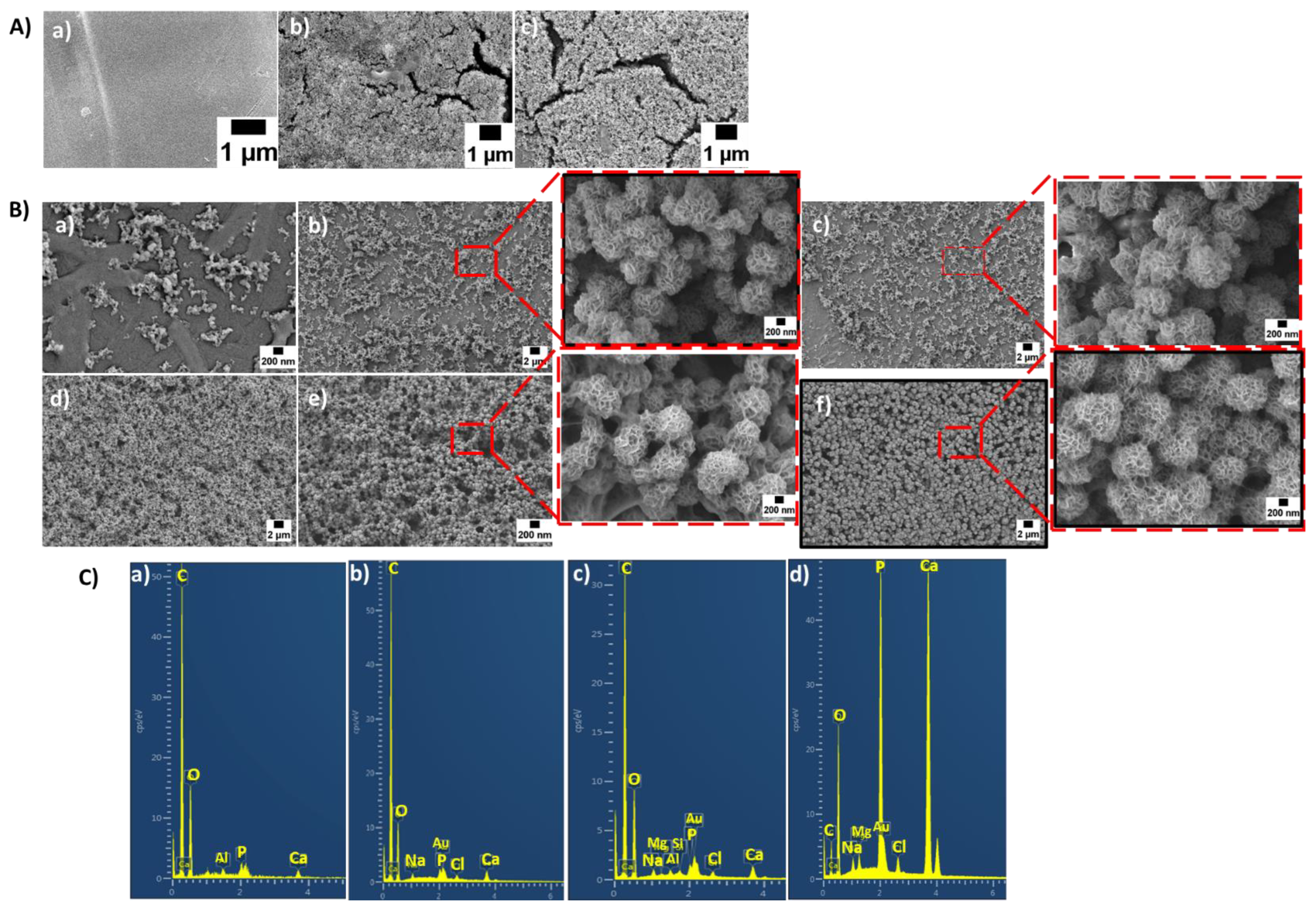
2.5.1. Surface Morphology, Porosity and BG Distribution
2.5.2. Contact Angle Measurement
2.5.3. In Vitro Degradation
2.5.4. Mechanical Properties
2.5.5. Bioactivity Study in Simulated Body Fluid
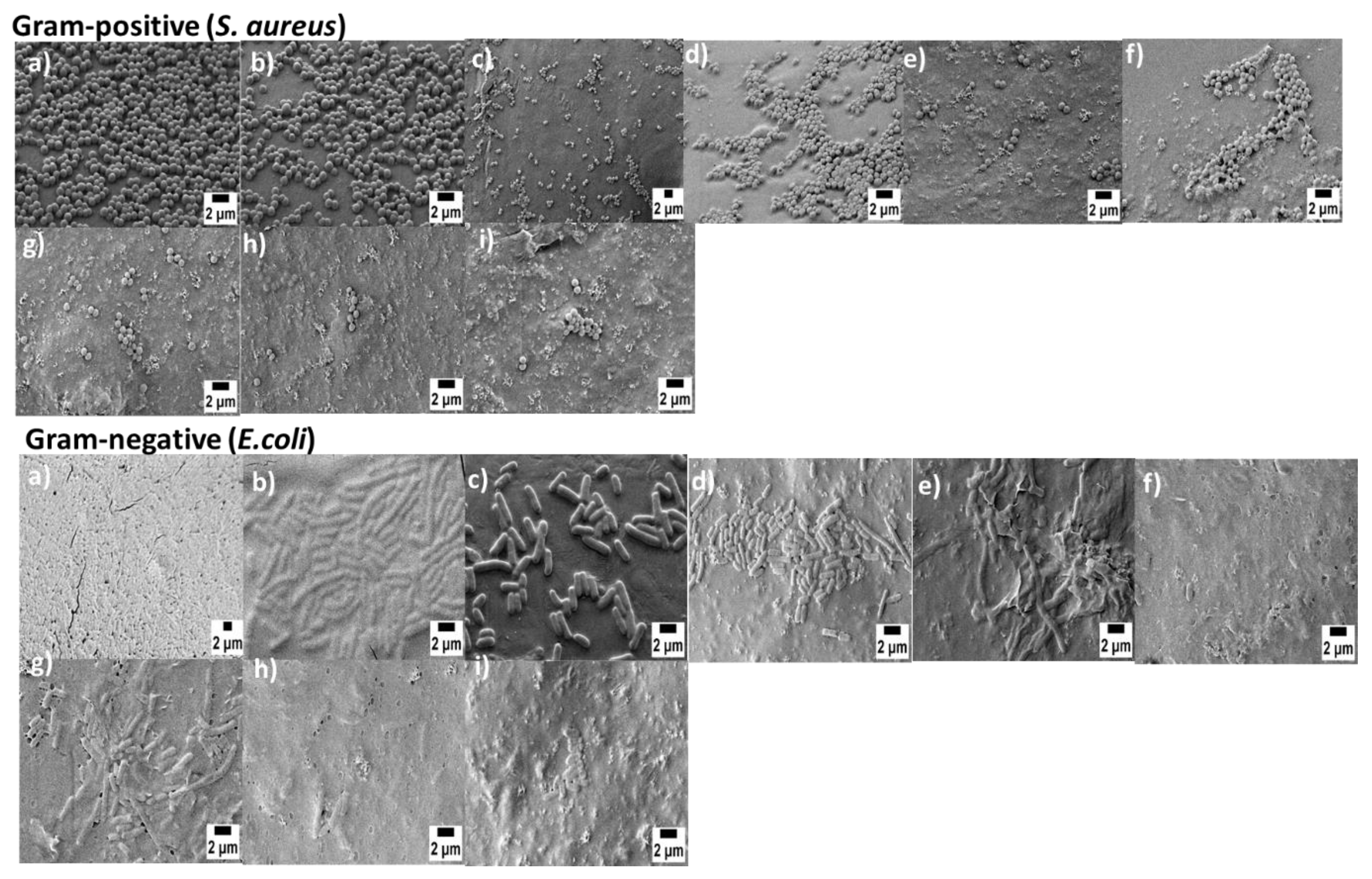
2.5.6. Antibacterial Study
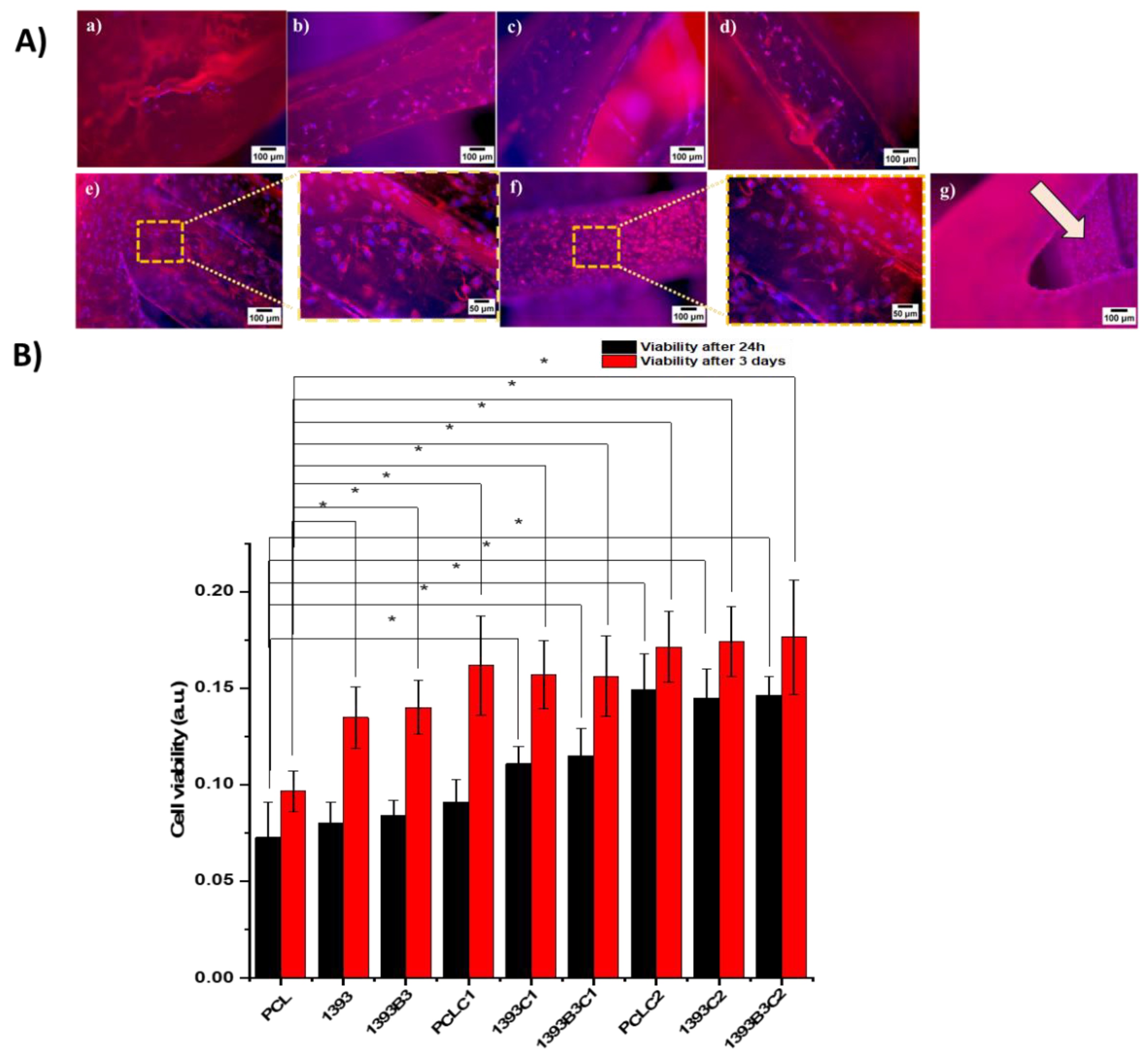
2.5.7. In Vitro Cytocompatibility
2.6. Statistical Analysis
3. Results and Discussion
3.1. Surface Morphology, BG Distribution and Porosity
3.2. Contact Angle Measurement
3.3. In Vitro Degradation
3.4. Mechanical Properties
3.5. In Vitro Bioactivity
3.6. Anti-Bacterial Study
3.7. In Vitro Cytocompatibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, H.; Zhang, H.; Guo, C.; Yang, K.; Chen, K.; Cheng, R.; Qian, N.; Sandler, N.; Zhang, Y.S.; et al. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials 2019, 190–191, 97–110. [Google Scholar] [CrossRef] [PubMed]
- De Witte, T.-M.; Fratila-Apachitei, L.E.; Zadpoor, A.A.; Peppas, N.A. Bone tissue engineering via growth factor delivery: From scaffolds to complex matrices. Regen. Biomater. 2018, 5, 197–211. [Google Scholar] [CrossRef]
- Pfister, A.; Landers, R.; Laib, A.; Hübner, U.; Schmelzeisen, R.; Mülhaupt, R. Biofunctional rapid prototyping for tissue-engineering applications: 3D bioplotting versus 3D printing. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 624–638. [Google Scholar] [CrossRef]
- Wu, C.; Luo, Y.; Cuniberti, G.; Xiao, Y.; Gelinsky, M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 2011, 7, 2644–2650. