Influence of Alkali-Activators on Acid Rain Resistance of Geopolymer-Recycled Pervious Concrete with Optimal Pore Size
Abstract
1. Introduction
2. Materials and Methods
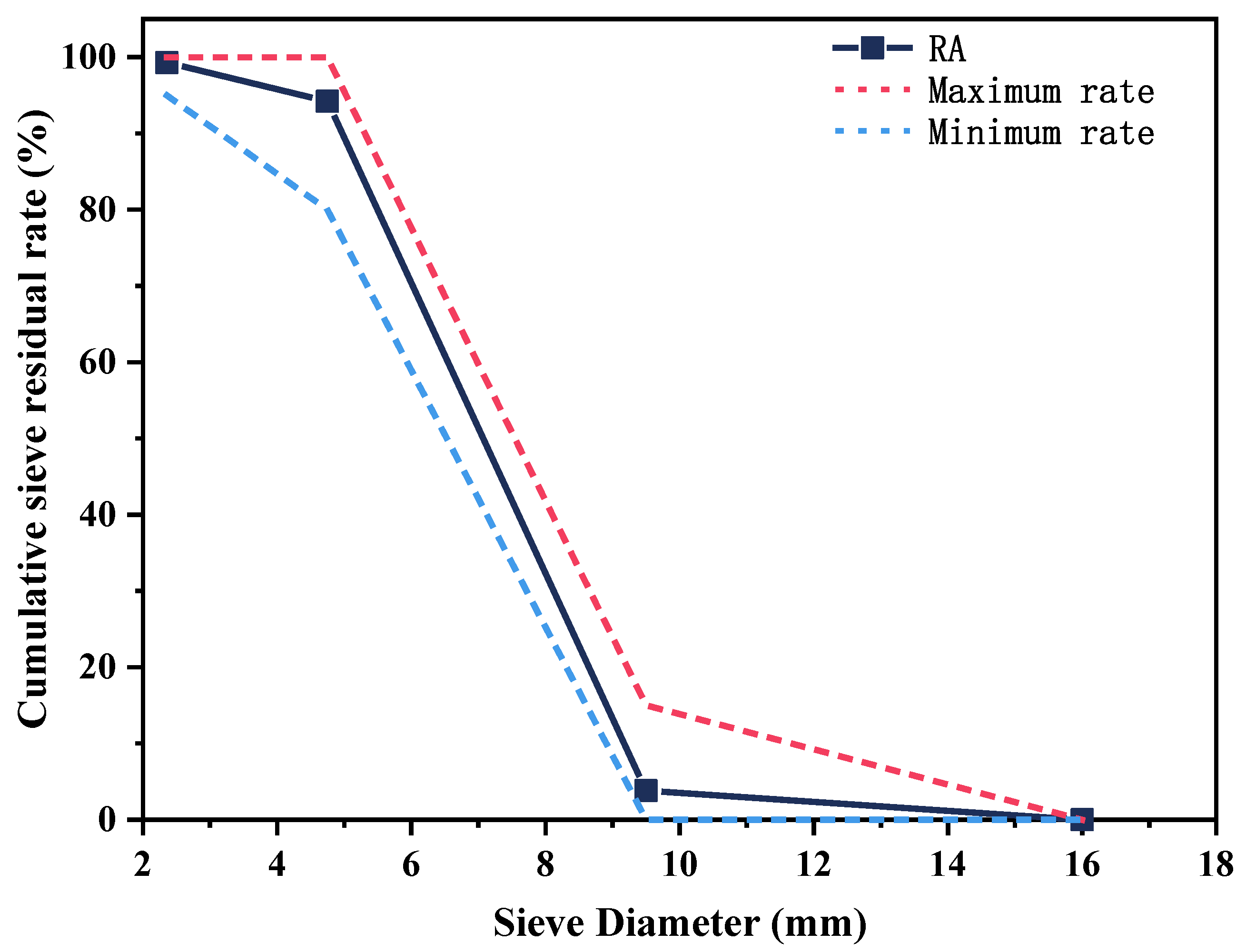
2.1. Raw Materials
