Mechanical Properties and Microstructure of Binary In-Sn Alloys for Flexible Low Temperature Electronic Joints
Abstract
:1. Introduction
2. Materials and Methods
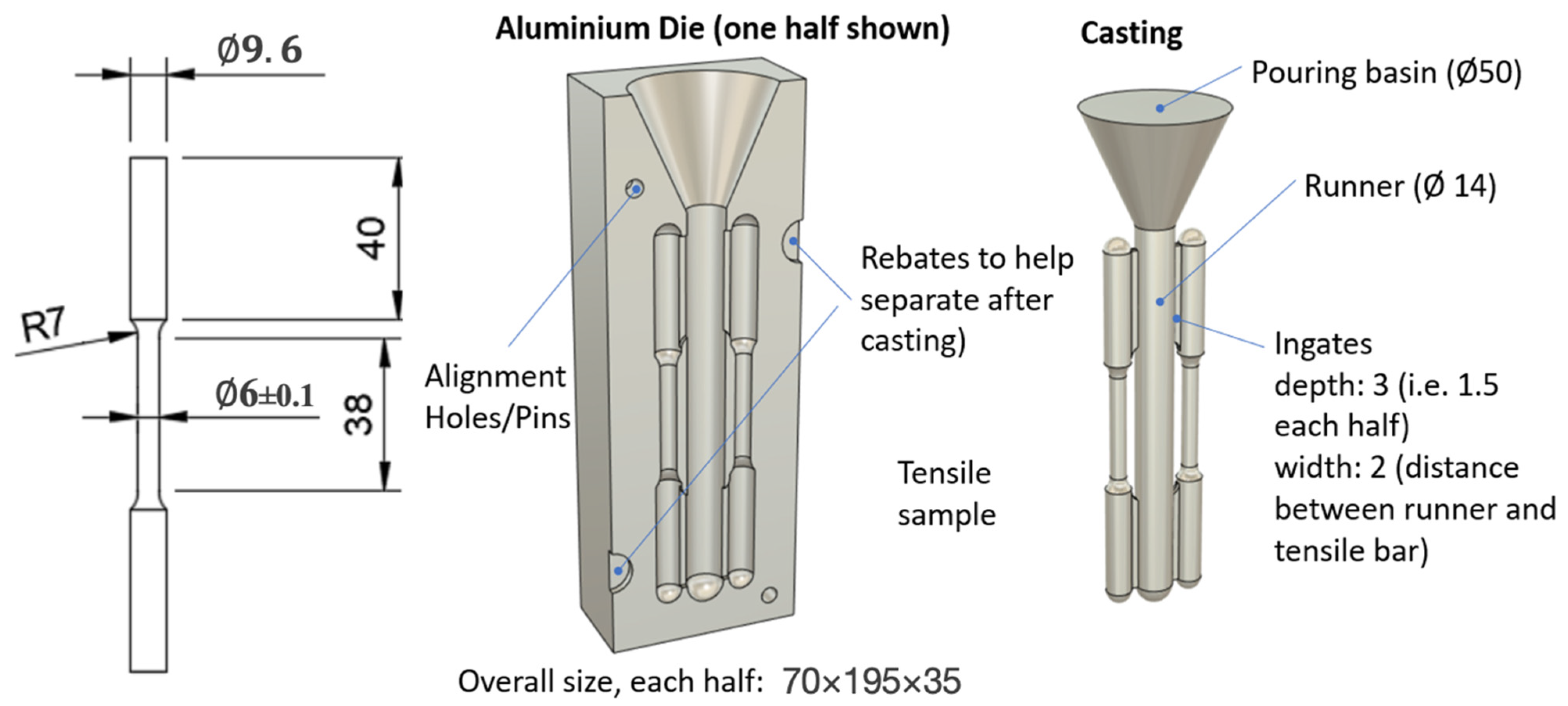
2.1. Materials and Samples Preparation
2.2. Mechanical Properties Test
