Influence of Different Aqueous Media on the Corrosion Behavior of B4C-Modified Lightweight Al-Mg-Si Matrix Composites
Abstract
1. Introduction
2. Materials and Methods
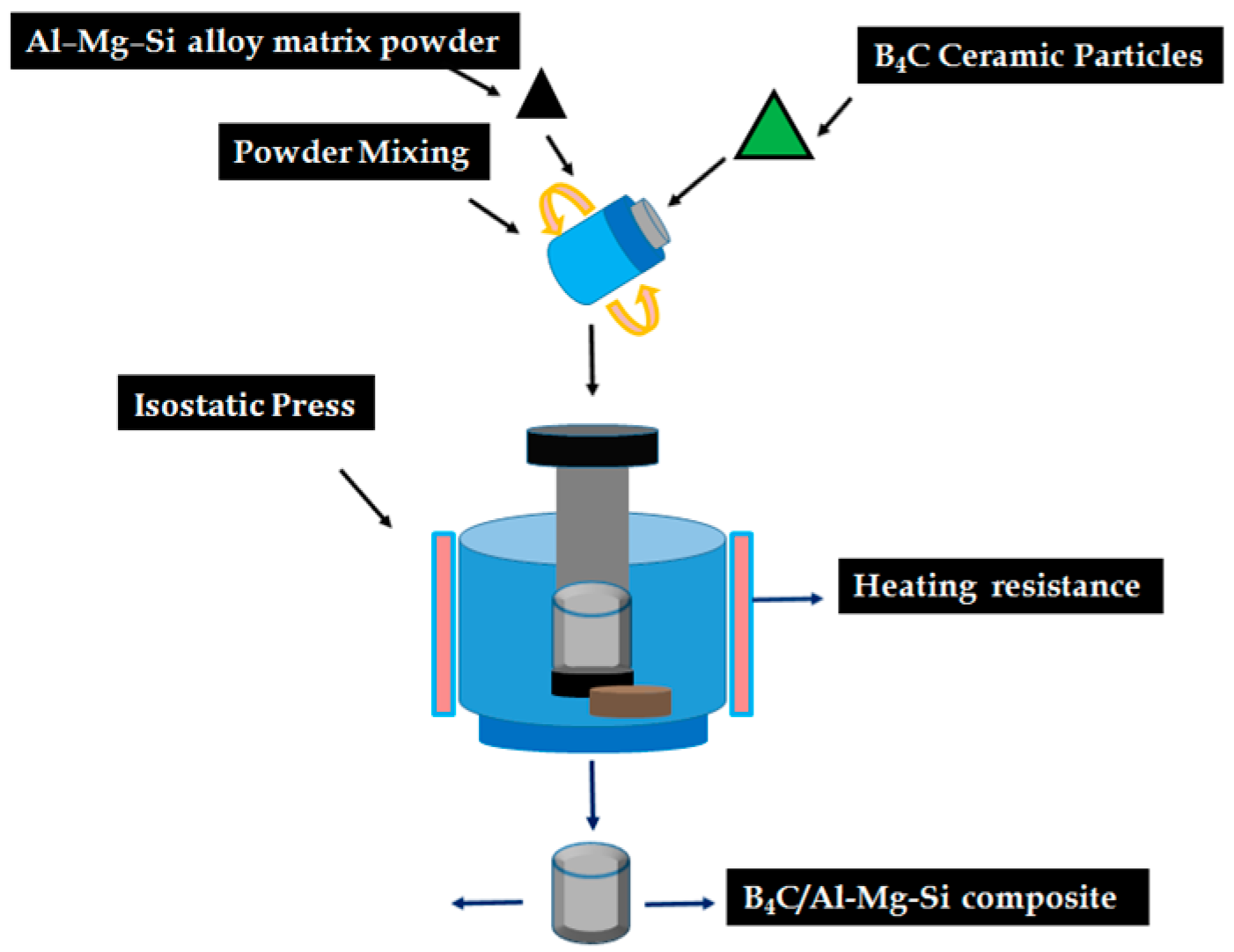
