Experimental and Theoretical Study of Ultra-Hard AlMgB14-TiB2 Composites: Structure, Hardness and Self-Lubricity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Measurements
3. Results
3.1. Combustion Temperature and Morphology of the AlMgB14-TiB2 Precursor
3.2. Phase Composition of the AlMgB14-TiB2 Composite
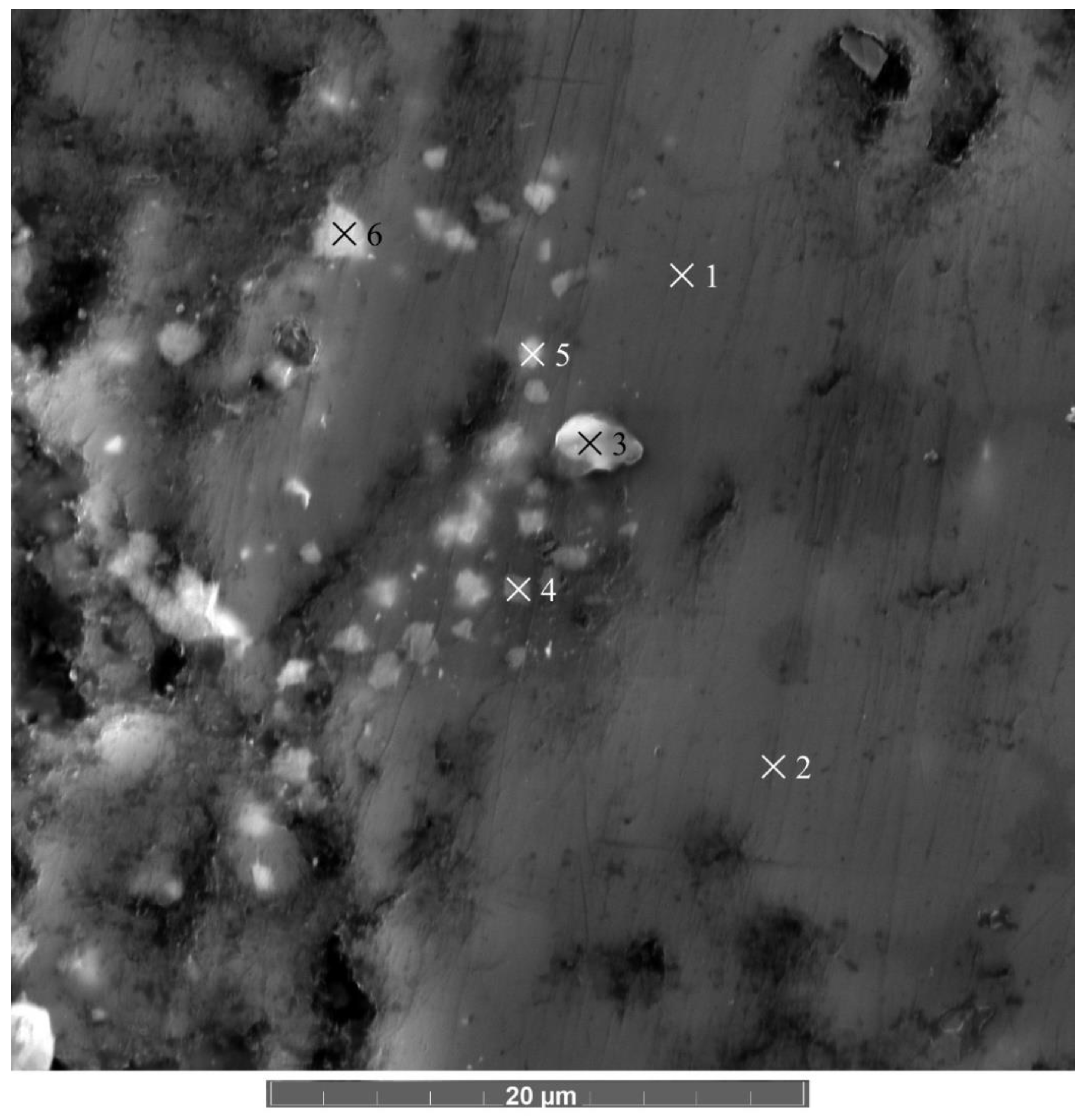