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone Tissue Engineering Using 3D Printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Thomas, D.J.; Jessop, Z.M.; Whitaker, I.S. 3D Bioprinting for Reconstructive Surgery: Techniques and Applications; Woodhead Publishing: Duxford, UK, 2017. [Google Scholar]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. In The Biomaterials: Silver Jubilee Compendium; Elsevier Science: Oxford, UK, 2000; pp. 175–189. [Google Scholar] [CrossRef]
- Ulag, S.; Kalkandelen, C.; Bedir, T.; Erdemir, G.; Kuruca, S.E.; Dumludag, F.; Ustundag, C.B.; Rayaman, E.; Ekren, N.; Kilic, B.; et al. Fabrication of three-dimensional PCL/BiFeO3 scaffolds for biomedical applications. Mater. Sci. Eng. B 2020, 261, 114660. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Kolan, K.; Liu, Y.; Baldridge, J.; Murphy, C.; Semon, J.; Day, D.; Leu, M. Solvent Based 3D Printing of Biopolymer/Bioactive Glass Composite and Hydrogel for Tissue Engineering Applications. Procedia CIRP 2017, 65, 38–43. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Portolés-Gil, N.; López-Periago, A.M.; Domingo, C.; Hosta-Rigau, L. Multi-layered polydopamine coatings for the immobilization of growth factors onto highly-interconnected and bimodal PCL/HA-based scaffolds. Mater. Sci. Eng. C 2020, 117, 111245. [Google Scholar] [CrossRef]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.; Thiré, R.M.D.S.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef]
- Xynos, I.D.; Hukkanen, M.; Batten, J.J.; Buttery, L.; Hench, L.L.; Polak, J.M. Bioglass ®45S5 Stimulates Osteoblast Turnover and Enhances Bone Formation In Vitro: Implications and Applications for Bone Tissue Engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Distler, T.; Fournier, N.; Grünewald, A.; Polley, C.; Seitz, H.; Detsch, R.; Boccaccini, A.R. Polymer-Bioactive Glass Composite Filaments for 3D Scaffold Manufacturing by Fused Deposition Modeling: Fabrication and Characterization. Front. Bioeng. Biotechnol. 2020, 8, 552. [Google Scholar] [CrossRef]