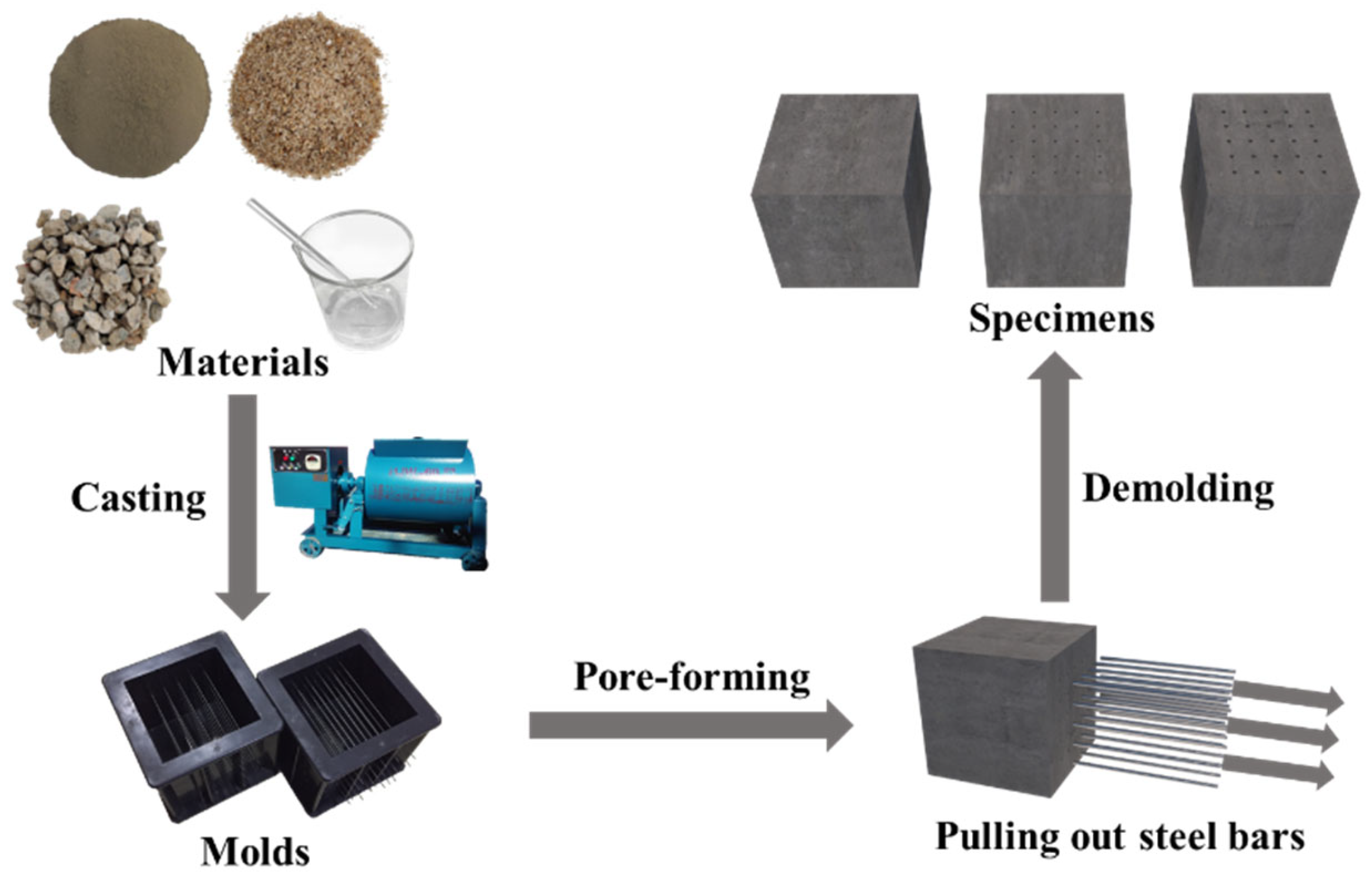
2.2. Mix Proportions and Preparation of Specimens
2.3. Measurements
2.3.1. Mechanical Properties
2.3.2. Permeability Coefficient
2.3.3. Acid Rain Resistance Test
3. Results and Discussion
3.1. Mechanical Properties and Water Permeability of GRPC
