1. Introduction
In many situations, the resistance against acid attack is of vital importance for the durability of concrete. Structures in agricultural, agro-food and biogas plants are often exposed to organic acids, such as acetic, citric or lactic acid, which attack the concrete with different intensities [
1]. Here, the solubility of acid salts formed during the degradation of the concrete matrix is an important factor to consider [
2]. Acetic acid was chosen for this study as it has an intermediate aggressiveness in decalcifying and dissolving almost all hydrated and anhydrous phases in the cement matrix. Thereby acetic acid does not form expansive salts as the even more corrosive citric acid [
2].
The type of binder also has a significant influence on the extent of deterioration of a cement paste matrix under acid attack. Concrete made of ordinary Portland cement (PC) usually has a lower acid resistance compared to concrete made of calcium aluminate cement (CAC) or mixtures of Portland cement and supplementary cementing materials (SCMs). The pozzolanic additives with high silicate (such as silica fume) and aluminate content (such as slag, metakaolin and fly ash) lead to a lower concentration of Portlandite in the hydrated paste and densification of the microstructure, both increasing the acid resistance [
3].
For PC exposed to acetic acid, the deterioration process begins with the dissolution of Portlandite from the cement matrix. With a further attack of protons from the acid species, other hydration products, such as the calcium silicate hydrate (CSH) phases, begin to decalcify, ultimately resulting in an amorphous silica gel with small amounts of aluminum and iron [
1]. Beneath the completely decalcified layer of silica gel, a partially degraded layer forms that are characterized by Portlandite dissolution only. This leads to a gradient of the pH with decreasing values from this layer towards the outer layer or the concrete surface [
3].
During the dissolution and decalcification process, the acetate ions form relatively weak complexes with calcium, aluminum and iron (II) ions, as well as stronger complexes with iron (III) ions [
3]. Precipitated ferric hydroxide appears as a characteristic light-brown discoloration at the deterioration front. The formation of a deteriorated layer, combined with the precipitation of acid salts and other secondary phases within this deteriorated layer, leads to an increasingly protective layer on the surface that can slow down the acid attack by limiting the amount of acid reaching the undamaged core [
4].
Modeling acid attack of cementitious materials is a challenging task due to the multi-component and multi-scale porous nature of cementitious materials [
5,
6,
7]. A fundamental modeling approach that couples the chemistry is needed, i.e., reaction thermodynamics and kinetics [
8,
9,
10] and transport phenomena with a morphological nature of the diffusion coefficient (e.g., porosity effects) [
11] and multi-scale distribution of mineral phases [
12]. To avoid enormous computational costs arising from an explicit description of heterogeneities, only homogenized (averaged) materials models are used, neglecting heterogeneity descriptions and fundamentals occurring across a range of scales. They rely on measurements of effective (overall lumped) parameters describing transport (e.g., effective diffusion coefficient), reaction rate and neutralization capacity, each of which results from lumping the many underlying fundamental processes. First, the thermodynamic description needs to be well-understood before considering the kinetics effects. The chemistry description requires equilibrium (thermodynamics), neutralization capacity and dissolution rates of individual phases, as well as the effect of different types and concentrations of acids. Recent reactive transport models consider a thermodynamic (TD) approach for describing the multiple homogenous and heterogeneous equilibria behavior [
8,
9,
13].
Usually, only selected acid concentrations or resulting pH values are investigated for acid exposed concretes. However, understanding the influence of different acid concentrations on the deterioration of hydrated cement paste is of key importance. Therefore, in this study, samples of hydrated cement paste were crushed and mixed into solutions of water and different amounts of acetic acid. The point at which equilibrium was reached was determined based on pH measurements. Afterward, the composition of the solution and potentially remaining solids were analyzed. To evaluate the ongoing reactions during an acid attack on cementitious materials or even to calculate material mass balance flows, it is not enough to focus on the altered cementitious samples only, but an additional chemical analysis of the fluid attack medium is crucial [
14,
15].