- Poh, P.S.; Hutmacher, D.W.; Holzapfel, B.M.; Solanki, A.K.; Stevens, M.M.; Woodruff, M.A. In vitro and in vivo bone formation potential of surface calcium phosphate-coated polycaprolactone and polycaprolactone/bioactive glass composite scaffolds. Acta Biomater. 2016, 30, 319–333. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yang, L.; Lin, M.; Ma, J.; Lu, X.; Lee, P.S. Polydopamine Spheres as Active Templates for Convenient Synthesis of Various Nanostructures. Small 2012, 9, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-T.; Lin, C.-C.; Chen, Y.-W.; Yeh, C.-H.; Fang, H.-Y.; Shie, M.-Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 56, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Han, P.; Liu, X.; Xu, M.; Tian, T.; Chang, J.; Xiao, Y. Mussel-inspired bioceramics with self-assembled Ca-P/polydopamine composite nanolayer: Preparation, formation mechanism, improved cellular bioactivity and osteogenic differentiation of bone marrow stromal cells. Acta Biomater. 2014, 10, 428–438. [Google Scholar] [CrossRef]
- Schuhladen, K.; Wang, X.; Hupa, L.; Boccaccini, A.R. Dissolution of borate and borosilicate bioactive glasses and the influence of ion (Zn, Cu) doping in different solutions. J. Non-Crystalline Solids 2018, 502, 22–34. [Google Scholar] [CrossRef]
- Ilyas, K.; Singer, L.; Akhtar, M.A.; Bourauel, C.P.; Boccaccini, A.R. Boswellia sacra Extract-Loaded Mesoporous Bioactive Glass Nano Particles: Synthesis and Biological Effects. Pharmaceutics 2022, 14, 126. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, J.; Li, W.; Dippold, D.; Wan, Y.; Boccaccini, A.R. Incorporation of Cu-Containing Bioactive Glass Nanoparticles in Gelatin-Coated Scaffolds Enhances Bioactivity and Osteogenic Activity. ACS Biomater. Sci. Eng. 2018, 4, 1546–1557. [Google Scholar] [CrossRef]