2.3. Thermal Properties Characterisation
2.4. Microstructural Analysis
3. Results and Discussion
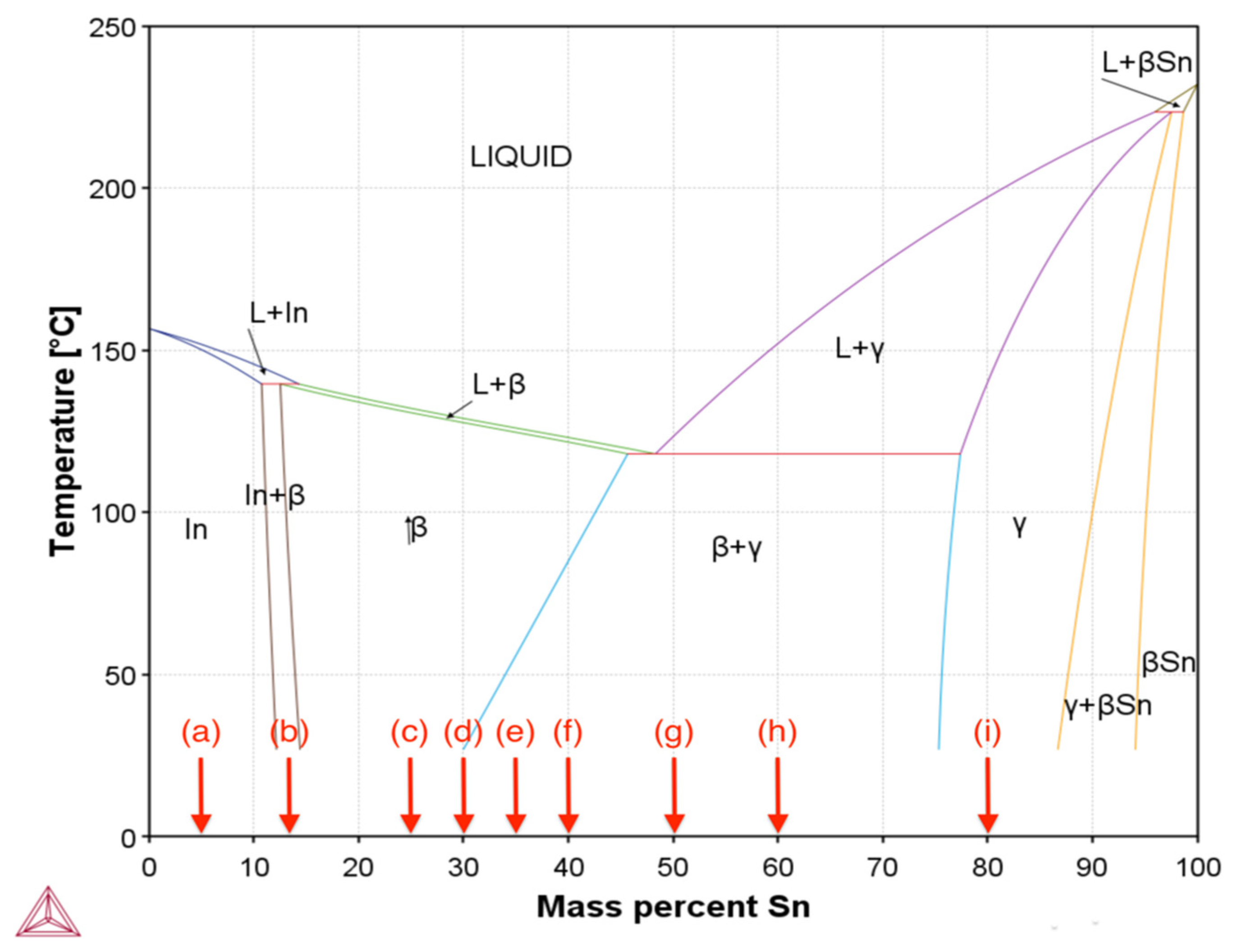
3.1. Thermal Properties and Microstructure Formation in In-Sn Solder Alloys
3.1.1. Melting Point Characterisation
3.1.2. Thermodynamics and Phase Formation