In this paper, the thermodynamic modeling of acidification and leaching of a realistic cement system was conducted in a newly developed Matlab code that links IPHREEQC with a recent TD database for cement hydration [
16] and acetic acid solution [
3,
8]. The leaching and acidification cycle consisted of closely simulating the batch experimental setups by adjusting the aggressive solution to hydrated solids ratios for thermodynamic (re-equilibration) calculations. The modeling approach is described in detail. The batch reaction model was developed to enable better estimation of the effects of different acids, their concentrations or even other hydrated paste compositions. The model was split into two sections. In the first step, the cement composition and water/cement ratio used in the experiments were used as input parameters for hydration calculations. In the second step, the resulting solid composition of the hydrated paste was exposed to solutions of water and the different concentrations of acetic acid, which is also used in the experimental part. The equilibrium results of the model are compared to the results of the batch reaction experiments to validate the model. The results of this validation provided improved input parameters for reactive transport models, which can be used to improve the accuracy of service-life predictions. These optimized predictions will help in the engineering process for designing, monitoring and maintaining concrete structures exposed to acid attacks.
3. Results
Before the final input parameters for the model were set, different configurations were tested. It was shown that, as a result of different w/c ratios (0.35, 0.40 and 1.00), no significant differences in the modeled composition of the hardened cement paste were observed after the first model step (hydration). For this reason, the w/c ratio could be set to 0.40, which is slightly higher compared with 0.35 in the experimental part. As described earlier, this w/c ratio is more realistic as the hardened cement paste (mixed with a w/c ratio of 0.35) can further hydrate during the water storage until the age of 28 days. Differences in (capillary) porosity of the hardened cement paste have no influence since the samples were finely ground before being added to the acid solutions, and the dosage was weight-based.
Aside from the different
w/
c ratios, three different options for the input of the cement composition were tested too. Input implementation for the cement composition via phases from the database according to the composition assessed by QXRD analysis and via XRF results, as well as the chemical composition of oxides determined by wet chemistry, MP-AES and CSD according to EN 196-2 and EN 451-1, showed no significant differences. However, the input by means of the oxides determined according to the standards seemed more accurate than both XRF and QXRD results, which was the reason for selecting this option. Modeling results of the phase compositions (in vol. %) of the hydrated cement paste from the hydration step (Model 1) are listed in
Table 3 for the mixture consisting purely of Portland cement (PC) and the mixture with Portland cement and 11% Silica Fume (PC + SF).
After pH equilibrium was reached, the samples showed different colors both for the liquid and solid fractions, as shown in
Figure 1 for selected samples of PC and PC + SF. For the liquid fraction of PC, three different colorations can be observed: colorless in the pH range of 12.6 to 6.4, light brown around 5.8 and, finally, red between 5.3 and 3.8. The solids even show four colors: light grey between pH 12.6 and 11.3, dark grey between 11.0 and 6.6, light brown between 6.4 and 5.3 followed by light grey from 4.8 to 3.8. For PC + SF, the liquid phase was colorless between pH 12.8 and 6.3, yellow around 5.5, dark red between 4.8 and 3.6 and pale red around 2.6. The related solids exhibited the following colorations: light grey between 12.8 and 11.4, dark grey between 11.1 and 10.3, light brown between 9.5 and 5.5, brown grey around 4.8, dark grey around 4.2 and light grey between 3.6 and 2.6.
The intense reddening of the liquid phases for low pH values is probably due to leached iron, which reacts with acetic acid to form red ferric acetate aqueous complex and further hydrolyzes to reddish brown ferric hydroxide colloid, which is very soluble [
19]. The colors of the solids are more difficult to interpret because they may be influenced by colored compounds subsequently precipitated during filtration due to the small amount of water used for washing (see the discussion concerning XRD during drying).
The pH of the suspensions after equilibrium is reached is a good indicator for the dissolution processes that have occurred at different acid concentrations. In
Figure 2a,b, the experimentally measured and the modeled pH is shown in combination with the modeled porosity for different acid concentrations.
Figure 2a shows an overview of the complete range of acid concentrations tested for both mixtures of PC and PC + SF. At acid concentrations below a few mmol of AcH per g hcp, the pH stays almost constant with a value higher than 12 for both mixtures as a result of the buffering effect of Portlandite. With increasing acid concentrations, the pH drops progressively, from gradual to abrupt changes. The pH of the pure PC mixtures starts to drop at higher acid concentrations than for the PC + SF mixture due to a higher content of Portlandite and has, therefore, a stronger buffering effect. The largest drop in the pH value occurs for acid concentrations between 5 and 20 mmol/g
(hcp). The resulting pH values and porosities for this range of acid concentrations are shown in detail in
Figure 2b. For both mixtures, a plateau appears at a pH value of around nine in the modeled curves correlating to a buffering effect. In
Figure 2c, this range of pH values matches the incongruent dissolution (mostly decalcification) of Tobermorite, which is the component of the CSH solid solution with the lowest Ca/Si ratio (see
Figure 2c).