- Arango-Ospina, M.; Lasch, K.; Weidinger, J.; Boccaccini, A.R. Manuka Honey and Zein Coatings Impart Bioactive Glass Bone Tissue Scaffolds Antibacterial Properties and Superior Mechanical Properties. Front. Mater. 2021, 7, 610889. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Esmaeilzadeh, J.; Hesaraki, S.; Hadavi, S.M.-M.; Ebrahimzadeh, M.H.; Esfandeh, M. Poly (d/l) lactide/polycaprolactone/bioactive glasss nanocomposites materials for anterior cruciate ligament reconstruction screws: The effect of glass surface functionalization on mechanical properties and cell behaviors. Mater. Sci. Eng. C 2017, 77, 978–989. [Google Scholar] [CrossRef]
- Ma, H.; Feng, C.; Chang, J.; Wu, C. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Yan, J.; Wu, R.; Liao, S.; Jiang, M.; Qian, Y. Applications of Polydopamine-Modified Scaffolds in the Peripheral Nerve Tissue Engineering. Front. Bioeng. Biotechnol. 2020, 8, 590998. [Google Scholar] [CrossRef] [PubMed]
- Chakrapani, V.Y.; Gnanamani, A.; Giridev, V.; Madhusoothanan, M.; Sekaran, G. Electrospinning of type I collagen and PCL nanofibers using acetic acid. J. Appl. Polym. Sci. 2012, 125, 3221–3227. [Google Scholar] [CrossRef]
- Xia, Y.; Zhou, P.; Cheng, X.; Xie, Y.; Liang, C.; Li, C.; Xu, S. Selective laser sintering fabrication of nano-hydroxyapatite/poly-ε-caprolactone scaffolds for bone tissue engineering applications. Int. J. Nanomed. 2013, 8, 4197–4213. [Google Scholar]