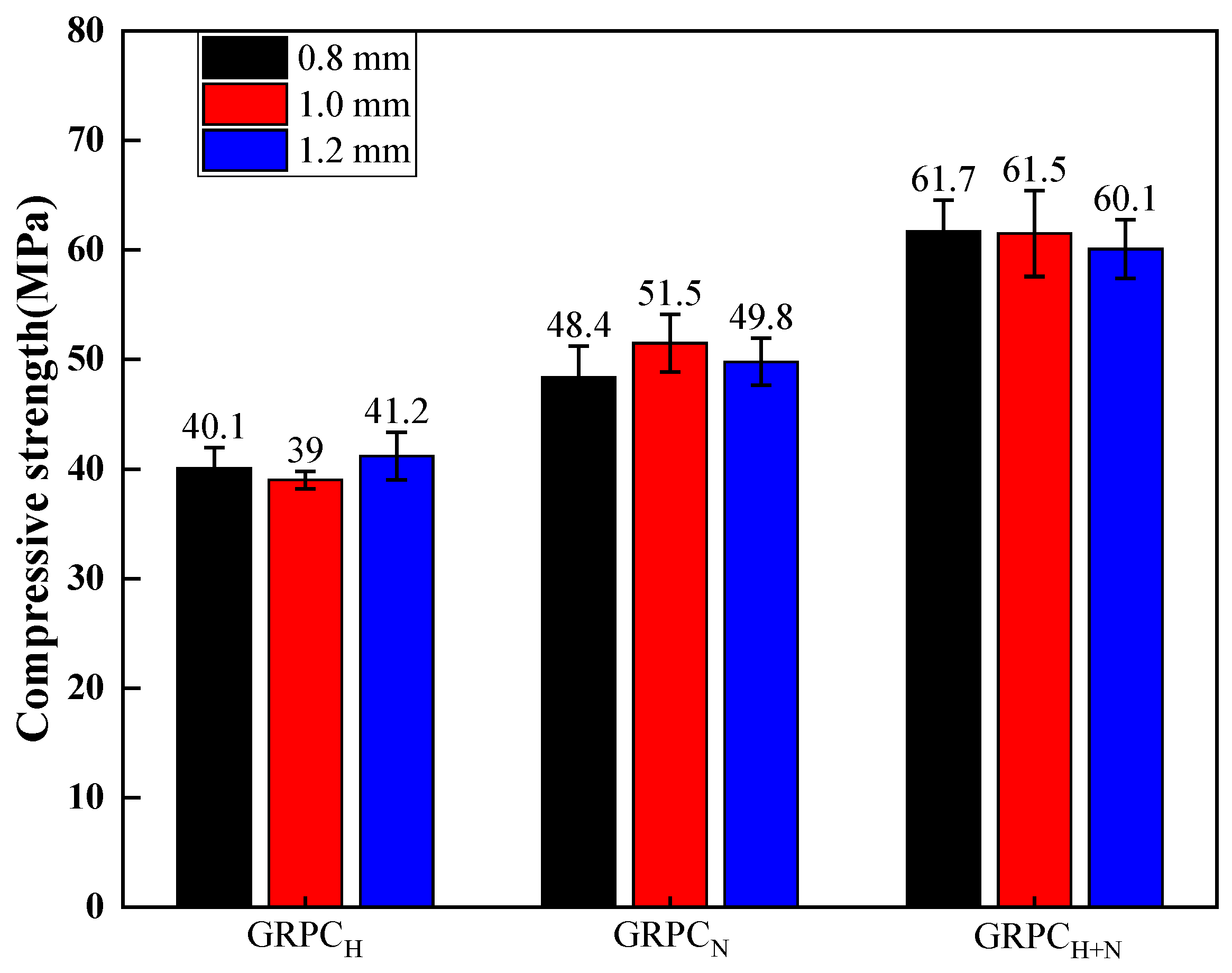
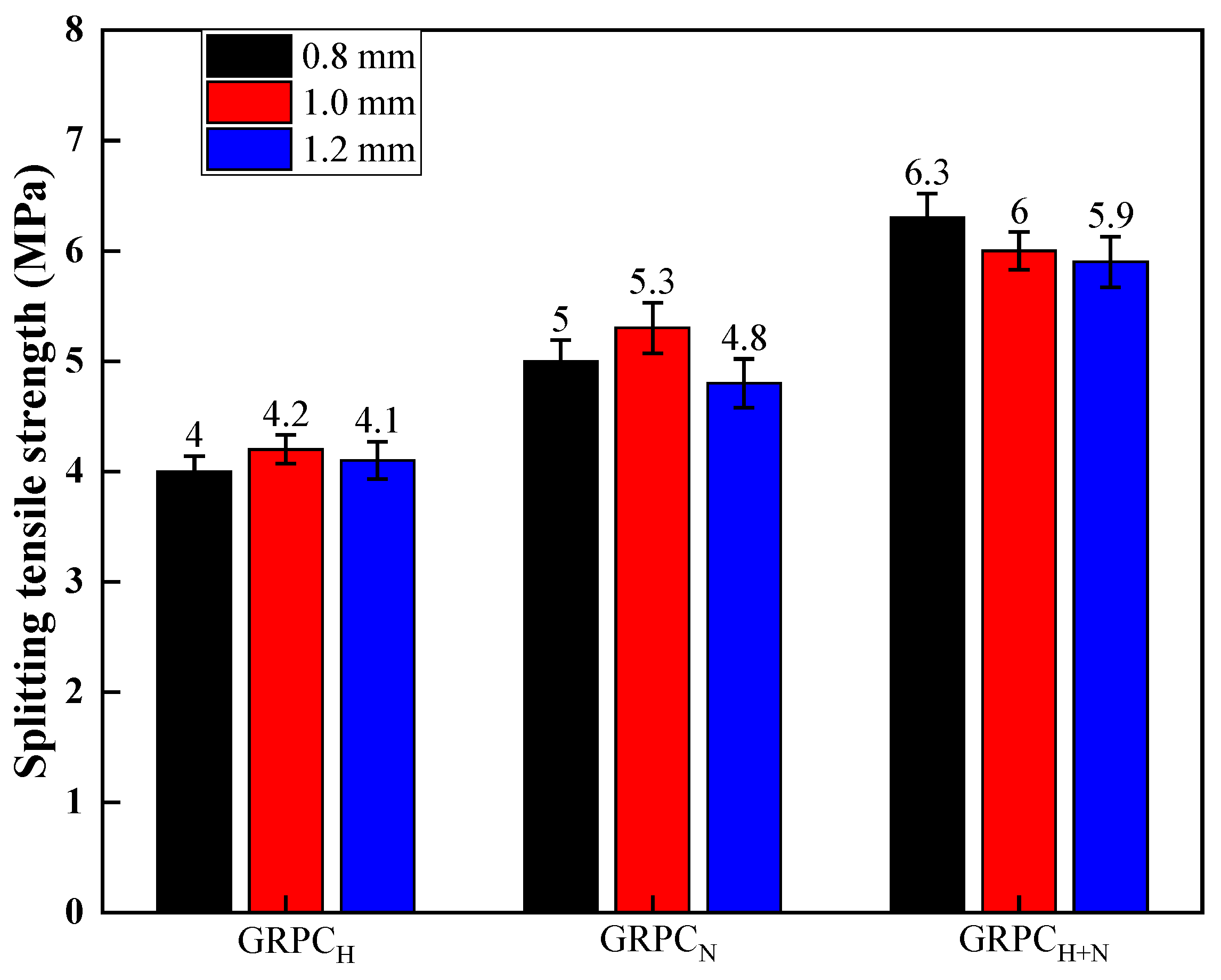
3.1.1. Mechanical Properties
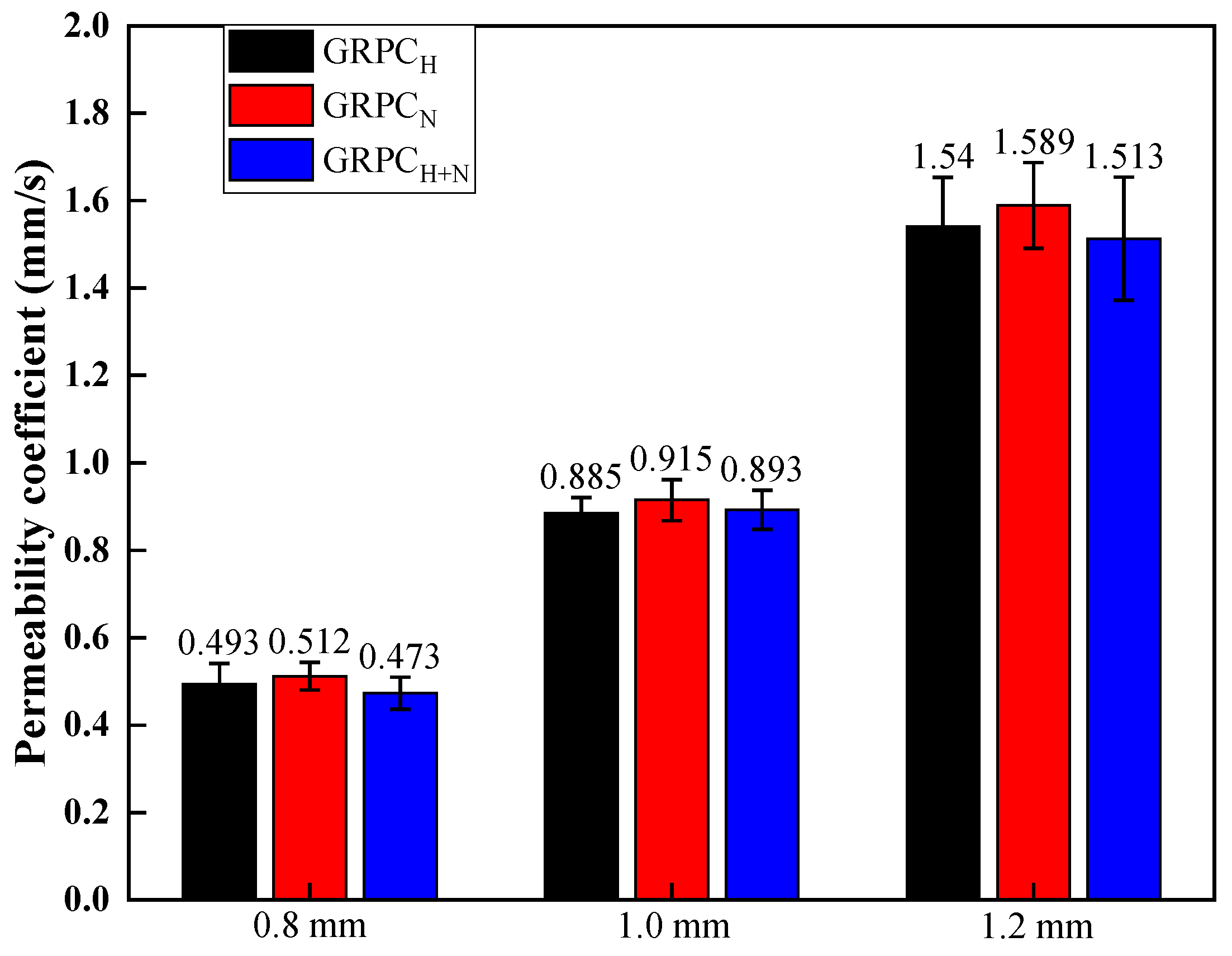
3.1.2. Water Permeability
3.2. Acid Rain Resistance of GRPC