The plateau in the PC + SF curve starts at lower acid concentrations and includes a wider range compared with the plateau in the PC curve. The earlier start of the plateau in the PC + SF curve is again resulting from the lower content of Portlandite, whereas the longer plateau correlates to the higher content of CSH phases (and also the Tobermorite end member) due to the pozzolanic reaction of the silica fume with Portlandite during the (initial, step 1) cement hydration. The measured pH curves from the experiments do not show an obvious plateau as the modeled curves do, indicating a limitation of the thermodynamic model (discussed in
Section 4). Adjacent to the first plateau, there is a second smaller plateau in the modeled pH curves correlating to the dissolution of Thaumasite (see
Figure 2c). Further increasing acid concentrations led to a relatively fast drop in pH until the curves started to flatten out for acid concentrations higher than 20 mmol/g
(hcp). For acid concentrations this high, the model predicts slightly lower pH values than the measurements, indicating the limitation of the used thermodynamic database for low pH regions. Despite the observed discrepancies, in total, the modeled curves are in good agreement with the measured pH values, as no fitting parameter was used in this study.
The modeled porosity for both mixtures is also shown in these figures to illustrate the connection between dissolution reactions and pH change. The biggest increase in porosity takes place in the same range of acid concentrations as the biggest drop in pH. This area also fits the area where most dissolution reactions take place. The porosity of the PC mixtures increases earlier (i.e., at lower acid concentrations) due to the higher content of Portlandite and its dissolution. Furthermore, after Portlandite dissolution, the increase in porosity has a steep incline in the area of the pH plateau related to the Tobermorite dissolution. After the depletion of Ca-based phases, the change in porosity is very small compared to the dissolution of amorphous silica. The porosities of the PC + SF mixtures show a slower increase at lower acid concentrations due to the lower content of Portlandite available for dissolution. With further increase in acid concentrations, the higher content of CSH phases (Tobermorite) and, after its decalcification, amorphous silica in the PC + SF mixture results in a gradual approach of the PC + SF curve to the PC curve (the difference at 20 mmol AcH still being more than 5 vol.%). Dissolution processes naturally include an increase in dissolved ions in the acid solution.
Figure 3,
Figure 4 and
Figure 5 show measured and modeled ion concentrations in the acid solutions for both PC and PC + SF mixtures and different acid concentrations.
Figure 3 shows the measured and modeled concentrations of calcium ions in different acid solutions. The initial incline of the curves is very similar for both mixtures and modeled as well as measured concentrations. Only at higher acid concentrations does the “final” plateau differs between the two mixtures, being higher for PC than for PC + SF, which is expected due to the respectively higher total content of calcium, which forms more aqueous Ca–acetate complexes. The comparison between the model and experiment for both mixtures shows small differences at higher acid concentrations. For the PC mixtures, the differences are very small, and due to a limited range of experimental data, it cannot be verified if the slight underestimation would also be present at acid concentrations higher than 60.2 mmol/g
(hcp). The deviations are more pronounced for PC + SF than PC, where the data also shows more scattering. The plateau of the modeled PC + SF curve is slightly higher than the measured values, from approximately 12 to 126 mmol/g
(hcp). However, the measured calcium contents at the highest acid concentrations (238 and 308 mmol/g
(hcp)) seem to match the modeled values.
Figure 4a,b show the amount of completely dissolved aluminum, iron and magnesium ions in solutions with PC or PC + SF and different acid concentrations. Each curve shows a characteristic threshold value, after which the initial very low amount of dissolved ions (for low acid concentrations) is followed by a relatively steep increase of dissolved ions in a small range of acid concentrations, leading to an almost constant plateau at the maximum concentration. The model captures the range of acid concentration with the biggest increase of dissolved ions in good agreement with measurements for all ions (Al, Fe and Mg) and both mixtures. However, the model overestimates the plateau of maximum ion concentration, indicating that more ions are being dissolved in the model compared to the experiment. The difference is particularly pronounced for aluminum ions. As the initial content of ions contained in the hydrated cement paste is equal for the model and experiment, this difference could be explained both by model uncertainty for low pH Al chemistry and/or measurement artifacts (precipitation of Ca- and Al-acetates during filtration). Experimental artifacts observed in measurement results (XRD, ion concentrations) are further discussed later.