3.3. Microstructure of the AlMgB14-TiB2 Composite
3.4. Hardness and Density of the AlMgB14-TiB2 Composite
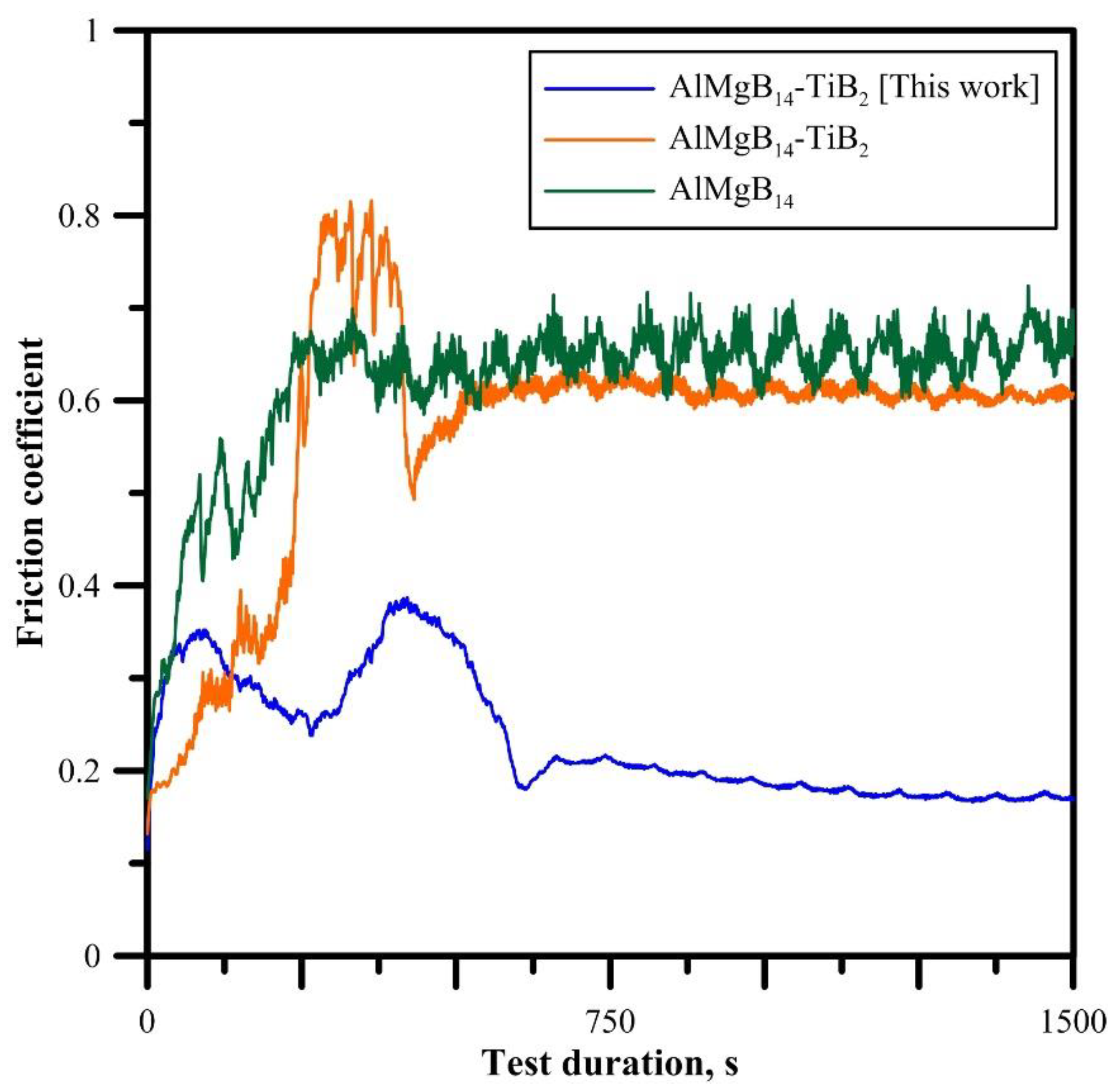
3.5. Tribological Behavior of the AlMgB14-TiB2 Composite
4. Discussion
4.1. Formation of the Al4B2O9 Phase
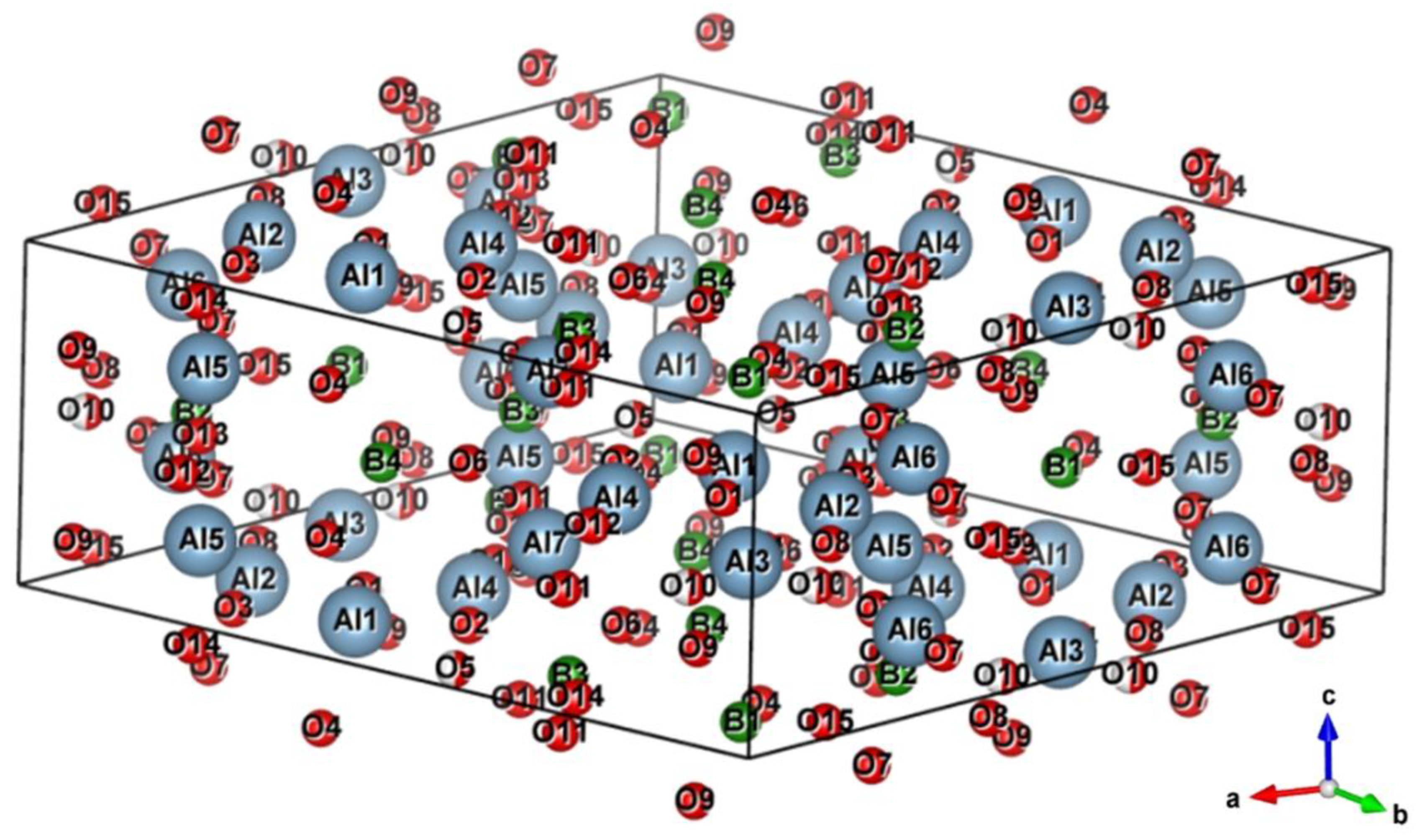
4.2. Structure and Phase Composition of the AlMgB14-TiB2 Composite
4.3. Mechanism of Self-Lubricity of the AlMgB14-TiB2 Composite
5. Conclusions
- The AlMgB14-TiB2 composite was spark plasma sintered from the SHS precursor.
- The main phases in the obtained composite are TiB2, AlMgB14 and Al4B2O9.
- The maximum hardness of the composite is 44.1 GPa at a density of 3.271 g/cm3.