- Mulyati, S.; Muchtar, S.; Arahman, N.; Syamsuddin, Y.; Nawi, N.I.M.; Harun, N.Y.; Bilad, M.R.; Firdaus, Y.; Takagi, R.; Matsuyama, H. Two-Step Dopamine-to-Polydopamine Modification of Polyethersulfone Ultrafiltration Membrane for Enhancing Anti-Fouling and Ultraviolet Resistant Properties. Polymers 2020, 12, 2051. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.; Sahranavard, M.; Zamanian, A. Immobilization of gelatin on the oxygen plasma-modified surface of polycaprolactone scaffolds with tunable pore structure for skin tissue engineering. J. Polym. Res. 2020, 27, 281. [Google Scholar] [CrossRef]
- Bi, L.; Cao, Z.; Hu, Y.; Song, Y.; Yu, L.; Yang, B.; Mu, J.; Huang, Z.; Han, Y. Effects of different cross-linking conditions on the properties of genipin-cross-linked chitosan/collagen scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2011, 22, 51–62. [Google Scholar] [CrossRef]
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and bioactive porous scaffold structures prepared using fused deposition modeling. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 610–619. [Google Scholar] [CrossRef]
- Jiang, W.; Shi, J.; Li, W.; Sun, K. Morphology, wettability, and mechanical properties of polycaprolactone/hydroxyapatite composite scaffolds with interconnected pore structures fabricated by a mini-deposition system. Polym. Eng. Sci. 2012, 52, 2396–2402. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Candarlioglu, P.L.; Miller, C.A.; Gentleman, E.; Stevens, M.M. Materials characterisation and cytotoxic assessment of strontium-substituted bioactive glasses for bone regeneration. J. Mater. Chem. 2010, 20, 8934–8941. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion. Biomaterials 1996, 17, 103–114. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ramaswamy, Y.; Kwik, D.; Zreiqat, H. The effect of strontium incorporation into CaSiO3 ceramics on their physical and biological properties. Biomaterials 2007, 28, 3171–3181. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.-C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 3, 3867–3910. [Google Scholar] [CrossRef]
