The Structure–Property Relationship of Pyrrolidinium and Piperidinium-Based Bromide Organic Materials
Abstract
:1. Introduction
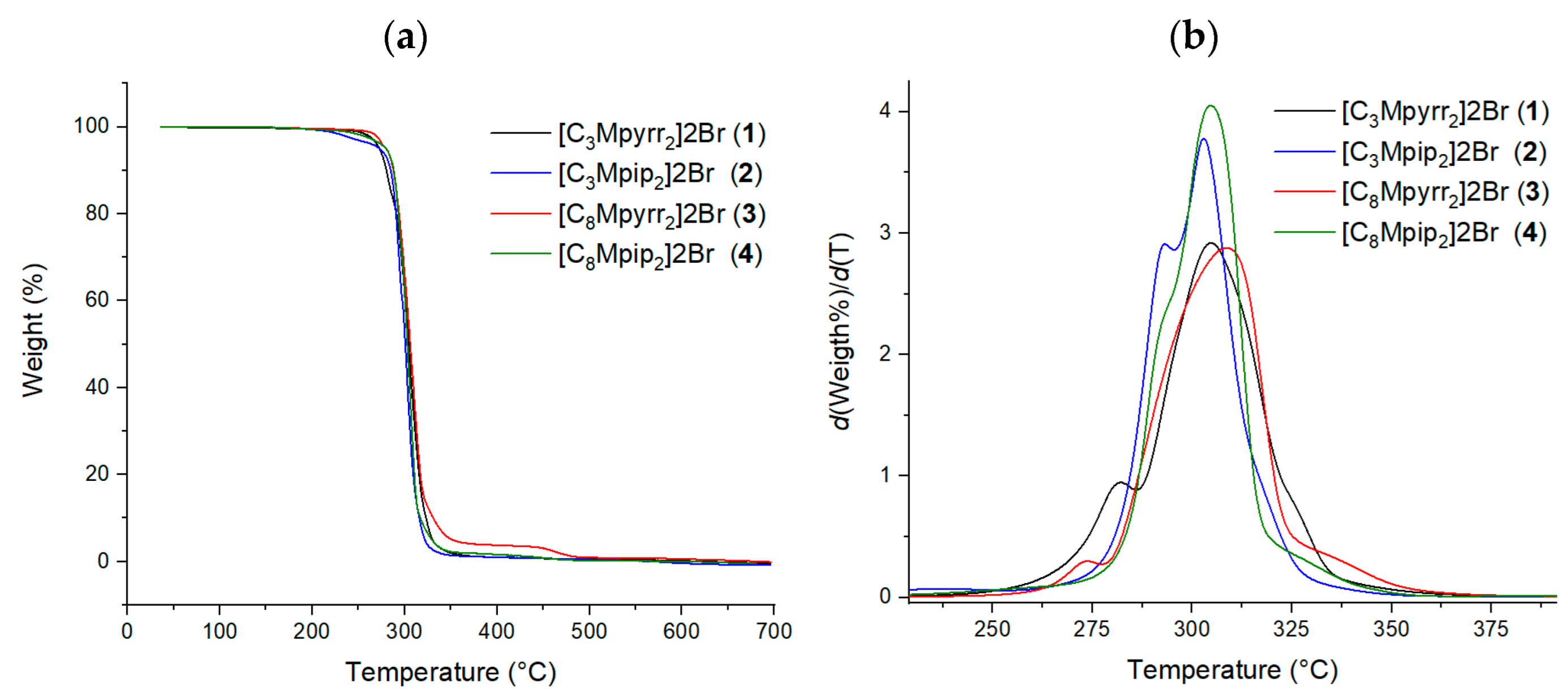
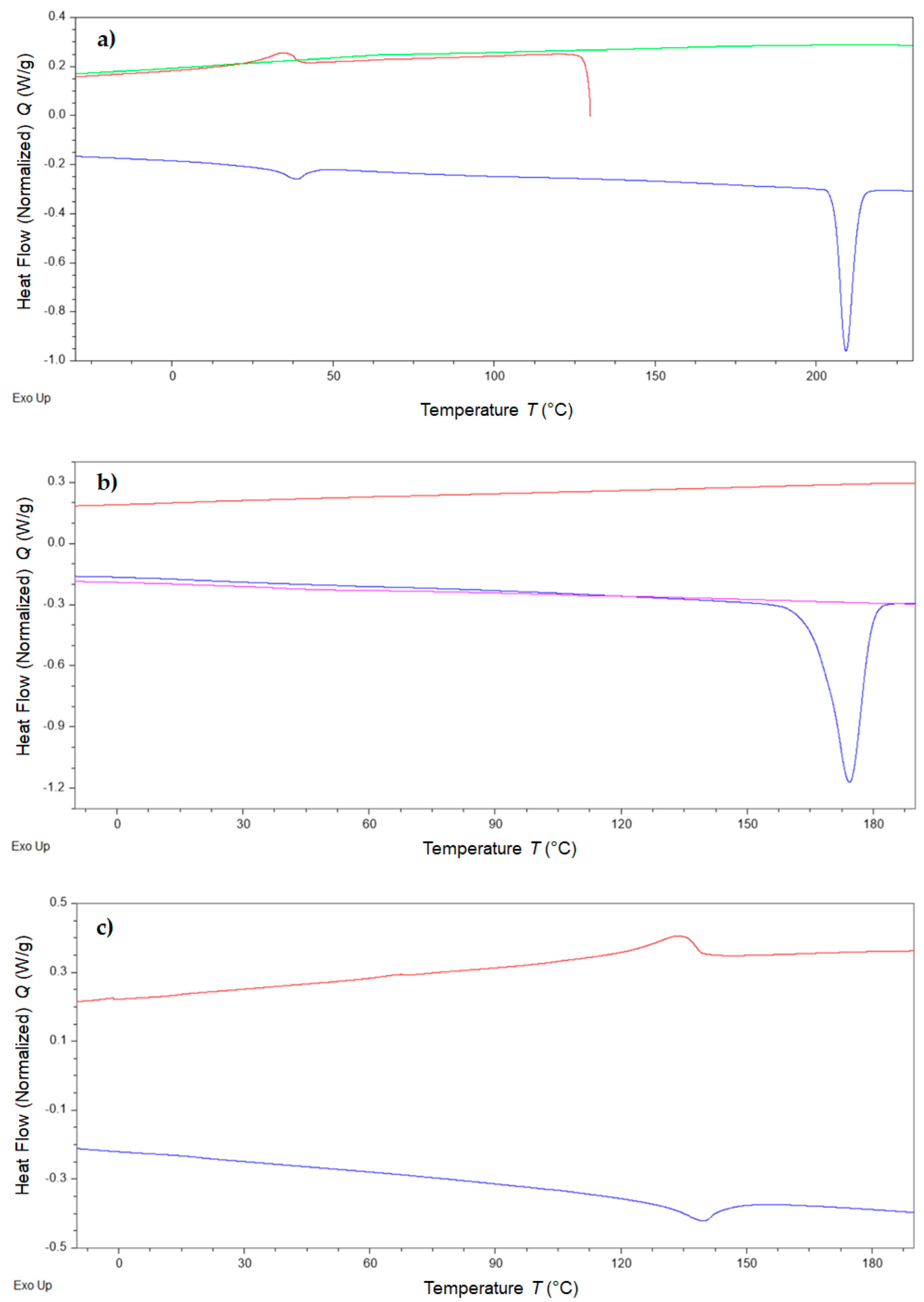
2. Results
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Compounds 1–4
3.2.1. Synthesis of Ionic Liquid 1 [C3Mpyrr2]2Br
3.2.2. Synthesis of Ionic Liquid 2 [C3Mpip2]2Br
3.2.3. Synthesis of Ionic Liquid 3 [C8Mpyrr2]2Br
3.2.4. Synthesis of Ionic Liquid 4 [C8Mpip2]2Br
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Piatti, E.; Guglielmero, L.; Tofani, G.; Mezzetta, A.; Guazzelli, L.; D’Andrea, F.; Roddaro, S.; Pomelli, C.S. Ionic Liquids for Electrochemical Applications: Correlation between Molecular Structure and Electrochemical Stability Window. J. Mol. Liq. 2022, 364, 120001. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical Properties and Structures of Room Temperature Ionic Liquids. 2. Variation of Alkyl Chain Length in Imidazolium Cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.-L.; Wu, K.-J.; He, C.-H. Thermal Conductivity of Ionic Liquids at Atmospheric Pressure: Database, Analysis, and Prediction Using a Topological Index Method. Ind. Eng. Chem. Res. 2014, 53, 7224–7232. [Google Scholar] [CrossRef]
- Zech, O.; Stoppa, A.; Buchner, R.; Kunz, W. The Conductivity of Imidazolium-Based Ionic Liquids from (248 to 468) K. B. Variation of the Anion. J. Chem. Eng. Data 2010, 55, 1774–1778. [Google Scholar] [CrossRef]
- Xue, Z.; Qin, L.; Jiang, J.; Mu, T.; Gao, G. Thermal, Electrochemical and Radiolytic Stabilities of Ionic Liquids. Phys. Chem. Chem. Phys. 2018, 20, 8382–8402. [Google Scholar] [CrossRef]
- Xu, C.; Cheng, Z. Thermal Stability of Ionic Liquids: Current Status and Prospects for Future Development. Processes 2021, 9, 337. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation Thermodynamics of the Tetraoctylphosphonium Bis(Trifluoromethansulfonyl)Imide([P8888]NTf2) and Tetraoctylphosphonium Nonafluorobutane-1-Sulfonate ([P8888]NFBS) Ionic Liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Radai, Z.; Kiss, N.Z.; Keglevich, G. An Overview of the Applications of Ionic Liquids as Catalysts and Additives in Organic Chemical Reactions. Curr. Org. Chem. 2018, 22, 533–556. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Deng, Y. Recent Advances in Ionic Liquid Catalysis. Green Chem. 2011, 13, 2619. [Google Scholar] [CrossRef]