- The presence of oxygen in the form of the Al4B2O9 phase led to unexpectable results: the friction coefficient of the obtained AlMgB14-TiB2 composite in dry conditions against the Al2O3 counter-specimen is 0.18. It is approximately four times lower than the friction coefficient of pure ceramic AlMgB14 (COF, 0.7).
- It was found that Al4B2O9 particles are responsible for the low friction coefficient: the elastic moduli of Al4B2O9 are significantly smaller than the elastic moduli of AlMgB14 and TiB2. Thus, during the friction, due to the plastic deformation of the tribo-contact region, Al4B2O9 particles are squeezed out onto the composite surface, form the lubricating layer and reduce the friction coefficient.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, X.; Yao, K.; Ouyang, J.; Tian, Y. Tribological characteristics and tribo-chemical mechanisms of Al–Mg–Ti–B coatings under water–glycol lubrication. Wear 2015, 326, 68–73. [Google Scholar] [CrossRef]
- Cook, B.A.; Harringa, J.L.; Anderegg, J.; Russell, A.M.; Qu, J.; Blau, P.J.; Elmoursi, A.A. Analysis of wear mechanisms in low-friction AlMgB14–TiB2 coatings. Surf. Coat. Technol. 2010, 205, 2296–2301. [Google Scholar] [CrossRef]
- Tian, Y.; Bastawros, A.F.; Lo, C.C.; Constant, A.P.; Russell, A.M.; Cook, B.A. Superhard self-lubricating AlMgB14 films for microelectromechanical devices. Appl. Phys. Lett. 2003, 83, 2781–2783. [Google Scholar] [CrossRef]
- Cook, B.A.; Harringa, J.L.; Lewis, T.L.; Russel, A.M. A new class of ultra-hard materials based on AlMgB14. Scr. Mater. 2000, 42, 597–602. [Google Scholar] [CrossRef]
- Russell, A.M.; Cook, B.A.; Harringa, J.L.; Lewis, T.L. Coefficient of thermal expansion of AlMgB14. Scr. Mater. 2002, 46, 629–633. [Google Scholar] [CrossRef]
- Matkovich, V.I.; Economy, J. Structure of MgAlB14 and a brief critique of structural relationships in higher borides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 616–621. [Google Scholar] [CrossRef]
- Lei, Y.; Meng, Q.; Zhuang, L.; Chen, S.; Hu, L.; Cheng, H. Friction and wear behavior of AlMgB14–TiB2 composite at elevated temperature. Tribol. Lett. 2014, 56, 435–442. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, J.; Li, F.; Zhu, S.; Li, W.; Yang, J.; Liu, W. Tribological study on a novel wear-resistant AlMgB14-Si composite. Ceram. Int. 2017, 43, 12362–12371. [Google Scholar] [CrossRef]
- Cheng, J.; Ma, J.; Li, F.; Qiao, Z.; Yang, J.; Liu, W. Dry-sliding tribological properties of Cu/AlMgB14 composites. Tribol. Lett. 2014, 55, 35–44. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Matveev, A.E.; Sokolov, S.D.; Boldin, M.S.; Vorozhtsov, A.B. AlMgB14–TiB2 composite materials obtained by self-propagating high-temperature synthesis and spark plasma sintering. Ceram. Int. 2020, 46, 22733–22737. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Nikitin, P.Y.; Vorozhtsov, A.B.; Perevislov, S.N.; Sokolov, S.D.; Ziatdinov, M.H. The use of intermetallic AlxMgy powder to obtain AlMgB14-based materials. Mater. Today Commun. 2020, 22, 100848. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr.-Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Evseev, N.S.; Matveev, A.E.; Nikitin, P.Y.; Abzaev, Y.A.; Zhukov, I.A. A theoretical and experimental investigation on the SHS synthesis of (HfTiCN)-TiB2 high-entropy composite. Ceram. Int. 2022, 48, 16010–16014. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Matveev, A.E.; Zhukov, I.A. Energy-effective AlMgB14 production by self-propagating high-temperature synthesis (SHS) using the chemical furnace as a source of heat energy. Ceram. Int. 2021, 47, 21698–21704. [Google Scholar] [CrossRef]