Figure 5 shows the concentration of total dissolved silicon ions for both PC and PC + SF at different acid concentrations. The modeled curves for the two mixtures show a similar progression with matching parts at low and high acid concentrations. At medium acid concentrations, compared with PC, the PC + SF (model and measurement) curves shift towards lower concentrations (similar to Ca and pH results). Both PC and PC + SF exhibit similar shapes with a steep incline followed by a short plateau before the curves drop. The PC + SF mixture shows an incline starting at lower concentrations due to the lower content of Portlandite compared to the PC mixture, which results in an earlier dissolution of CSH phases, including silicon ions. The relatively short ‘one step’ peak in the model curve is due to the lack of a more detailed description of the CSH (Tobermorite end-member) incongruent dissolution, which, according to the experiments, occurs more gradually and shifts towards lower pH values. The almost constant plateau after the ‘one step’ peak is wider for PC + SF as a result of the higher content of CSH phases or, in general, silicon ions in the initial hydrated cement paste compared to the PC mix without silica fume. The experimentally measured values seem to have a differing shape of the curve with almost no dissolved silicon ions for low acid concentrations. The increase in ion concentrations with increasing acid concentrations, including a small plateau at lower silicon levels, in the measured curve is not as steep as it is for the modeled curve. After the low plateau, the ion concentrations increase to a higher plateau over a wider range of acid concentrations compared to the modeled curves. The measured values show a decrease of dissolved ions for high acid concentrations correlating to the model. Thus, the model predicted the difference well between the PC and PC + SF mixtures regarding the onset and initial part of the increase in the concentration of dissolved ions, which are in good agreement with the measurements.
In addition to the analysis of the acid solution, the composition of the remaining solids was investigated after reaching equilibrium.
Figure 6a,b shows the comparison of the composition of oxides in the remaining solids obtained by XRF measurement and model predictions. The main oxides CaO, SiO
2, Al
2O
3, Fe
2O
3 and MgO are to be complemented by the loss of ignition (LOI). LOI consists mainly of water (H
2O) and carbon dioxide (CO
2) that are initially bound to the remaining (degraded) solid samples and released during the calcination treatment procedure before XRF measurement. However, the comparison of the model and experiment was challenging in the low pH range, mainly due to the model assumptions for precipitation of anhydrous SiO
2, which in reality has an unknown (relatively high) amount of water content. More details, also including other challenges in modeling and experimental artifacts, are discussed in
Section 4. For the PC mixture,
Figure 6a shows good agreement in chemical compositions of the remaining solids between the experiment and the model, only at lower acid concentrations up to around 15 mmol/g
(hcp). When exceeding this acid concentration, the model LOI and all oxide elements, except for SiO
2, exhibit a fast drop to zero, while amorphous SiO
2 becomes the only solid phase rapidly reaching 100 wt. %. In the measured data points, this systematic change in LOI is not visible. Here the oxides remain more or less constant at acid concentrations higher than 15 mmol/g
(hcp).
The PC + SF mixture in
Figure 6b shows a similar progression of up to around 13 mmol/g
(hcp), which is slightly lower than pure PC; the modeled curves match the measured oxide composition. At higher concentrations, the LOI and the oxides, except for SiO
2, drop to zero, while SiO
2 reaches 100 wt. %. Again, the measured oxide fractions remain almost constant for higher acid concentrations.
Aside from the chemical composition, the model also predicted the phase (mineralogical) composition of the remaining solids. Such modeling results are shown in
Figure 7a,b as volume fractions in relation to the initially added hydrated cement paste. In this way, the increase in total porosity with increasing acid concentration due to dissolution processes was quantified.
The differences obtained due to the SF addition match the differences already shown in the above figures. The most obvious difference is the bigger fraction of Portlandite in the mixture without silica fume. In PC + SF, the proportion of Portlandite is reduced due to the pozzolanic reaction with the amorphous SiO2 from the silica fume. The volume fraction of CSH phases is increased compared with the PC mixture as a result of this reaction. The higher content of Portlandite and the resulting increased OH buffering at low acid concentrations leads to the beginning of the decalcification of CSH phases and the dissolution of other shifted phases towards higher acid concentrations for the PC samples, as compared with the PC + SF samples. The lower content of CSH phases in the PC samples leads to a narrower range of acid concentrations at which the (incongruent) dissolution takes place. For both mixtures at acid concentrations around 22 mmol/g(hcp), the remaining solids consist purely of amorphous silica (SiO2), whose water content was not captured by the model.