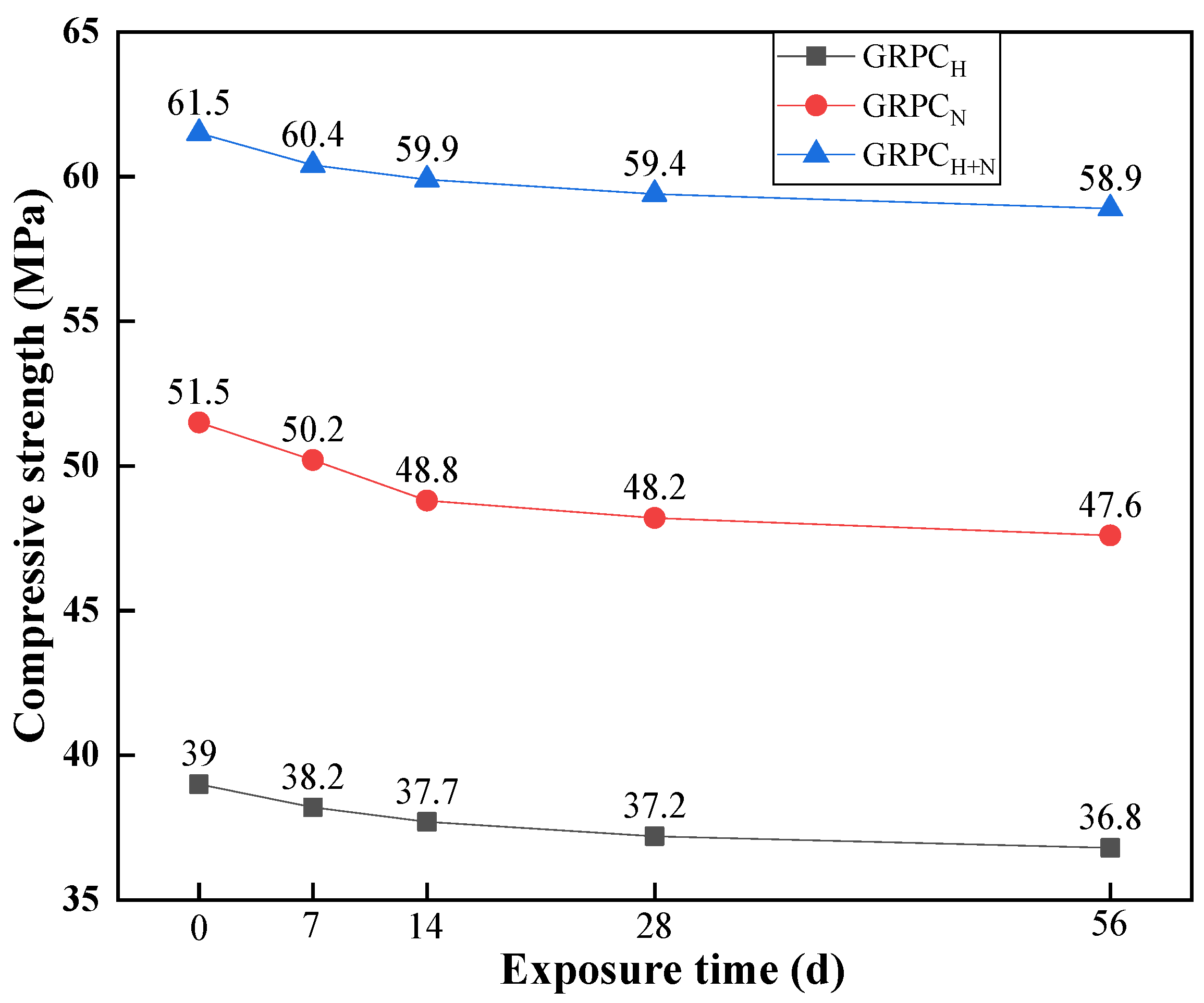
3.2.1. Compressive Strength
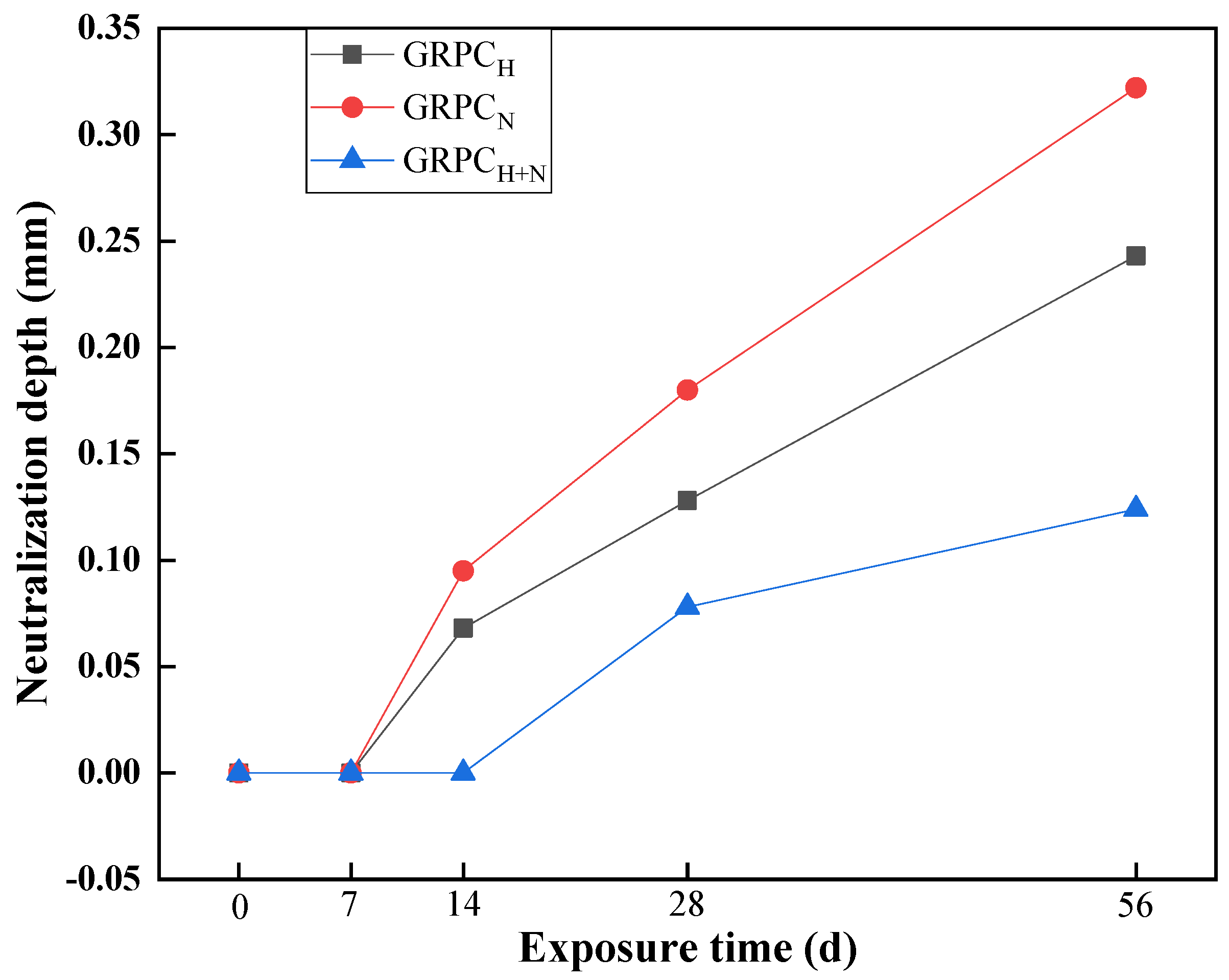
3.2.2. Neutralization Depth
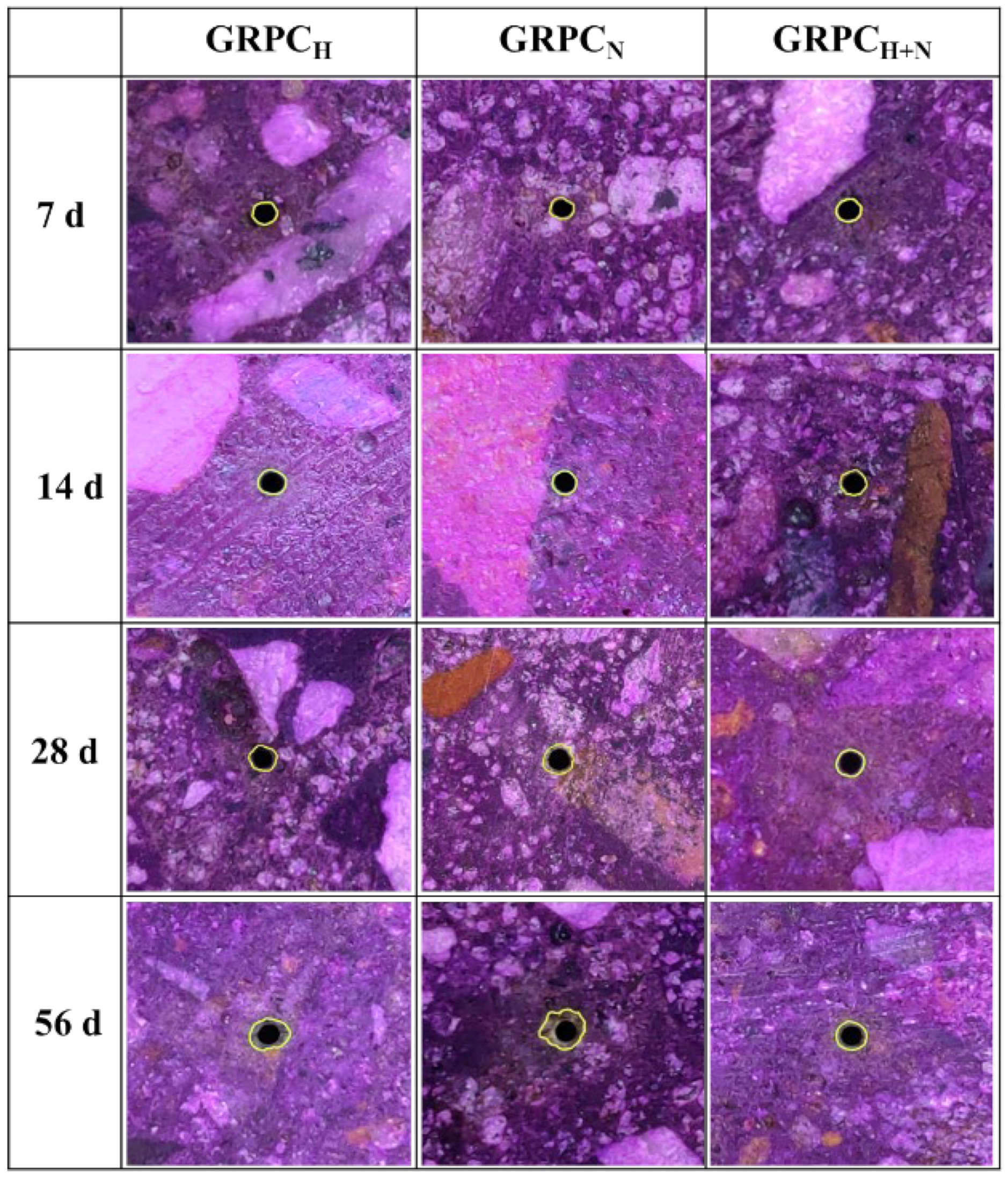
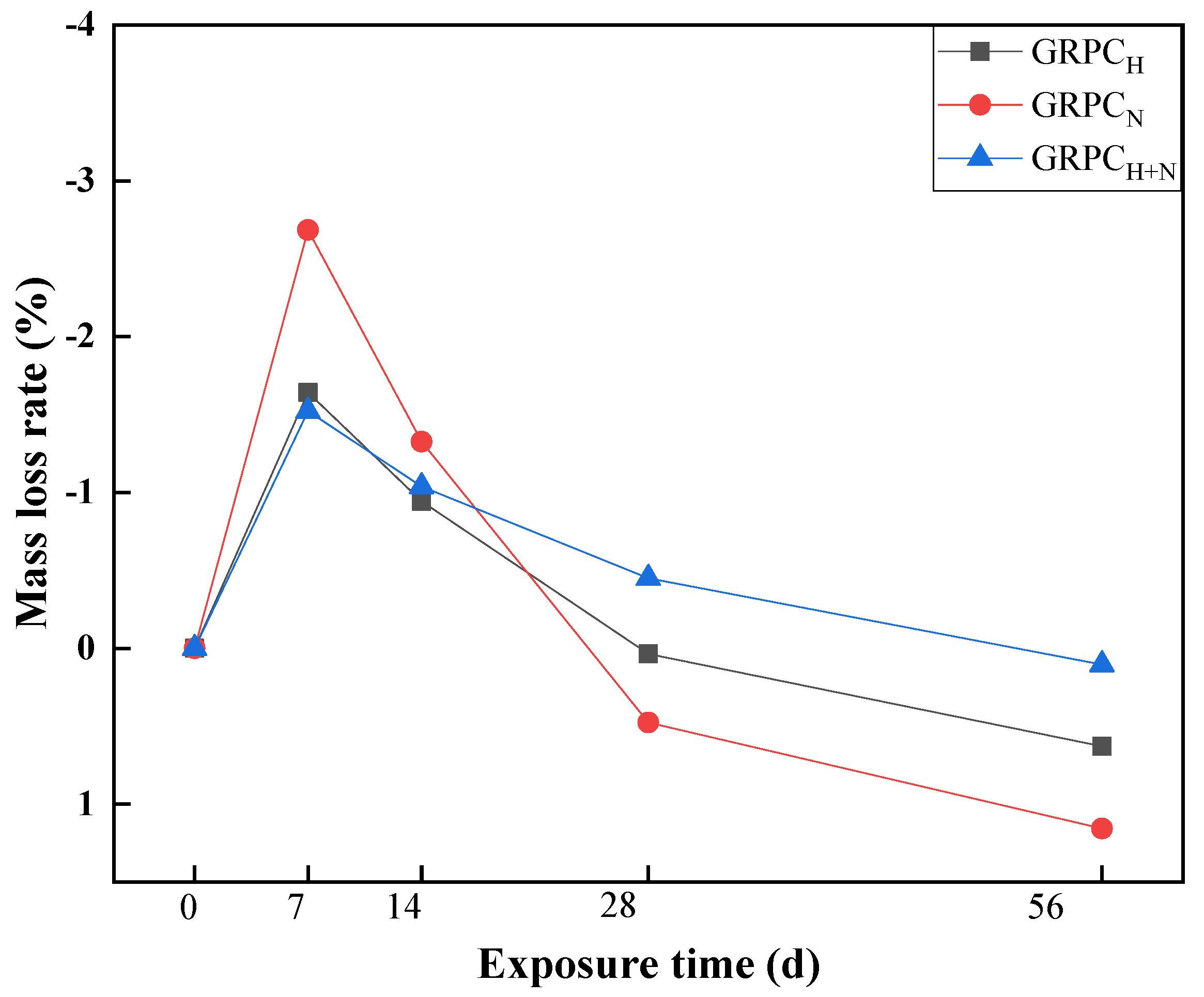
3.2.3. Mass Loss
4. Conclusions
- (1)
- The mechanical properties of GRPC were excellent, except for GRPCH with the pore size of 1.0 mm. The compressive strength of other specimens surpassed 40 MPa and the splitting tensile strength exceeded 4 MPa, meeting the requirements of light and medium traffic roads’ load-bearing capacity. The compressive strength of GRPCH+N reached more than 60.1 MPa, and the splitting tensile strength attained more than 5.9 MPa, meeting the strength requirement of a road for heavy traffic load.