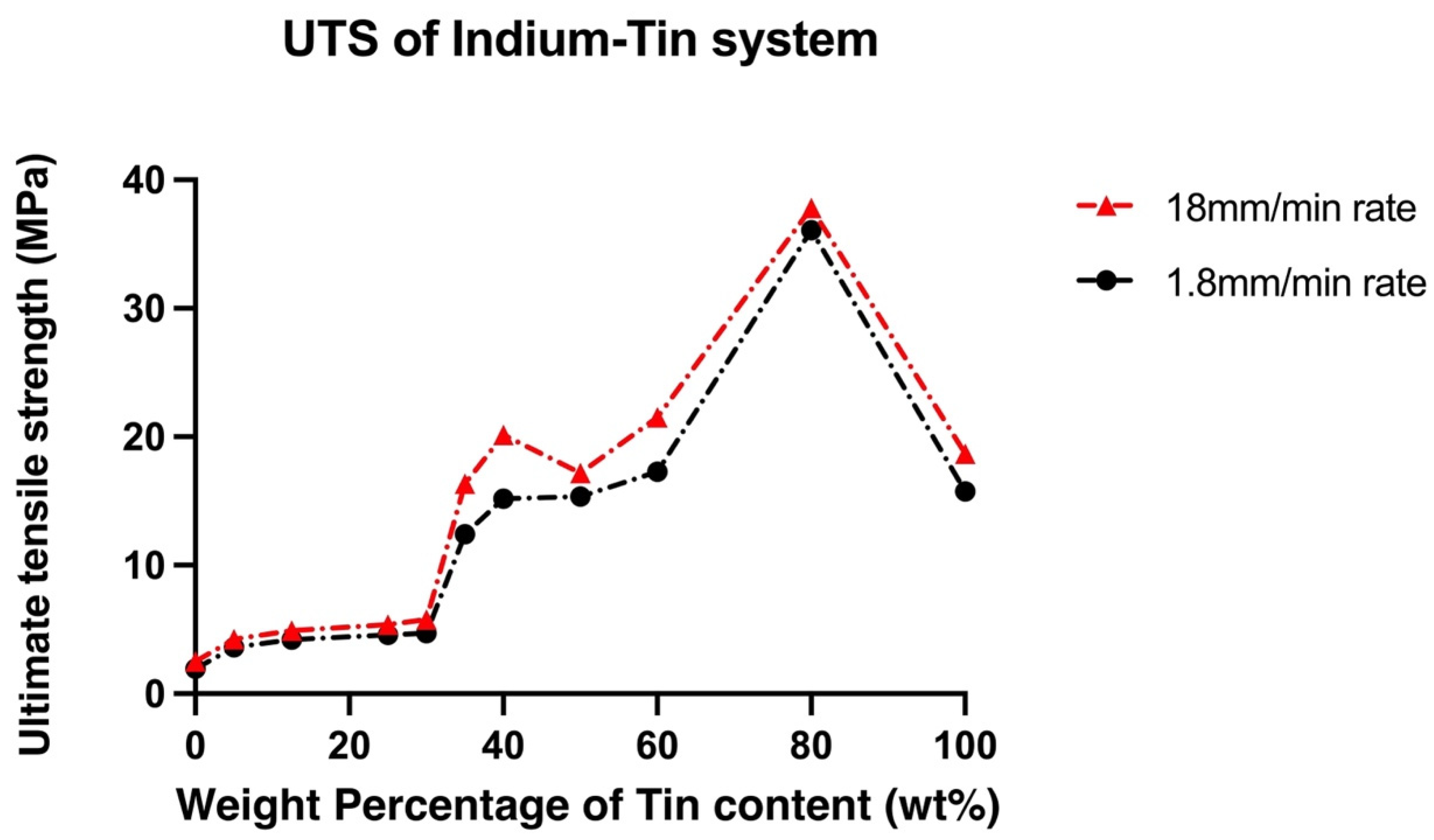
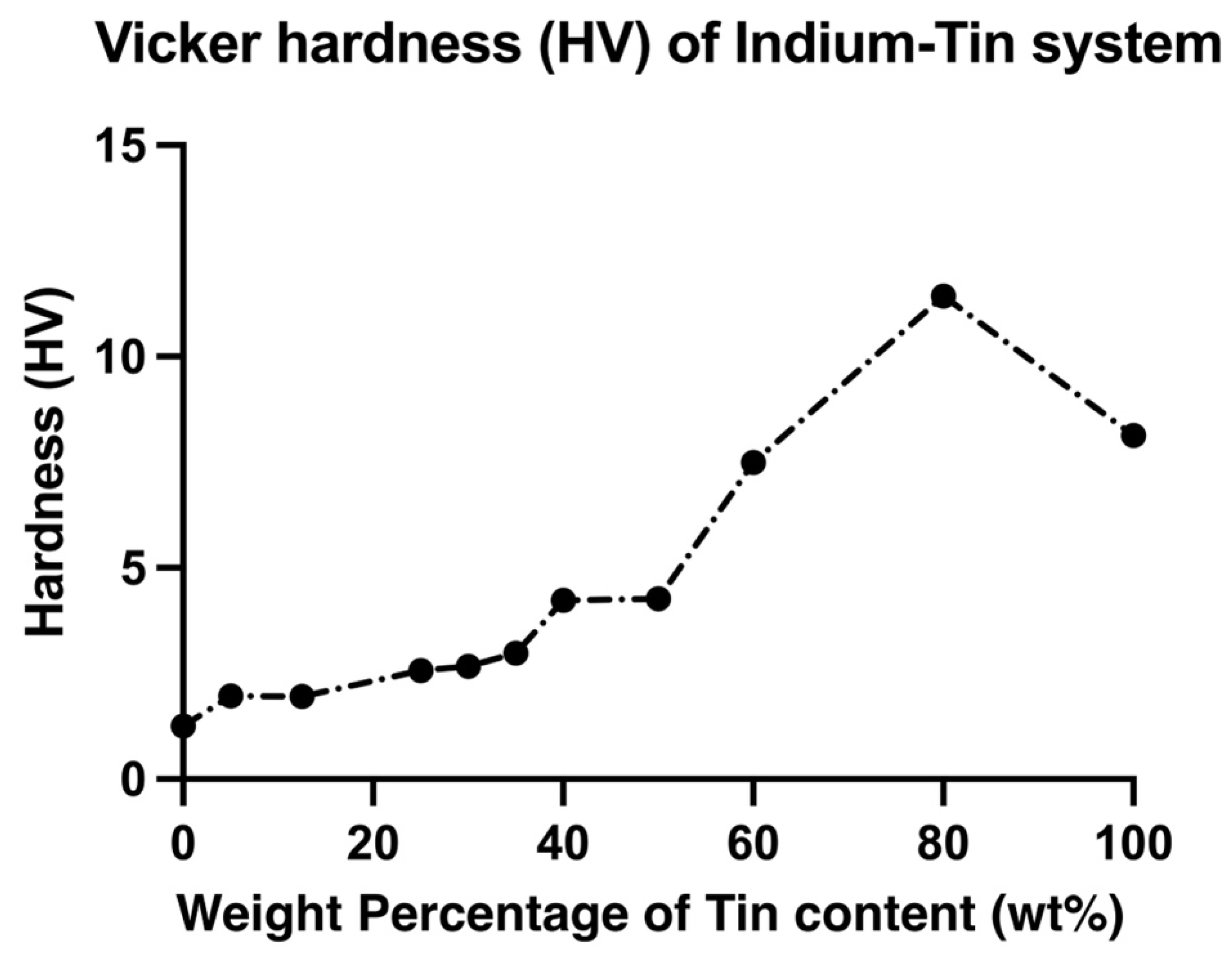
3.2. Mechanical Properties
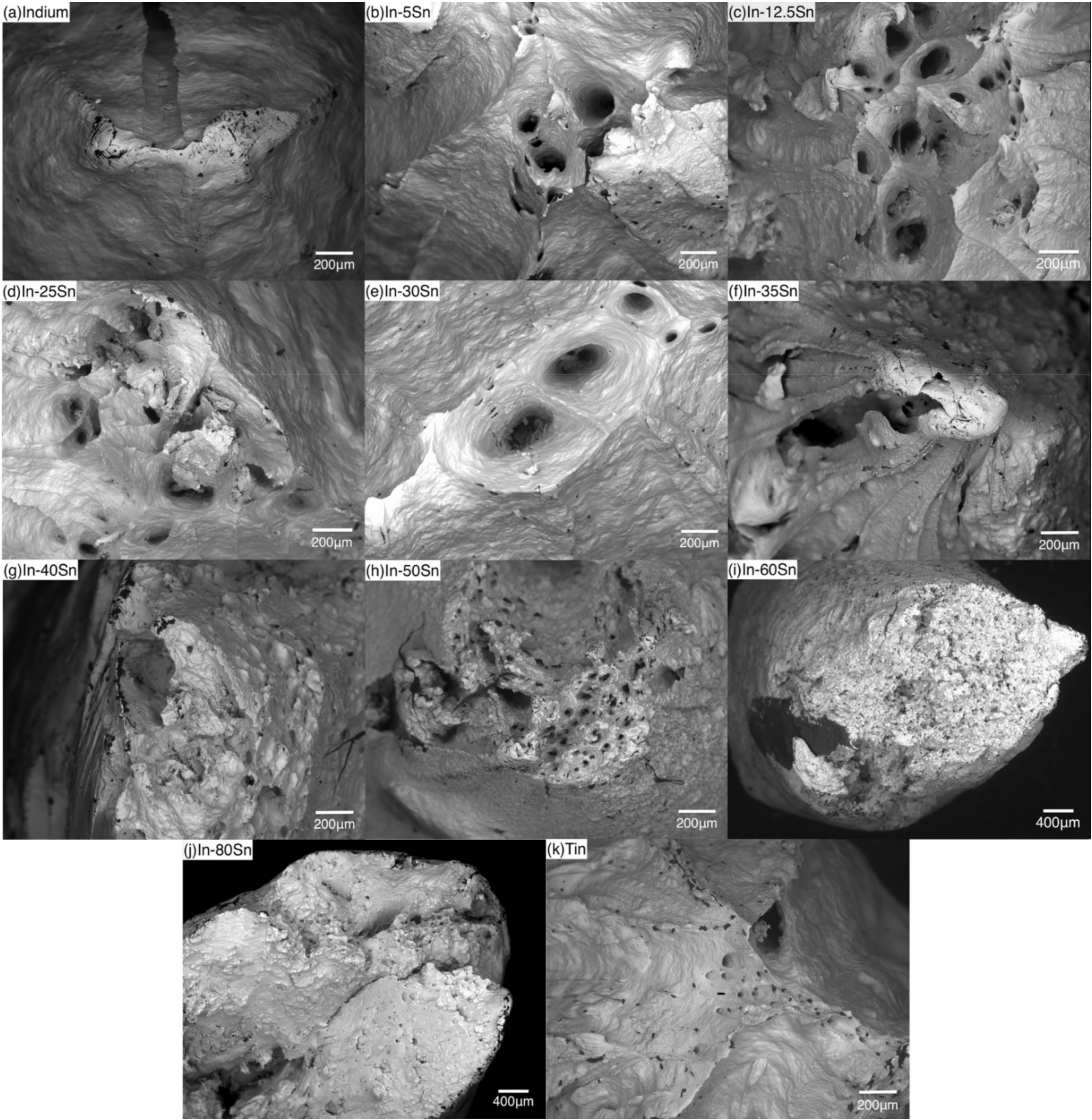
3.2.1. Fracture Characterisation
3.2.2. Strain Rate Effect
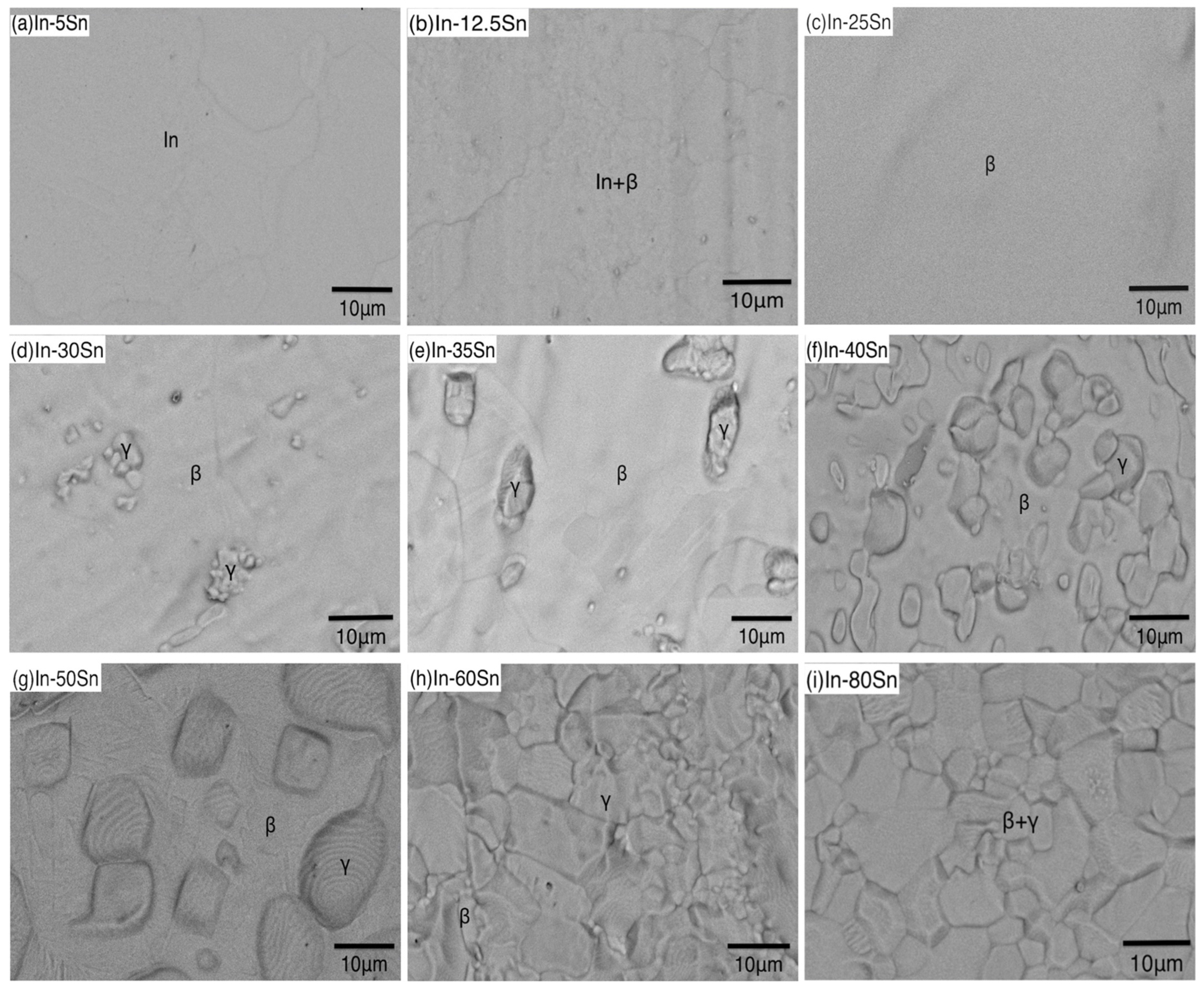
3.2.3. Microstructure
3.3. Microstructural Characterisation
4. Conclusions
- Different volume fractions of β and γ phases in In-Sn alloys had a significant effect on the mechanical properties; as compared to the β phase, the island-like γ phase is harder and less ductile. The increase of Sn content in the In-Sn alloys that were tested was associated with an increase in the ultimate tensile strength and hardness, and the highest UTS of 37.8 MPa has been measured in a composition of In-80Sn. Furthermore, ductile fracture was observed in all samples and good elongation was measured in most compositions.
- The DSC results showed a melting point range of 119.3 °C to 194.9 °C which are relatively low for solder materials. The calculated enthalpy values revealed that the formation of γ-InSn4 is associated with more heat and requires more energy than the β-In3Sn phase.