As the amount of Portlandite has a large effect on the dissolution behavior of the samples at low acid concentrations, the Portlandite content of the initial input and the remaining solids was measured via STA for selected acid concentrations in the lower range. The results from STA measurements of PC and PC + SF samples are listed together with the corresponding values obtained from the model in
Table 4. The measured values for the initial powder samples without leaching in water are lower than the modeled ones, which can be explained by the incomplete hydration after 28 days in the experiment. The relation shifts to higher Portlandite content in the measurements when acid is introduced due to the incomplete dissolution reactions compared to the model. However, the values after an acid attack, and especially the acid concentrations at which Portlandite is dissolved, show completely good agreement between the measurements and model.
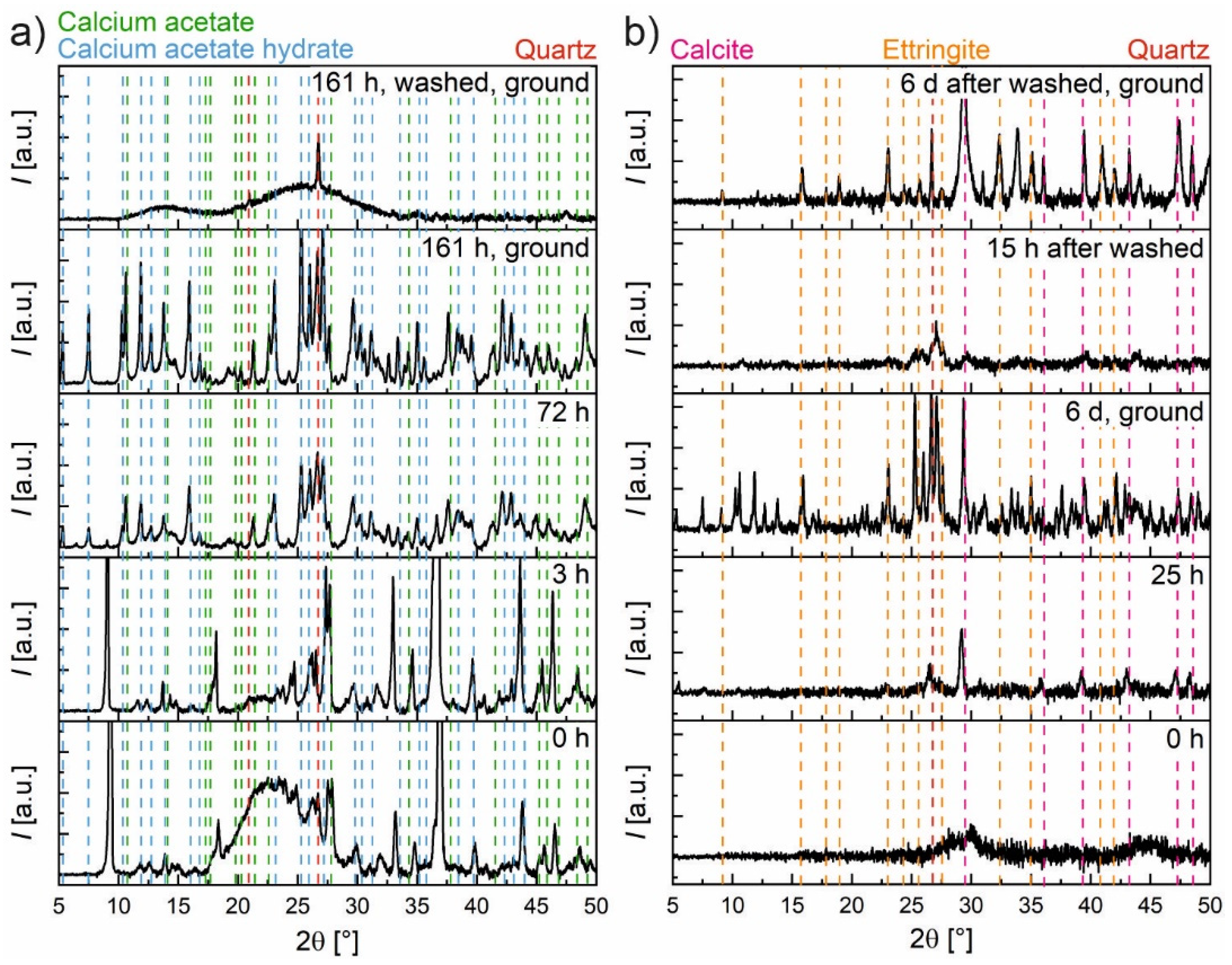
After filtration, the dried solid fractions were investigated by means of XRD.