- (2)
- The permeability coefficient depended mainly on the pore size and had little correlation with the matrix properties. The average permeability coefficients of GRPC with pore sizes of 0.8, 1, and 1.2 mm were 0.5, 0.9, and 1.55 mm/s. Considering that the pore size of 0.8 mm was prone to clogging, which affected the permeability of GRPC, the smaller permeability coefficient had the better effect on acid rain purification. Therefore, the optimal pore size of GRPC was 1 mm.
- (3)
- When specimens were exposed to acid solution, the pores of GRPC absorbed the acid solution and reacted with some of the RA connected with the pores, resulting in a reduction of compressive strength. However, the gypsum generated by the reaction blocked the pores to inhibit further corrosion, keeping the stability of the compressive strength.
- (4)
- The GRPC activated by the mixture of NaOH and Na2SiO3 generated the more stable amorphous three-dimensional network structure, endowing GRPCH+N with better mechanical properties and acid corrosion resistance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.; He, C.; Fu, J.; Xu, D. Enhancement of wear and corrosion resistance of polyvinyl chloride/sorghum straw-based composites in cyclic sea water and acid rain conditions. Constr. Build. Mater. 2019, 223, 133–141. [Google Scholar] [CrossRef]
- Lu, C.; Wang, W.; Zhou, Q.; Wei, S.; Wang, C. Mechanical behavior degradation of recycled aggregate concrete after simulated acid rain spraying. J. Clean. Prod. 2020, 262, 121237. [Google Scholar] [CrossRef]
- Fany, F.; Huz, Q.; Zhangy, Z.; Liu, J.L. Deterioration of compressive property of concrete under simulated acid rain environment. Constr. Build. Mater. 2010, 24, 1975–1983. [Google Scholar] [CrossRef]
- Mahdikhani, M.; Bamshad, O.; Fallah Shirvani, M. Mechanical properties and durability of concrete specimens containing nano silica in sulfuric acid rain condition. Constr. Build. Mater. 2018, 167, 929–935. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Liu, G.; Peng, X. Preparation and performance evaluation of an innovative pervious concrete pavement. Constr. Build. Mater. 2017, 138, 479–485. [Google Scholar] [CrossRef]
- Qaidi, S.; Najm, H.M.; Abed, S.M.; Ahmed, H.U.; Al Dughaishi, H.; Al Lawati, J.; Sabri, M.M.; Alkhatib, F.; Milad, A. Fly Ash-Based Geopolymer Composites: A Review of the Compressive Strength and Microstructure Analysis. Materials 2022, 15, 7098. [Google Scholar] [CrossRef]
- Amran, Y.H.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Ahmad Zaidi, F.H.; Ahmad, R.; Al Bakri Abdullah, M.M.; Abd Rahim, S.Z.; Yahya, Z.; Li, L.Y.; Ediati, R. Geopolymer as underwater concreting material: A review. Constr. Build. Mater. 2021, 291, 123276. [Google Scholar] [CrossRef]
- Ren, B.; Zhao, Y.; Bai, H.; Kang, S.; Zhang, T.; Song, S. Eco-friendly geopolymer prepared from solid wastes: A critical review. Chemosphere 2021, 267, 128900. [Google Scholar] [CrossRef]
- Assi, L.; Carter, K.; Deaver, E.; Anay, R.; Ziehl, P. Sustainable concrete: Building a greener future. J. Clean. Prod. 2018, 198, 1641–1651. [Google Scholar] [CrossRef]
- Assi, L.N.; Carter, K.; Deaver, E.; Ziehl, P. Review of availability of source materials for geopolymer/sustainable concrete. J. Clean. Prod. 2020, 263, 121477. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, H.J.; Zhou, C.H.; Wang, H. Geopolymer from kaolin in China: An overview. Appl. Clay Sci. 2016, 119, 31–41. [Google Scholar] [CrossRef]
- Nuaklong, P.; Sata, V.; Chindaprasirt, P. Influence of recycled aggregate on fly ash geopolymer concrete properties. J. Clean. Prod. 2016, 112, 2300–2307. [Google Scholar] [CrossRef]
- Arafa, S.; Milad, A.; Yusoff, N.I.M.; Al-Ansari, N.; Yaseen, Z.M. Investigation into the permeability and strength of pervious geopolymer concrete containing coated biomass aggregate material. J. Mater. Res. Technol. 2021, 15, 2075–2087. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Jitsangiam, P.; Chalee, W.; Rattanasak, U. Case study of the application of pervious fly ash geopolymer concrete for neutralization of acidic wastewater. Case Stud. Constr. Mater. 2021, 15, e00770. [Google Scholar] [CrossRef]
- Sata, V.; Wongsa, A.; Chindaprasirt, P. Properties of pervious geopolymer concrete using recycled aggregates. Constr. Build. Mater. 2013, 42, 33–39. [Google Scholar] [CrossRef]
- Lu, J.-X.; Yan, X.; He, P.; Poon, C.S. Sustainable design of pervious concrete using waste glass and recycled concrete aggregate. J. Clean. Prod. 2019, 234, 1102–1112. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, P.; Liu, H.; Wang, X.; Ge, W.; Hua, M. Resistance to Sulfuric Acid Corrosion of Geopolymer Concrete Based on Different Binding Materials and Alkali Concentrations. Materials 2021, 14, 7109. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- GB/T 25177-2010; Recycled Coarse Aggregate for Concrete. China Building Industry Press: Beijing, China, 2010.
- Kränzlein, E.; Pöllmann, H.; Krcmar, W. Metal powders as foaming agents in fly ash based geopolymer synthesis and their impact on the structure depending on the Na/Al ratio. Cem. Concr. Compos. 2018, 90, 161–168. [Google Scholar] [CrossRef]
- GB/T 50081-2019; Standard Test Methods for Physical and Mechanical Properties of Concrete. China Building Industry Press: Beijing, China, 2019.
- CJJ/T 135-2009; Technical Specification for Pervious Cement Concrete Pavement. China Building Industry Press: Beijing, China, 2009.