- Javaherian, M.; Saghanezhad, S.J. Synthesis, Characterization and Applications of Dicationic Ionic Liquids in Organic Synthesis. Mini Rev. Org. Chem. 2020, 17, 450–464. [Google Scholar] [CrossRef]
- Guglielmero, L.; Langroudi, M.M.; Al Khatib, M.; de Oliveira, M.A.C.; Mecheri, B.; de Leo, M.; Mezzetta, A.; Guazzelli, L.; Giglioli, R.; Epifanio, A.D.; et al. Electrochemical and Spectroscopic Study of Vanadyl Acetylacetonate–Ionic Liquids Interactions. Electrochim. Acta 2021, 373, 137865. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mero, A.; Mezzetta, A.; Tofani, G.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Novel Access to Ionic Liquids Based on Trivalent Metal–EDTA Complexes and Their Thermal and Electrochemical Characterization. J. Mol. Liq. 2021, 340, 117210. [Google Scholar] [CrossRef]
- Watanabe, M.; Thomas, M.L.; Zhang, S.; Ueno, K.; Yasuda, T.; Dokko, K. Application of Ionic Liquids to Energy Storage and Conversion Materials and Devices. Chem. Rev. 2017, 117, 7190–7239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahadori, L.; Boyd, R.; Warrington, A.; Shafeeyan, M.S.; Nockemann, P. Evaluation of Ionic Liquids as Electrolytes for Vanadium Redox Flow Batteries. J. Mol. Liq. 2020, 317, 114017. [Google Scholar] [CrossRef]
- Jónsson, E. Ionic Liquids as Electrolytes for Energy Storage Applications—A Modelling Perspective. Energy Storage Mater. 2020, 25, 827–835. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic Liquids and Cellulose: Dissolution, Chemical Modification and Preparation of New Cellulosic Materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Berga, L.; Bruce, I.; Nicol, T.W.J.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E.S.J. Cellulose Dissolution and Regeneration Using a Non-Aqueous, Non-Stoichiometric Protic Ionic Liquid System. Cellulose 2020, 27, 9593–9603. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Brzęczek-Szafran, A.; Gaida, B.; Blacha-Grzechnik, A.; Matuszek, K.; Chrobok, A. Bioderived Ionic Liquids and Salts with Various Cyano Anions as Precursors for Doped Carbon Materials. Int. J. Mol. Sci. 2021, 22, 10426. [Google Scholar] [CrossRef] [PubMed]
- Soares, B.F.; Nosov, D.R.; Pires, J.M.; Tyutyunov, A.A.; Lozinskaya, E.I.; Antonov, D.Y.; Shaplov, A.S.; Marrucho, I.M. Tunning CO2 Separation Performance of Ionic Liquids through Asymmetric Anions. Molecules 2022, 27, 413. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.K.; Moshikur, R.M.; Wakabayashi, R.; Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocompatible Ionic Liquid Surfactant-Based Microemulsion as a Potential Carrier for Sparingly Soluble Drugs. ACS Sustain. Chem. Eng. 2020, 8, 6263–6272. [Google Scholar] [CrossRef]
- Florio, W.; Becherini, S.; D’Andrea, F.; Lupetti, A.; Chiappe, C.; Guazzelli, L. Comparative Evaluation of Antimicrobial Activity of Different Types of Ionic Liquids. Mater. Sci. Eng. C 2019, 104, 109907. [Google Scholar] [CrossRef]
- Stachowiak, W.; Wysocki, M.; Niemczak, M. “Bitter” Results: Toward Sustainable Synthesis of the Most Bitter Substances, Denatonium Saccharinate and Denatonium Benzoate, Starting from a Popular Anesthetic, Lidocaine. J. Chem. Educ. 2022, 99, 1604–1611. [Google Scholar] [CrossRef]
- Anderson, J.L.; Ding, R.; Ellern, A.; Armstrong, D.W. Structure and Properties of High Stability Geminal Dicationic Ionic Liquids. J. Am. Chem. Soc. 2005, 127, 593–604. [Google Scholar] [CrossRef] [Green Version]
- Shirota, H.; Mandai, T.; Fukazawa, H.; Kato, T. Comparison between Dicationic and Monocationic Ionic Liquids: Liquid Density, Thermal Properties, Surface Tension, and Shear Viscosity. J. Chem. Eng. Data 2011, 56, 2453–2459. [Google Scholar] [CrossRef]
- Pitawala, J.; Matic, A.; Martinelli, A.; Jacobsson, P.; Koch, V.; Croce, F. Thermal Properties and Ionic Conductivity of Imidazolium Bis(Trifluoromethanesulfonyl)Imide Dicationic Ionic Liquids. J. Phys. Chem. B 2009, 113, 10607–10610. [Google Scholar] [CrossRef]