Figure 8 shows parts of the obtained patterns of hardened cement paste with and without silica fume after leaching with water and with two low concentrations of acetic acid, respectively. In all four samples, Ettringite, Hydrotalcite, Calcite and a small amount of Quartz were found. Portlandite was only detectable for the samples without silica fume with acid concentrations of zero and in a lower quantity of 2.4 mmol/g
(hcp). These observed phases are also in good agreement with the phases predicted by the model (
Figure 7). All predicted crystalline phases were found except for the very small amount of K
2SO
4. The observed decreased fraction of Portlandite is comparable to the finding in the STA measurements and the prediction of the model. Although, according to the model and STA measurements, small amounts of Portlandite should additionally be present for the hardened cement paste with silica fume at 0 mmol/g
(hcp) and for the one without silica fume at 4.8 mmol/g
(hcp). Due to the considerable amount of background scattering, these small fractions could not be determined with XRD.
The filtrate obtained by filtration was used to characterize the ions in the solution in an attempt to ensure that the composition was related to the ones in the solids. Furthermore, only a small and constant amount of water was used for washing in order to prevent the additional dissolution of solids, which should be in equilibrium and remain part of the solid fraction. However, it was observed that with this experimental procedure, the filtration and washing process has a considerable influence on the results of the composition of the solid fraction.
This can be seen in
Figure 9a, where XRD measurements were conducted at different times for a sample without silica fume initially in equilibration with diluted acetic acid (350 mmol/g
(hcp)). Immediately after the wet sludge is removed from the storage tanks, despite the background correction, a pronounced amorphous scattering is visible in addition to the peaks of the crystalline phases. After three hours of drying in the XRD sample holder, this amorphous scattering almost vanished. After a total of about 72 h of drying at room conditions in this XRD sample holder, some peaks disappeared while some new ones appeared, mainly calcium acetate and calcium acetate hydrates. No significant changes took place thereafter in the investigated time period until 161 h. As expected, grounding this sample results in more distinct peaks, which mostly vanish after washing with an extensive amount of water. In the end, Quartz mainly remains. Only the phases of the last sample are in agreement with the known chemistry of altered hardened cement paste and the model prediction. Without thorough washing, different acetates (mainly calcium acetates and calcium acetate hydrates, in other samples also aluminum acetate hydroxide and Gypsum) are always present, depending on the water content.
In
Figure 9b, a sample of PC + SF with a much lower acid concentration (11.2 mmol/g
(hcp)) is shown. The wet sludge (0 h) shows no significant peaks. Drying this wet sludge for 25 h led to more pronounced peaks (mainly Calcite). After drying for 6 d and subsequent grounding, more peaks appeared (mostly calcium acetate hydrates), which now became better detectable due to the improved signal-to-noise ratio. A parallel sample was washed with 360 mL of water immediately after sampling and first measured after 15 h of drying and again after 6 d of drying in a desiccator over silica gel as well as subsequent grounding. It can be seen that washing resulted in fewer peaks than in the dried sludge, mainly Ettringite, Calcite and Quartz. If calcium and aluminum acetate (hydrates) are still present, they were only in traces. Thereby, it appears that washing the initial 12.5 g hardened cement paste with 360 mL of water is sufficient to prevent artifacts in the composition of the solids by subsequent precipitation. However, the model predicts, in contradiction to these observations, the presence of Hydrotalcite, Straetlingite and Thaumasite and the absence of Ettringite (see
Figure 7b). It indeed seems to be the case that the ‘sample 6 d, ground’ contains less or no Ettringite and instead some Thaumasite (most peaks can be attributed to calcium acetate and calcium acetate hydrate,
Figure 7a). Therefore, washing, on the one hand, prevented the enrichment of the solid phase with subsequently precipitated acetate (hydrates) or removed them, but, on the other hand, it had an influence on the phase composition probably by changing ion concentrations and pH values and thus the stability of phases.
The solids investigated in this study via XRF represent a similar state as the XRD pattern after ‘161 h, ground’ in
Figure 9a, whereas XRD patterns in other studies [
1,
3,
6,
19,
20,
21,
22] investigated bulk samples, which are more comparable to the example after ‘161 h, washed, ground’ in this figure.