- Chi, M.; Li, N.; Cui, J.; Karlin, S.; Rohr, N.; Sharma, N.; Thieringer, F.M. Biomimetic, mussel-inspired surface modification of 3D-printed biodegradable polylactic acid scaffolds with nano-hydroxyapatite for bone tissue engineering. Front. Bioeng. Biotechnol. 2022, 10, 1646. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Ding, Y.; Scheithauer, E.C.; Goudouri, O.-M.; Grünewald, A.; Detsch, R.; Agarwal, S.; Boccaccini, A.R. Antibacterial 45S5 Bioglass®-based scaffolds reinforced with genipin cross-linked gelatin for bone tissue engineering. J. Mater. Chem. B 2015, 3, 3367–3378. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Brauer, D.S.; Jones, J.R.; Law, R.V.; Hill, R.G. Influence of strontium for calcium substitution in bioactive glasses on degradation, ion release and apatite formation. J. R. Soc. Interface 2012, 9, 880–889. [Google Scholar] [CrossRef]
- Chen, X.; Meng, Y.; Li, Y.; Zhao, N. Investigation on bio-mineralization of melt and sol–gel derived bioactive glasses. Appl. Surf. Sci. 2008, 255, 562–564. [Google Scholar] [CrossRef]
- Khvostenko, D.; Hilton, T.; Ferracane, J.; Mitchell, J.; Kruzic, J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M.; Sedghi, A. RETRACTED: A comparative study on the in vitro formation of hydroxyapatite, cytotoxicity and antibacterial activity of 58S bioactive glass substituted by Li and Sr. Mater. Sci. Eng. C 2018, 91, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, M.; Lee, P.-N.; Lee, C.-K. Stepwise assembly of multimetallic nanoparticles via self-polymerized polydopamine. J. Mater. Chem. 2011, 21, 12316–12320. [Google Scholar] [CrossRef]