- JTG D40-2011; Specifications for Design of Highway Cement Concrete Pavement. China Communications Publishing House: Beijing, China, 2011.
- Strieder, H.L.; Dutra, V.F.P.; Graeff, Â.G.; Núñez, W.P.; Merten, F.R.M. Performance evaluation of pervious concrete pavements with recycled concrete aggregate. Constr. Build. Mater. 2022, 315, 125384. [Google Scholar] [CrossRef]
- Wu, X.; Shen, Y.; Hu, L. Performance of geopolymer concrete activated by sodium silicate and silica fume activator. Case Stud. Constr. Mater. 2022, 17, e01513. [Google Scholar] [CrossRef]
- Cai, J.; Chen, J.; Shi, J.; Tian, Q.; Xu, G.; Du, Y. A novel approach to evaluate the clogging resistance of pervious concrete. Case Stud. Constr. Mater. 2022, 16, e00864. [Google Scholar] [CrossRef]
- Starke, P.; Wallmeyer, C.; Rölver, S.; Göbel, P.; Coldewey, W.G. Development of a new laboratory evaporation measurement device as decision support for evaporation-optimized building. Build. Environ. 2011, 46, 2552–2561. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.; Lukey, A.G.C.; van Deventer, J.S.J. In Situ ATR-FTIR Study of the Early Stages of Fly Ash Geopolymer Gel Formation. Langmuir 2007, 23, 9076–9082. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; van Deventer, J.S.J. Acid resistance of inorganic polymer binders. 1. Corrosion rate. Mater. Struct. 2012, 45, 1–14. [Google Scholar] [CrossRef]
- Xie, Y.; Lin, X.; Ji, T.; Liang, Y.; Pan, W. Comparison of corrosion resistance mechanism between ordinary Portland concrete and alkali-activated concrete subjected to biogenic sulfuric acid attack. Constr. Build. Mater. 2019, 228, 117071. [Google Scholar] [CrossRef]
- Chen, K.; Wu, D.; Xia, L.; Cai, Q.; Zhang, Z. Geopolymer concrete durability subjected to aggressive environments—A review of influence factors and comparison with ordinary Portland cement. Constr. Build. Mater. 2021, 279, 122496. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Khan, H.A.; Khan, M.S.H.; Castel, A.; Sunarho, J. Deterioration of alkali-activated mortars exposed to natural aggressive sewer environment. Constr. Build. Mater. 2018, 186, 577–597. [Google Scholar] [CrossRef]
- Valencia-Saavedra, W.G.; Mejía de Gutiérrez, R.; Puertas, F. Performance of FA-based geopolymer concretes exposed to acetic and sulfuric acids. Constr. Build. Mater. 2020, 257, 119503. [Google Scholar] [CrossRef]
- Krivenko, P.V.; Kovalchuk, G.Y. Directed synthesis of alkaline aluminosilicate minerals in a geocement matrix. J. Mater. Sci. 2007, 42, 2944–2952. [Google Scholar] [CrossRef]
- Kuri, J.C.; Sarker, P.K.; Shaikh, F.U.A. Sulphuric acid resistance of ground ferronickel slag blended fly ash geopolymer mortar. Constr. Build. Mater. 2021, 313, 125505. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Influence of the slag content on the chloride and sulfuric acid resistances of alkali-activated fly ash/slag paste. Cem. Concr. Compos. 2016, 72, 168–179. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Charkhtab Moghaddam, S.; Madandoust, R.; Jamshidi, M.; Nikbin, I.M. Mechanical properties of fly ash-based geopolymer concrete with crumb rubber and steel fiber under ambient and sulfuric acid conditions. Constr. Build. Mater. 2021, 281, 122571. [Google Scholar] [CrossRef]
- Khan, H.A.; Castel, A.; Khan, M.S.H. Corrosion investigation of fly ash based geopolymer mortar in natural sewer environment and sulphuric acid solution. Corros. Sci. 2020, 168, 108586. [Google Scholar] [CrossRef]

| Components | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | K2O | MgO |
|---|---|---|---|---|---|---|---|
| Weight (%) | 44.18 | 26.92 | 9.34 | 1.34 | 11.02 | 1.39 | 1.88 |
| Mixes | Fly Ash | Recycled Coarse Aggregate | Sand | Na2SiO3 | NaOH | Water |
|---|---|---|---|---|---|---|
| GRPCH | 541.3 | 912.7 | 559.4 | - | 80 | 198 |
| GRPCN | 541.3 | 655.4 | 401.7 | 476.2 | - | 58.9 |
| GRPCH+N | 541.3 | 784.1 | 480.6 | 238.1 | 40 | 128.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Yang, W.; Duan, Z.; Liu, H.; Hua, M.; Deng, Q. Influence of Alkali-Activators on Acid Rain Resistance of Geopolymer-Recycled Pervious Concrete with Optimal Pore Size. Materials 2022, 15, 8368. https://doi.org/10.3390/ma15238368
Ma Q, Yang W, Duan Z, Liu H, Hua M, Deng Q. Influence of Alkali-Activators on Acid Rain Resistance of Geopolymer-Recycled Pervious Concrete with Optimal Pore Size. Materials. 2022; 15(23):8368. https://doi.org/10.3390/ma15238368
Chicago/Turabian StyleMa, Quan, Wei Yang, Zhenhua Duan, Hui Liu, Minqi Hua, and Qi Deng. 2022. "Influence of Alkali-Activators on Acid Rain Resistance of Geopolymer-Recycled Pervious Concrete with Optimal Pore Size" Materials 15, no. 23: 8368. https://doi.org/10.3390/ma15238368
APA StyleMa, Q., Yang, W., Duan, Z., Liu, H., Hua, M., & Deng, Q. (2022). Influence of Alkali-Activators on Acid Rain Resistance of Geopolymer-Recycled Pervious Concrete with Optimal Pore Size. Materials, 15(23), 8368. https://doi.org/10.3390/ma15238368