Therefore, it can be concluded that the amount of calcium in the solids determined in this study tends to be too high, whereas the calcium in the solution tends to be too low. This error due to the filtration and washing process is expected to be negligible for low concentrations of acetic acid (there are almost no acetates and acetate hydrates detectable in
Figure 8) and to increase with an increasing concentration of acetic acid and, therefore, the increasing presence of acetate ions (see
Figure 6).
After these considerations, the question arises of which experimental procedure is most suitable for investigating the composition of the solids and solution in the equilibrium state. We suggest using finely ground hardened cement paste instead of bulk specimens if the information on the phases and concentrations at thermodynamic equilibrium or on the kinetics of the reactions involved are to be gained. In the case of bulk samples, they will probably develop different zones with different chemical compositions of the solids due to the influence of diffusion. Thus, the solution will not be in equilibrium with only a single state of altered cement paste. Investigating the composition of the solution can be performed by pipetting a small and defined volume before filtration, which ensures that the actual equilibrium state of the solution is investigated. Only then should the filtration be carried out, which should be accompanied by extensive washing. The amount of water should be sufficient to dissolute all acetates. Here it would be good to measure the combined volume of solution and also to determine the composition of this combined solution, which should be comparable to the result prior to the filtration process. After this, the composition of the solids should be determined. Nevertheless, determining the exact composition of the solids is much more challenging than for the solution since the washing procedure can lead to dissolution, which then has to be accounted for by calculation. This allows for determining the realistic chemical composition of the solids, although it should be stressed that the actual phase composition can hardly be determined since the investigations by means of XRD cannot be carried out in situ in equilibrium with the surrounding solution, and, therefore, the phase inventory can be changed by sampling and preparation.
4. Discussion
In this paper, the thermodynamic modeling of acidification and leaching of a realistic cement system was conducted in the newly developed Matlab code that links IPHREEQC with a recent TD database for cement hydration [
16] and acetic acid solution [
3,
8]. The leaching and acidification cycle consisted of closely simulating batch experimental setups by adjusting the aggressive solution to hydrated solids ratios for thermodynamic re-equilibration calculations. Therefore, numerous batch experiments with hardened cement paste powder were conducted to determine the composition in equilibrium over a wide range of acid concentrations and pH.
Validation of the TD model is the first fundamental step in modeling acid attack of cementitious materials, as the much more challenging reactive-transport models [
5,
6] should couple the chemistry, i.e., reaction thermodynamics and kinetics [
8,
9,
10] coupled with the transport phenomena. The main link between chemistry and transport is mostly (over) simplified by using an (apparent) diffusion coefficient that should consider the changes in porosity [
11]. Typically, the acidic acid attack on cement-based materials is investigated by using large samples such as cylinders or cubes [
1,
3,
6,
19,
20,
21,
22]. Therefore, the changes in chemical composition and structural and mechanical properties can be studied. However, if one is specifically interested in the chemical composition in thermodynamic equilibrium, this experimental setup has disadvantages. First, chemical reactions are influenced by the rate of diffusion of the ions from the surface of the samples to the inner parts or vice versa. Second, there can be solely one state of thermodynamic equilibrium investigated for a given combination of sample size and volume of the solution, and this is the case when the whole sample has reacted. Dyer [
3] employed an inverse modeling approach to (empirically) estimate the apparent diffusion coefficient based on the measured pH increase of the exposure (external) solution. However, such an estimate of the apparent diffusion coefficients does not consider the time nor space dependency of the microstructural changes (dominantly porosity). It represents an overall diffusion rate throughout the whole specimen, both the degraded (decalcified) and sound (unaffected) cement paste, without considering the changes in porosity and diffusion coefficients throughout the different layers. Thus, first, the thermodynamic description needs to be well understood by adopting the equilibration of powdered cement in acidic solutions of varying concentrations, which represent fundamental inputs to tackle the transport and kinetic effects in the bulk samples.