- Baheiraei, N.; Eyni, H.; Bakhshi, B.; Najafloo, R.; Rabiee, N. Effects of strontium ions with potential antibacterial activity on in vivo bone regeneration. Sci. Rep. 2021, 11, 8745. [Google Scholar] [CrossRef] [PubMed]
- Declercq, H.A.; Desmet, T.; Berneel, E.E.; Dubruel, P.; Cornelissen, M.J. Synergistic effect of surface modification and scaffold design of bioplotted 3-D poly-ε-caprolactone scaffolds in osteogenic tissue engineering. Acta Biomater. 2013, 9, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Seyednejad, H.; Gawlitta, D.; Dhert, W.J.A.; van Nostrum, C.F.; Vermonden, T.; Hennink, W.E. Preparation and characterization of a three-dimensional printed scaffold based on a functionalized polyester for bone tissue engineering applications. Acta Biomater. 2011, 7, 1999–2006. [Google Scholar] [CrossRef]
- Jo, S.; Kang, S.M.; Park, S.A.; Kim, W.D.; Kwak, J.; Lee, H. Enhanced Adhesion of Preosteoblasts inside 3DPCL Scaffolds by Polydopamine Coating and Mineralization. Macromol. Biosci. 2013, 13, 1389–1395. [Google Scholar] [CrossRef]
- Ghorbani, F.; Ghalandari, B.; Khan, A.L.; Li, D.; Zamanian, A.; Yu, B. Decoration of electrical conductive polyurethane-polyaniline/polyvinyl alcohol matrixes with mussel-inspired polydopamine for bone tissue engineering. Biotechnol. Prog. 2020, 36, e3043. [Google Scholar] [CrossRef]
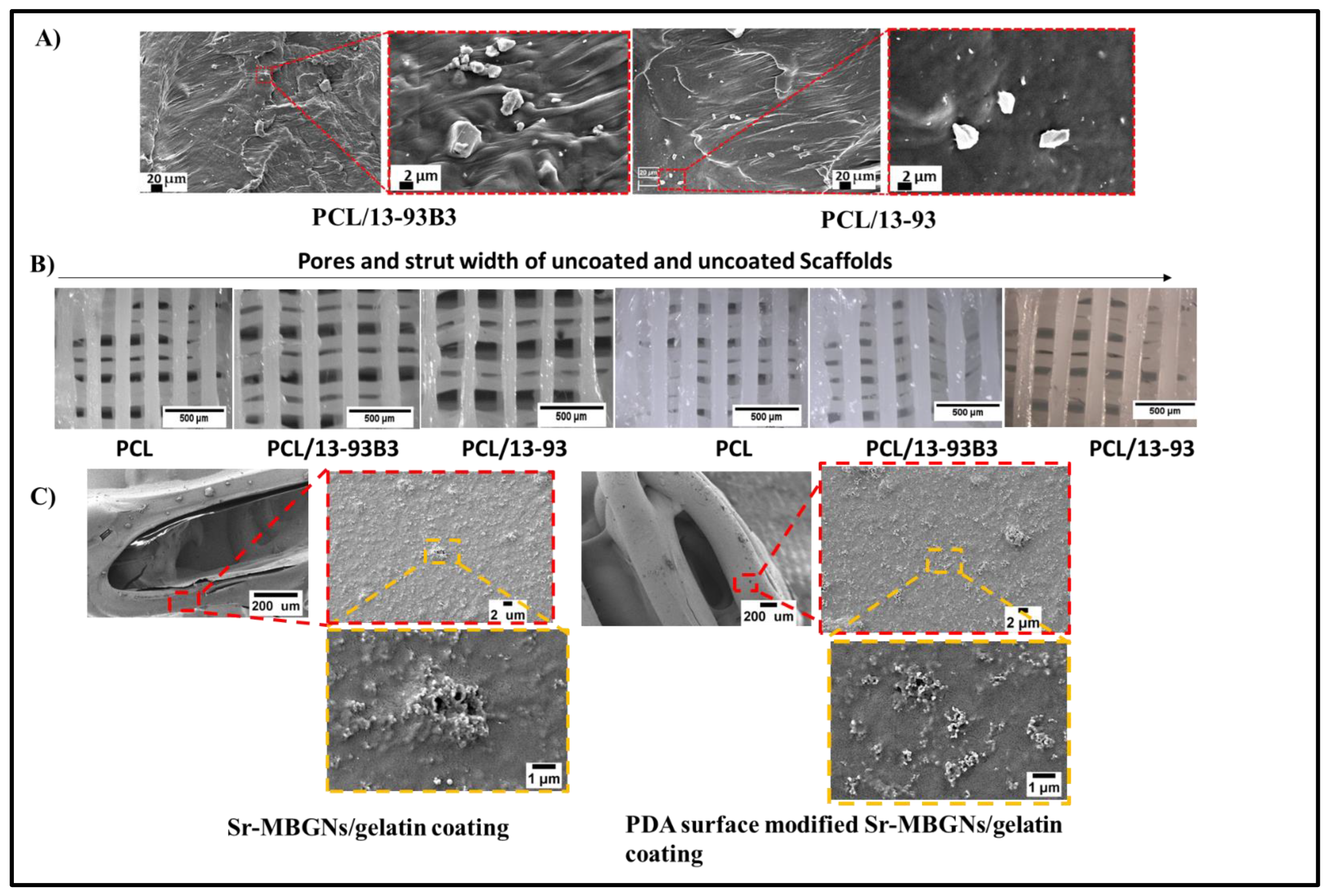
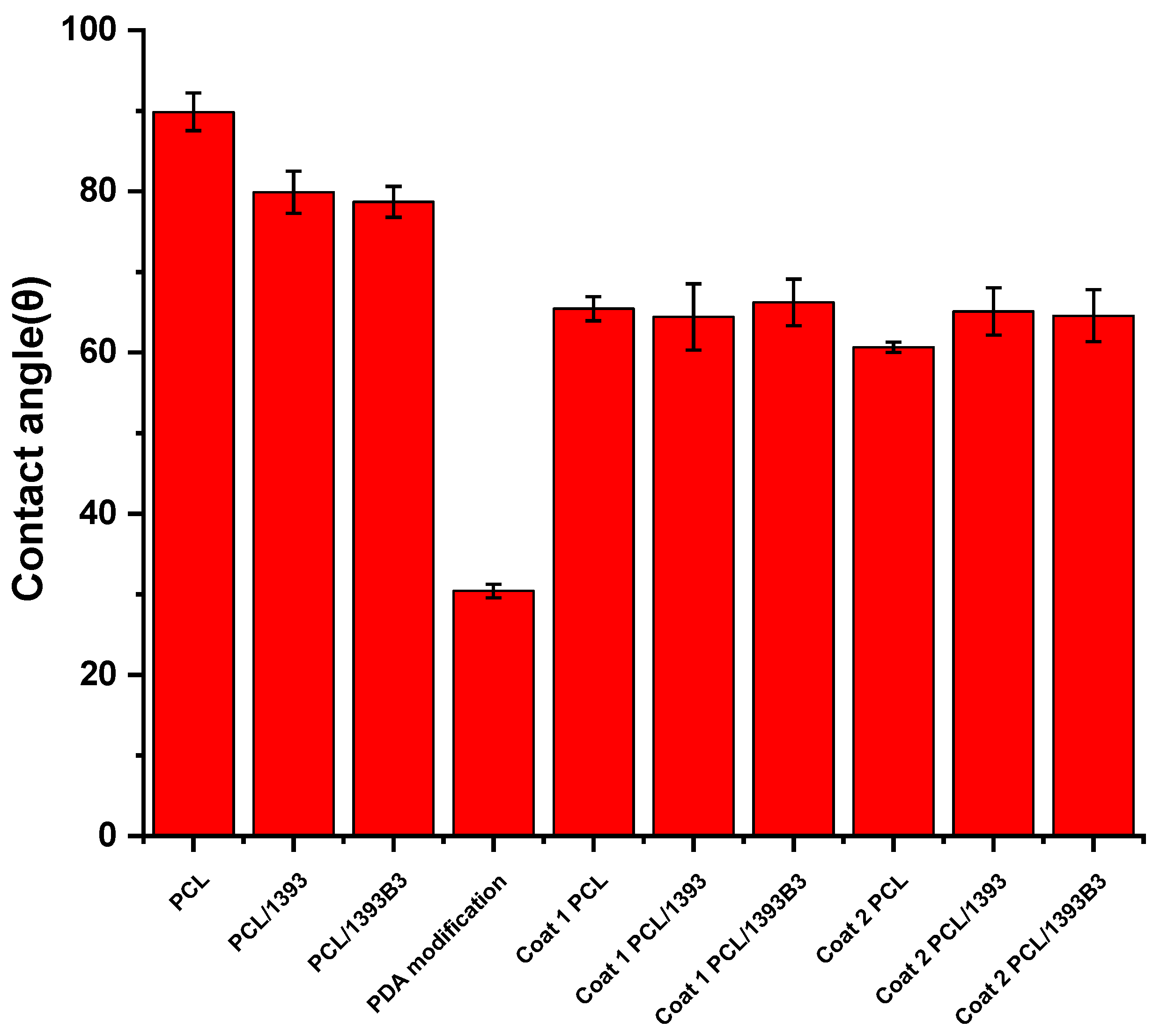

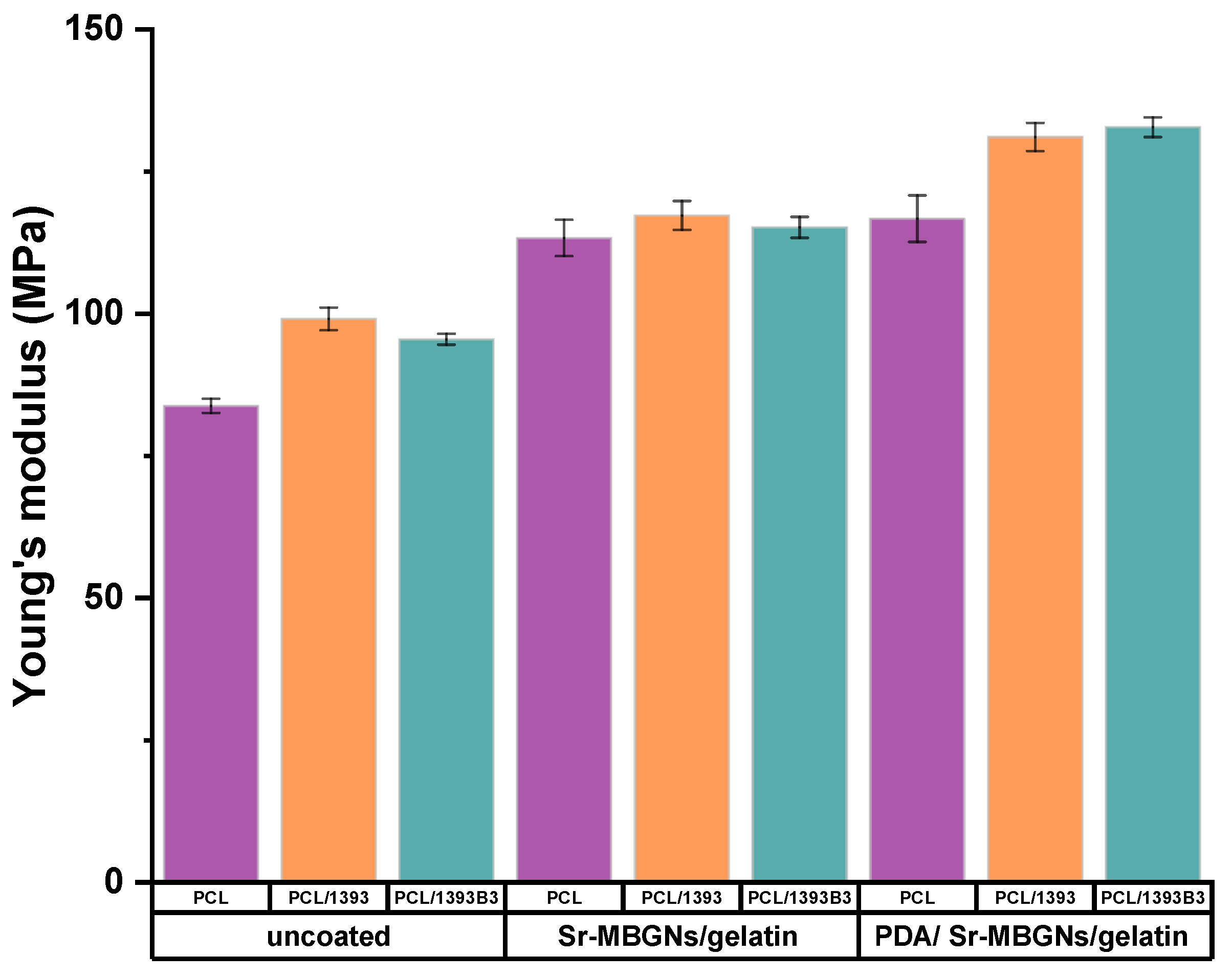
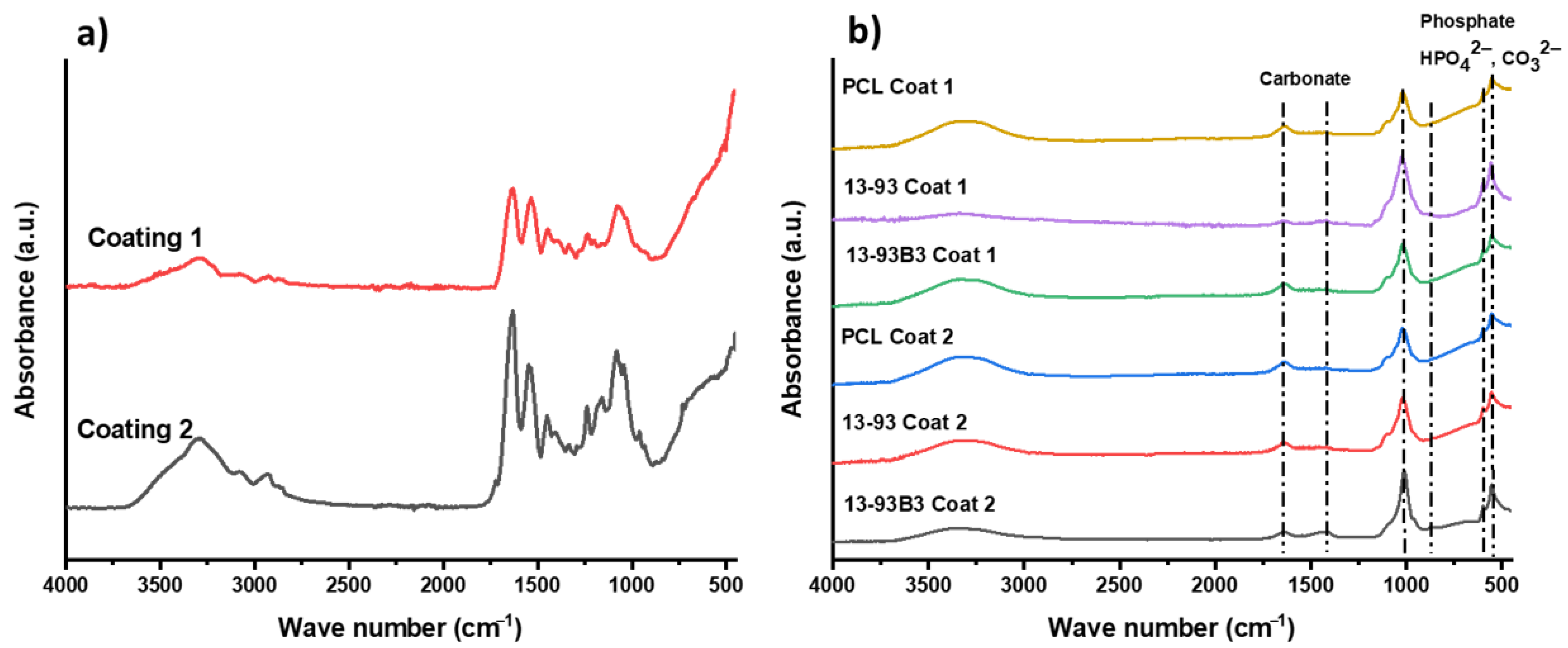

| Composition (wt%) | 13-93 | 13-93B3 |
|---|---|---|
| Na2O | 5.5 | 5.5 |
| K2O | 11.1 | 11.1 |
| MgO | 4.6 | 4.6 |