- In-Sn alloys have numerous applications as solder alloys for low-temperature processing and low temperature applications, especially where high ductility is required. The data from this study provides a good foundation for the further research into these alloys and gives some confidence on the expected mechanical properties. However, more mechanical property testing under cryogenic conditions as well as characterising the thermal cycling stability are required.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gain, A.K.; Zhang, L. Effects of Ni nanoparticles addition on the microstructure, electrical and mechanical properties of Sn-Ag-Cu alloy. Materialia 2019, 5, 100234. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, K.N. Structure and properties of lead-free solders bearing micro and nano particles. Mater. Sci. Eng. R Rep. A Rev. J. 2014, 82, 1–32. [Google Scholar] [CrossRef]
- Date, M.; Shoji, T.; Fujiyoshi, M.; Sato, K.; Tu, K.N. Ductile-to-brittle transition in Sn–Zn solder joints measured by impact test. Scr. Mater. 2004, 51, 641–645. [Google Scholar] [CrossRef]
- Gain, A.K.; Zhang, L. Effect of Ag nanoparticles on microstructure, damping property and hardness of low melting point eutectic tin–bismuth solder. J. Mater. Sci. Mater. Electron. 2017, 28, 15718–15730. [Google Scholar] [CrossRef]
- Morris, J.W.; Goldstein, J.L.F.; Mei, Z. Microstructure and mechanical properties of Sn-In and Sn-Bi solders. JOM 1993, 45, 25–27. [Google Scholar] [CrossRef]
- Wada, O. Optoelectronic Integration: Physics, Technology and Applications; Springer: Boston, MA, USA, 1994; pp. 1–16. [Google Scholar]
- Mousavi, T.; Darby, W.; Aksoy, C.; Davies, T.; Brittles, G.; Grovenor, C.; Speller, S. Novel Superconducting Joints for Persistent Mode Magnet Applications. MRS Adv. 2016, 1, 3483–3488. [Google Scholar] [CrossRef]
- Souriau, J.C.; Morales, J.M.H.; Castagné, L.; Simon, G.; Amara, K.; Boutaud, B. Miniaturization and Biocompatible Encapsulation for Implantable Biomedical Silicon Devices. ECS J. Solid State Sci. Technol. 2015, 4, P445–P450. [Google Scholar] [CrossRef]
- Chang, R.W.; McCluskey, P.F. Reliability assessment of indium solder for low temperature electronic packaging. Cryogenics 2009, 49, 630–634. [Google Scholar] [CrossRef]
- Reed, R.P.; McCowan, C.N.; Walsh, R.P.; Delgado, L.A.; McColskey, J.D. Tensile strength and ductility of indium. Mater. Sci. Engineering. A Struct. Mater. Prop. Microstruct. Process. 1988, 102, 227–236. [Google Scholar] [CrossRef]
- Plötner, M.; Donat, B.; Benke, A. Deformation properties of indium-based solders at 294 and 77 K. Cryogenics 1991, 31, 159–162. [Google Scholar] [CrossRef]
- Deshpande, M.; Chaudhari, R.; Narayanan, P.R.; Kale, H. Evaluation of Shear Properties of Indium Solder Alloys for Cryogenic Applications. J. Mater. Eng. Perform. 2021, 30, 7958–7966. [Google Scholar] [CrossRef]
- Tudryn, C.D. Solder joint fatigue study under low temperature Martian conditions. In Proceedings of the IEEE Aerospace Conference, Big Sky, MT, USA, 4–11 March 2006; p. 9. [Google Scholar]
- Chuang, T.H.; Yu, C.L.; Chang, S.Y.; Wang, S.S. Phase identification and growth kinetics of the intermetallic compounds formed during In-49Sn/Cu soldering reactions. J. Electron. Mater. 2002, 31, 640–645. [Google Scholar] [CrossRef]
- Faizov, S.; Sarafanov, A.; Erdakov, I.; Gromov, D.; Svistun, A.; Glebov, L.; Bykov, V.; Bryk, A. On the Direct Extrusion of Solder Wire from 52In-48Sn Alloy. Machines 2021, 9, 93. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L.; Lu, K.J.; Zhang, Y.C.; Xu, Y.H.; Xu, F.; Gao, H.M. IMC Growth and Mechanical Properties of Cu/In-48Sn/Cu Solder Joints. J. Electron. Mater. 2021, 50, 3326–3333. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, K.N. Low melting point solders based on Sn, Bi, and In elements. Mater. Today Adv. 2020, 8, 100115. [Google Scholar] [CrossRef]
- Le Han, D.; Shen, Y.-A.; Jin, S.; Nishikawa, H. Microstructure and mechanical properties of the In–48Sn–xAg low-temperature alloy. J. Mater. Sci. 2020, 55, 10824–10832. [Google Scholar] [CrossRef]
- Mousavi, T.; Aksoy, C.; Grovenor, C.R.M.; Speller, S.C. Microstructure and superconducting properties of Sn-In and Sn-In-Bi alloys as Pb-free superconducting solders. Supercond. Sci. Technol. 2015, 29, 15012–15013. [Google Scholar] [CrossRef]
- Wernick, J.H.; Matthias, B.T. Superconductivity in the In–Sn System. J. Chem. Phys. 1961, 34, 2194–2195. [Google Scholar] [CrossRef]
- ASTM Standard E8/E8M–21; Standard Test Methods for Tension Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2021.