During the acid attack of cementitious materials, the pH of the pore solution decreases, increasing the solubility equilibrium concentrations, e.g., of calcium, silicon and aluminum ions in the cement paste matrix, according to Le Chatelier’s principle of equilibrium law. This is because, during the acid attack, the ingress of protons removes OH
− from the equilibrium by forming water molecules (water dissociation constant). Likewise, organic acid anions, e.g., the acetate focused in this study, form aqueous complexes, namely metal-acetates, resulting in more dissolution of the corresponding solids. The main objective of this study is to validate the equilibrium results of the model by comparison with the results of the batch reaction experiments specially designed and performed in the current paper to reach this objective. Despite the observed discrepancies, we argue that the model curves are in good overall agreement with measurement values for the equilibrium concentrations of the dissolved ions (
Figure 2,
Figure 3,
Figure 4 and
Figure 5) and undissolved solids (
Figure 6 and
Figure 7).
Figure 10 shows the deviations between the model and experiment calculated as the relative root square mean error (rRSME).
For the ion concentrations in the solution, the total deviations were calculated for each ion over the full range of acid concentrations, whereas the deviations between oxide compositions of the remaining solids obtained from XRD measurements and modeling were only calculated for lower acid concentrations up to 14.8 mmol/g(hcp). For higher acid concentrations, the discussed challenges in modeling the LOI made comparison impossible. The deviation of the pH curves is calculated for the full range as well as for the lower range of acid concentrations. To objectively judge the agreement, one should also consider that all model parameters are fundamentally and independently chosen based on chemical composition and the thermodynamic database, and no ‘fitting’ calibration was adapted throughout the whole study.
The sequential dissolution of the phases, Portlandite -> AFm -> CSH -> AFt was in agreement with previous findings for Ca leaching [
2,
3,
23] and is followed by the dissolution of Straetlingite and Thaumasite, which is in agreement with our XRD data. The model was not fully able to predict a gradual smooth decrease of the pH as observed by the measured curves (
Figure 2,
Figure 3,
Figure 4 and
Figure 5). Instead, an obvious plateau indicated a limitation of the thermodynamic model, primarily related to the chosen CSH3T solid solution model. This discrepancy was most obvious when predicting Si concentrations in solution, which still correctly predicts the onset but happens in a too-short pH range, with a one-step-like peak overestimate of the measurement (
Figure 5). This is directly related to the limitation of the CSH3T (Tobermorite end-member) incongruent dissolution, which in reality occurs more gradually, involving the dissolution of more intermediate phases over a wider pH range. For a better agreement with measurements, a more detailed CSH model is needed to capture the incongruent nature of the CSH dissolution even better. The discrete solid solution CSH model proposed by Walker et al. [
24] would be a way to improve predictions (pH, Ca and Si concentrations, as well as more realistic changes in molar volume) that are suitable for implementation in IPHREEQC. However, such a model lacks a description of known CSH structural features and uptake of other elements (e.g., alkalies, aluminum, etc.). To capture both solubility and structural features, Lothenbach et al. [
16] recommended the use of recent truly multi-site mixing models for CSH, but they are still the subject of ongoing research. Moreover, such advanced models are not possible to implement in the used version of IPHREEQC software(Version 3.6.2-15100).
Aside from improvements in modeling CSH solubility, the highest discrepancy observed for the dissolved aluminum ions could not only be attributed to the lack of better model parameters but also to the measurement artifacts, such as precipitation of calcium acetate hydrate and AlOH(acetate)
2 during filtration. The lack of an adequate thermodynamic description would primarily be related to the uncertainties in modeling precipitation of different types of aluminum hydroxide gels/crystals and/or AlOH(acetate)
2 precipitation and complexation reactions [
3]. Improvement of model predictions would require either lower solubility of aluminum precipitates or less stable Al complexation (i.e., lower formation constants).
Regarding the composition of the remaining solids, significant discrepancies between the model and measurements were obtained only in the low pH range, which involved the precipitation of anhydrous SiO2. This is mainly due to the model assumptions for LOI predictions which do not include the unknown amount of water content in the silica gel, while the type of aluminate and iron precipitates are questionable. Namely, aside from the selected AlOHam and FeOOHam, precipitation of other gel phases could be expected as well as AlOH(acetate)2 and FeOH(acetate)2. The LOI predictions are based only on water, CO2 and sulfate release from mineral hydrates (no acetate salts), while no water was assigned to the anhydrous SiO2 (Amor_Sl). Thus, the LOI of the remaining solids is particularly difficult to predict for the very low pH range. This is because the carbon and water release in the samples may originate from the acetate salts, carbonation and presence of unknown water amounts in silica-based and other (Fe- and Al-based) gels. In particular, we highlight here the artifacts experimentally observed (XRD results) related to the precipitations (e.g., acetate salts) occurring during the filtration process.