- Vieira, J.C.B.; Villetti, M.A.; Frizzo, C.P. Thermal Stability and Decomposition Mechanism of Dicationic Imidazolium-Based Ionic Liquids with Carboxylate Anions. J. Mol. Liq. 2021, 330, 115618. [Google Scholar] [CrossRef]
- Ferdeghini, C.; Guazzelli, L.; Pomelli, C.S.; Ciccioli, A.; Brunetti, B.; Mezzetta, A.; Vecchio Ciprioti, S. Synthesis, Thermal Behavior and Kinetic Study of N-Morpholinium Dicationic Ionic Liquids by Thermogravimetry. J. Mol. Liq. 2021, 332, 115662. [Google Scholar] [CrossRef]
- Avila, J.; Lepre, L.F.; Goloviznina, K.; Guazzelli, L.; Pomelli, C.S.; Chiappe, C.; Pádua, A.; Costa Gomes, M. Improved Carbon Dioxide Absorption in Double-Charged Ionic Liquids. Phys. Chem. Chem. Phys. 2021, 23, 23130–23140. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zhang, C.; Dong, R.; Shi, Y.; Wang, Y.; Bai, Y.; Zhang, J.; Cai, M.; Zhou, F.; Liu, W. Physicochemical and Tribological Properties of Gemini-Type Halogen-Free Dicationic Ionic Liquids. Friction 2021, 9, 344–355. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the Effect of the Dicationic Ionic Liquid Structure on the Cycloaddition of CO2 to Epoxides. J. CO2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, D.-K.; Shin, C.-H.; Park, Y.-K.; Wright, P.A.; Lee, W.M.; Hong, S.B. Synthesis, Characterization, and Catalytic Properties of Zeolites IM-5 and NU-88. J. Catal. 2003, 215, 151–170. [Google Scholar] [CrossRef]
- Pernak, J.; Niemczak, M.; Rzemieniecki, T.; Marcinkowska, K.; Praczyk, T. Dicationic Herbicidal Ionic Liquids Comprising Two Active Ingredients Exhibiting Different Modes of Action. J. Agric. Food Chem. 2022, 70, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Mezzetta, A.; Perillo, V.; Guazzelli, L.; Chiappe, C. Thermal Behavior Analysis as a Valuable Tool for Comparing Ionic Liquids of Different Classes. J. Therm. Anal. Calorim. 2019, 138, 3335–3345. [Google Scholar] [CrossRef]
- Armstrong, D.; Anderson, J. High Stability Diionic Liquid Salts. U.S. Patent US 2006/0025598A1, 2 February 2006. [Google Scholar]