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.; Sundman, B. Thermo-Calc & DICTRA, computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar] [CrossRef]
- Sigworth, G.; Cáceres, C. Quality Issues in Aluminum Net-Shape Castings. AFS Trans. 2004, 112, 1–15. [Google Scholar]
- BS EN ISO 11357-5:2014: Plastics; Differential Scanning Calorimetry (DSC): Determination of Characteristic Reaction-Curve Temperatures and Times, Enthalpy of Reaction and Degree of Conversion. British Standards Institute: London, UK, 2014.
- Singh, A.; MhdNoor, E.E. MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2015; p. 02003. [Google Scholar]
- Kossakowski, P. Influence of initial porosity on strength properties of S235JR steel at low stress triaxiality. Arch. Civ. Eng. 2012, 58, 293–308. [Google Scholar] [CrossRef] [Green Version]
- Callister, W.D., Jr.; Rethwisch, D.G. Materials Science and Engineering, 9th ed.; SI version; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Eid, E.A.; Fouda, A.N.; Duraia, E.-S.M. Effect of adding 0.5 wt% ZnO nanoparticles, temperature and strain rate on tensile properties of Sn–5.0 wt% Sb–0.5 wt% Cu (SSC505) lead free solder alloy. Mater. Sci. Eng. A Struct. Mater. Prop. Microstruct. Process. 2016, 657, 104–114. [Google Scholar] [CrossRef]
- Fan, H.; Wang, Q.; El-Awady, J.A.; Raabe, D.; Zaiser, M. Strain rate dependency of dislocation plasticity. Nat. Commun. 2021, 12, 1845. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, A.; Fayek, S.A.; Sobhy, M.; Nassr, E.; Mousa, M.M.; Saad, G. Effect of ZnO nanoparticles addition on thermal, microstructure and tensile properties of Sn–3.5 Ag–0.5 Cu (SAC355) solder alloy. J. Mater. Sci. Mater. Electron. 2013, 24, 3210–3218. [Google Scholar] [CrossRef]

| Test Sample | Onset temperature (°C) | Peak I (°C) | Peak II (°C) | Enthalpy ΔH (J/g) |
|---|---|---|---|---|
| In-5Sn | 151.2 | 152.1 | - | 27.3 |
| In-12.5Sn | 141.8 | 143.5 | - | 25.4 |
| In-25Sn | 131.8 | 132.9 | - | 23.0 |
| In-30Sn | 128.4 | 129.2 | - | 23.2 |
| In-35Sn | 125.5 | 126.7 | - | 22.3 |
| In-40Sn | 121.7 | 123.0 | - | 21.2 |
| In-50Sn | 118.7 | 119.3 | - | 25.2 |
| In-60Sn | 118.6 | 119.2 | 150.7 | 28.9 |
| In-80Sn | 175.3 | 194.9 | - | 47.8 |
| Test Sample | Peak I (°C) | Peak II (°C) | Peak III (°C) |
|---|---|---|---|
| In-5Sn | 149.7 | - | - |
| In-12.5Sn | 141.1 | 138.6 | - |
| In-25Sn | 130.3 | - | - |
| In-30Sn | 126.6 | - | - |
| In-35Sn | 123.3 | - | - |
| In-40Sn | 120.7 | 26.2 | - |
| In-50Sn | 116.1 | - | - |
| In-60Sn | 142.7 | 116.9 | 48.5 |
| In-80Sn | 186.6 | 117.2 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Tan, X.F.; McDonald, S.D.; Nogita, K. Mechanical Properties and Microstructure of Binary In-Sn Alloys for Flexible Low Temperature Electronic Joints. Materials 2022, 15, 8321. https://doi.org/10.3390/ma15238321
Zhou J, Tan XF, McDonald SD, Nogita K. Mechanical Properties and Microstructure of Binary In-Sn Alloys for Flexible Low Temperature Electronic Joints. Materials. 2022; 15(23):8321. https://doi.org/10.3390/ma15238321
Chicago/Turabian StyleZhou, Jiye, Xin Fu Tan, Stuart D. McDonald, and Kazuhiro Nogita. 2022. "Mechanical Properties and Microstructure of Binary In-Sn Alloys for Flexible Low Temperature Electronic Joints" Materials 15, no. 23: 8321. https://doi.org/10.3390/ma15238321