- Matveev, A.E.; Zhukov, I.A.; Ziatdinov, M.H.; Zhukov, A.S. Planetary Milling and Self-Propagating High-Temperature Synthesis of Al-TiB2 Composites. Materials 2020, 13, 1050. [Google Scholar] [CrossRef]
- Matveev, A.; Promakhov, V.; Nikitin, P.; Babaev, A.; Vorozhtsov, A. Effect of Mechanical Activation of Al-Ti-B Powder Mixture on Phase Composition and Structure of Al-TiB2 Composite Materials Obtained by Self-Propagating High-Temperature Synthesis (SHS). Materials 2022, 15, 2668. [Google Scholar] [CrossRef]
- Nikitin, P.; Zhukov, I.; Matveev, A.; Sokolov, S.; Grigoriev, M.; Vorozhtsov, A. On the Structure and Properties of AlMgB14-TiB2 Composites Obtained from SHS Powders by Spark Plasma Sintering. Materials 2021, 14, 5521. [Google Scholar] [CrossRef]
- Ahmed, A.; Bahadur, S.; Cook, B.A.; Peters, J. Mechanical properties and scratch test studies of new ultra-hard AlMgB14 modified by TiB2. Tribol. Int. 2006, 39, 129–137. [Google Scholar] [CrossRef]
- Kevorkijan, S.D.; Škapin, M.; Jelen, K.; Krnel, A. Meden, Cost-effective synthesis of AlMgB14–xTiB2. J. Eur. Ceram. Soc. 2007, 27, 493–497. [Google Scholar] [CrossRef]
- Jiang, J.; Xie, J.; Zhong, H.; Dong, F.; Liu, N.; Tang, W.; Zhu, H.; Zhang, J. Synthesis and mechanical properties of AlMgB14–Al composite. J. Alloys Compd. 2020, 818, 152910. [Google Scholar] [CrossRef]
- Xie, Z.; DeLucca, V.; Haber, R.A.; Restrepo, D.T.; Todd, J.; Blair, R.G.; Orlovskaya, N. Aluminum magnesium boride: Synthesis, sintering and microstructure. Adv. Appl. Ceram. 2017, 116, 341–347. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Boldin, M.S.; Perevislov, S.N.; Chuvil’deev, V.N. Spark plasma sintering, phase composition, and properties of AlMgB14 ceramic materials. Rus. J. Inorg. Chem. 2021, 66, 1252–1256. [Google Scholar] [CrossRef]
- Nikitin, P.Y.; Zhukov, I.A.; Vorozhtsov, A.B. Decomposition mechanism of AlMgB14 during the spark plasma sintering. J. Mater. Res. Technol. 2020, 11, 687–692. [Google Scholar] [CrossRef]
- Mazhnik, E.; Oganov, A.R. Application of machine learning methods for predicting new superhard materials. J. Appl. Phys. 2020, 128, 075102. [Google Scholar] [CrossRef]
- Mazhnik, E.; Oganov, A.R. A model of hardness and fracture toughness of solids. J. Appl. Phys. 2019, 126, 125109. [Google Scholar] [CrossRef]
- Jianxin, D.; Xing, A.; Zhaoqian, L. Friction and wear behavior of Al2O3/TiB2 composite against cemented carbide in various atmospheres at elevated temperature. Wear 1996, 195, 128–132. [Google Scholar] [CrossRef]
- Jianxin, D.; Tongkun, C.; Lili, L. Self-lubricating behaviors of Al2O3/TiB2 ceramic tools in dry high-speed machining of hardened steel. J. Eur. Ceram. Soc. 2005, 25, 1073–1079. [Google Scholar] [CrossRef]