- Guglielmero, L.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; D’Andrea, F.; Chiappe, C. Systematic Synthesis and Properties Evaluation of Dicationic Ionic Liquids, and a Glance into a Potential New Field. Front. Chem. 2018, 6, 612. [Google Scholar] [CrossRef] [PubMed]

| DILs | H2O | MeOH | EtOH | iPrOH | CH3CN | (CH3)2CO | EtOAc | CH2Cl2 |
|---|---|---|---|---|---|---|---|---|
| [C3Mpyrr2]2Br (1) | >2.5 | 2.526 | 1.470 | 0.309 | 0.0387 | <0.003 | <0.003 | <0.003 |
| [C3Mpip2]2Br (2) | >2.5 | 0.678 | 0.060 | <0.003 | <0.003 | <0.003 | <0.003 | <0.003 |
| [C8Mpyrr2]2Br (3) | >2.5 | 1.180 | 0.627 | 0.017 | 0.0134 | <0.002 | <0.002 | <0.002 |
| [C8Mpip2]2Br (4) | >2.5 | 0.852 | 0.263 | 0.013 | 0.0086 | <0.002 | <0.002 | <0.002 |
| Compound | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| DIL | [C3Mpyrr2]2Br | [C3Mpip2]2Br | [C8Mpyrr2]2Br | [C8Mpip2]2Br | |
| TGA | Tstart 5% (°C) | 272.8 | 272.3 | 280.3 | 279.8 |
| Tonset (°C) | 271.2 | 286.4 | 287.9 | 285.9 | |
| Tpeak (°C) | 304.7 | 302.9 | 308.6 | 304.6 | |
| DSC | Tm (°C) | 209.2 | - | 174.3 | - |
| ΔmH(kJ/mol) | 7.35 | 22.08 | |||
| Tc (°C) | - | - | - | - | |
| ΔcH(kJ/mol) | |||||
| Ts-s (heating) (°C) | 30.7 | - | - | 141.4 | |
| Δs-s(h)H(kJ/mol) | 1.07 | 3.00 | |||
| Ts-s (cooling) (°C) | 34.2 | - | - | 135.8 | |
| Δs-s(c)H(kJ/mol) | 1.18 | 3.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdeghini, C.; Mezzetta, A.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L.; Guglielmero, L. The Structure–Property Relationship of Pyrrolidinium and Piperidinium-Based Bromide Organic Materials. Materials 2022, 15, 8483. https://doi.org/10.3390/ma15238483
Ferdeghini C, Mezzetta A, D’Andrea F, Pomelli CS, Guazzelli L, Guglielmero L. The Structure–Property Relationship of Pyrrolidinium and Piperidinium-Based Bromide Organic Materials. Materials. 2022; 15(23):8483. https://doi.org/10.3390/ma15238483
Chicago/Turabian StyleFerdeghini, Claudio, Andrea Mezzetta, Felicia D’Andrea, Christian Silvio Pomelli, Lorenzo Guazzelli, and Luca Guglielmero. 2022. "The Structure–Property Relationship of Pyrrolidinium and Piperidinium-Based Bromide Organic Materials" Materials 15, no. 23: 8483. https://doi.org/10.3390/ma15238483