| CaO | 18.5 | 18.5 |
| SiO2 | 56.6 | 0 |
| P2O4 | 3.7 | 3.7 |
| B2O3 | 0 | 56.6 |
| Sample | Strut Width (μm) | Pore Size (μm) |
|---|---|---|
| PCL | 293 ± 15 | 284 ± 8 |
| PCL/13-93 | 267 ± 19 | 299 ± 40 |
| PCL/13-93B3 | 214 ± 19 | 350 ± 37 |
| Samples | Before Coating (%) | After Sr-MBGNs/Gelatin Coating (%) | After PDA/Sr-MBGNs/Gelatin Coating (%) |
|---|---|---|---|
| PCL | 72 ± 1 | 72 ± 1 | 71 ± 1 |
| PCL/13-93 | 78 ± 2 | 76 ± 1 | 77.3 ± 0.6 |
| PCL/13-93B3 | 79.3 ± 0.8 | 77.8 ± 0.7 | 77.2 ± 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, K.; Akhtar, M.A.; Ammar, E.B.; Boccaccini, A.R. Surface Modification of 3D-Printed PCL/BG Composite Scaffolds via Mussel-Inspired Polydopamine and Effective Antibacterial Coatings for Biomedical Applications. Materials 2022, 15, 8289. https://doi.org/10.3390/ma15238289
Ilyas K, Akhtar MA, Ammar EB, Boccaccini AR. Surface Modification of 3D-Printed PCL/BG Composite Scaffolds via Mussel-Inspired Polydopamine and Effective Antibacterial Coatings for Biomedical Applications. Materials. 2022; 15(23):8289. https://doi.org/10.3390/ma15238289
Chicago/Turabian StyleIlyas, Kanwal, Muhammad Asim Akhtar, Ezzeddine Ben Ammar, and Aldo R. Boccaccini. 2022. "Surface Modification of 3D-Printed PCL/BG Composite Scaffolds via Mussel-Inspired Polydopamine and Effective Antibacterial Coatings for Biomedical Applications" Materials 15, no. 23: 8289. https://doi.org/10.3390/ma15238289
APA StyleIlyas, K., Akhtar, M. A., Ammar, E. B., & Boccaccini, A. R. (2022). Surface Modification of 3D-Printed PCL/BG Composite Scaffolds via Mussel-Inspired Polydopamine and Effective Antibacterial Coatings for Biomedical Applications. Materials, 15(23), 8289. https://doi.org/10.3390/ma15238289