| Raw Precursors | Particle Size, μm | Purity, % |
|---|---|---|
| Ti | 140 | ≥99.2 |
| Amorphous B | 0.6 | ≥98.7 |
| Al12Mg17 | 15 | ≥99.2 |
| Phase | State | a, Å | b, Å | c, Å | α = β | γ | V, Å3 | E, eV |
|---|---|---|---|---|---|---|---|---|
| TiB2 | Reference | 3.009 | 3.009 | 3.262 | 90.00 | 120.00 | 25.578 | −210.90 |
| Refined | 3.033 | 3.033 | 3.240 | 90.00 | 120.00 | 25.816 | −210.89 | |
| AlMgB14 | Reference | 5.848 | 8.115 | 10.313 | 90.00 | 90.00 | 489.419 | −7149.38 |
| Refined | 5.690 | 8.050 | 10.357 | 90.00 | 90.00 | 474.448 | −7148.67 | |
| Al4B2O9 | Reference | 14.792 | 15.028 | 5.534 | 90.00 | 90.96 | 1230.15 | −34471.7 |
| Refined | 14.460 | 15.036 | 5.475 | 90.00 | 85.51 | 1152.61 | −34469.3 |
| Composition | Hardness Value, GPa | Reference |
|---|---|---|
| AlMgB14 + 50 wt% TiB2 | 40–46 | [4] |
| AlMgB14 + 30 wt% Si | 27 | [8] |
| AlMgB14-5Al | 22.4 | [21] |
| AlMgB14 | 26.7 | [22] |
| AlMgB14 + 50 wt% TiB2 | 37.4 | This work |
| Phase | Young’s Modulus | Young’s Modulus (Experimental Value) * | Bulk Modulus | Shear Modulus |
|---|---|---|---|---|
| TiB2 | 491.8 | 480.1 | 245.9 | 210.8 |
| AlMgB14 | 424.7 | 237.3 | 182.9 | |
| Al4B2O9 | 206.2 | 132.0 | 83.2 |
| Element | Mark 1 | Mark 2 | Mark 3 | Mark 4 | Mark 5 | Mark 6 |
|---|---|---|---|---|---|---|
| wt% | ||||||
| B | 84.4 | 93.1 | 66.7 | 49.0 | 55.8 | 43.9 |
| Al | 8.8 | 8.5 | 8.7 | 17.7 | 15.0 | 20.9 |
| Mg | 6.1 | 6.0 | 5.9 | 9.3 | 7.5 | 9.7 |
| Ti | 0.4 | 2.1 | 0.4 | 0.4 | 0.3 | 0.3 |
| O | − | 0.2 | 18.3 | 23.5 | 21.3 | 25.1 |
| Estimated phase | AlMgB14 | AlMgB14/TiB2 | Al4B2O9 | Al4B2O9 | Al4B2O9 | Al4B2O9 |
| Composition | Friction Coefficient | Reason of Low COF | Reference |
|---|---|---|---|
| AlMgB14 | 0.38 | − | [11] |
| AlMgB14-TiB2 | 0.45−0.65 | − | [7] |
| AlMgB14-TiB2 | 0.12 (800 °C) | TiO2 particles | [7] |
| AlMgB14-Si | 0.19−0.28 | 316L counter-specimen Si particles | [8] |
| AlMgB14-Cu | 0.2 | AlMgB14 particles | [9] |
| AlMgB14-TiB2 | 0.18 | Al4B2O9 particles | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitin, P.; Zhukov, I.; Tkachev, D.; Abzaev, Y.; Marchenko, E.; Vorozhtsov, A. Experimental and Theoretical Study of Ultra-Hard AlMgB14-TiB2 Composites: Structure, Hardness and Self-Lubricity. Materials 2022, 15, 8450. https://doi.org/10.3390/ma15238450
Nikitin P, Zhukov I, Tkachev D, Abzaev Y, Marchenko E, Vorozhtsov A. Experimental and Theoretical Study of Ultra-Hard AlMgB14-TiB2 Composites: Structure, Hardness and Self-Lubricity. Materials. 2022; 15(23):8450. https://doi.org/10.3390/ma15238450
Chicago/Turabian StyleNikitin, Pavel, Ilya Zhukov, Dmitrii Tkachev, Yurii Abzaev, Ekaterina Marchenko, and Alexander Vorozhtsov. 2022. "Experimental and Theoretical Study of Ultra-Hard AlMgB14-TiB2 Composites: Structure, Hardness and Self-Lubricity" Materials 15, no. 23: 8450. https://doi.org/10.3390/ma15238450
APA StyleNikitin, P., Zhukov, I., Tkachev, D., Abzaev, Y., Marchenko, E., & Vorozhtsov, A. (2022). Experimental and Theoretical Study of Ultra-Hard AlMgB14-TiB2 Composites: Structure, Hardness and Self-Lubricity. Materials, 15(23), 8450. https://doi.org/10.3390/ma15238450