2.1. Materials and Fabrication of B4C/Al-Mg-Si AMCs Composites
2.2. Electrochemical Corrosion Characterization
2.3. Materials Characterization
3. Results
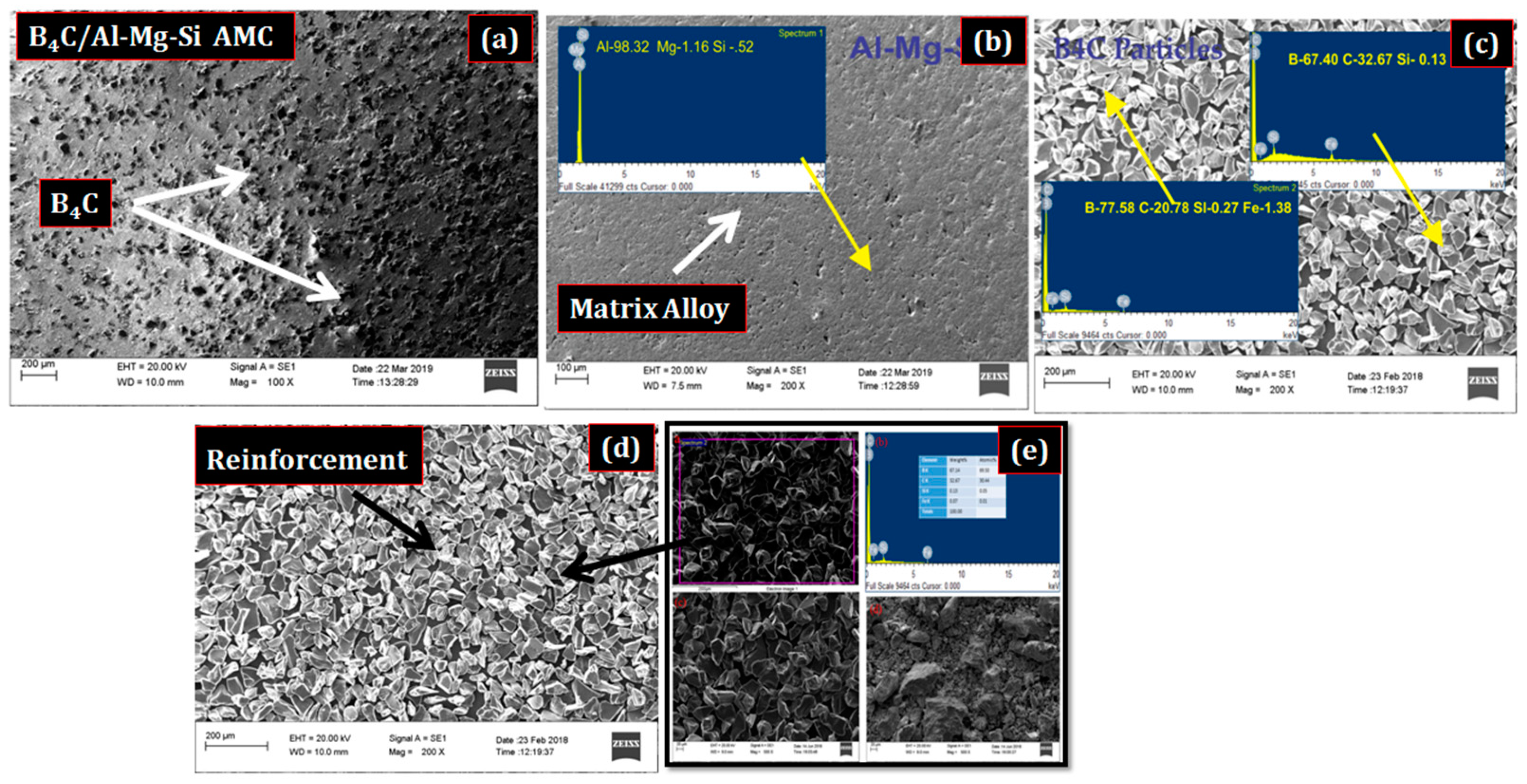
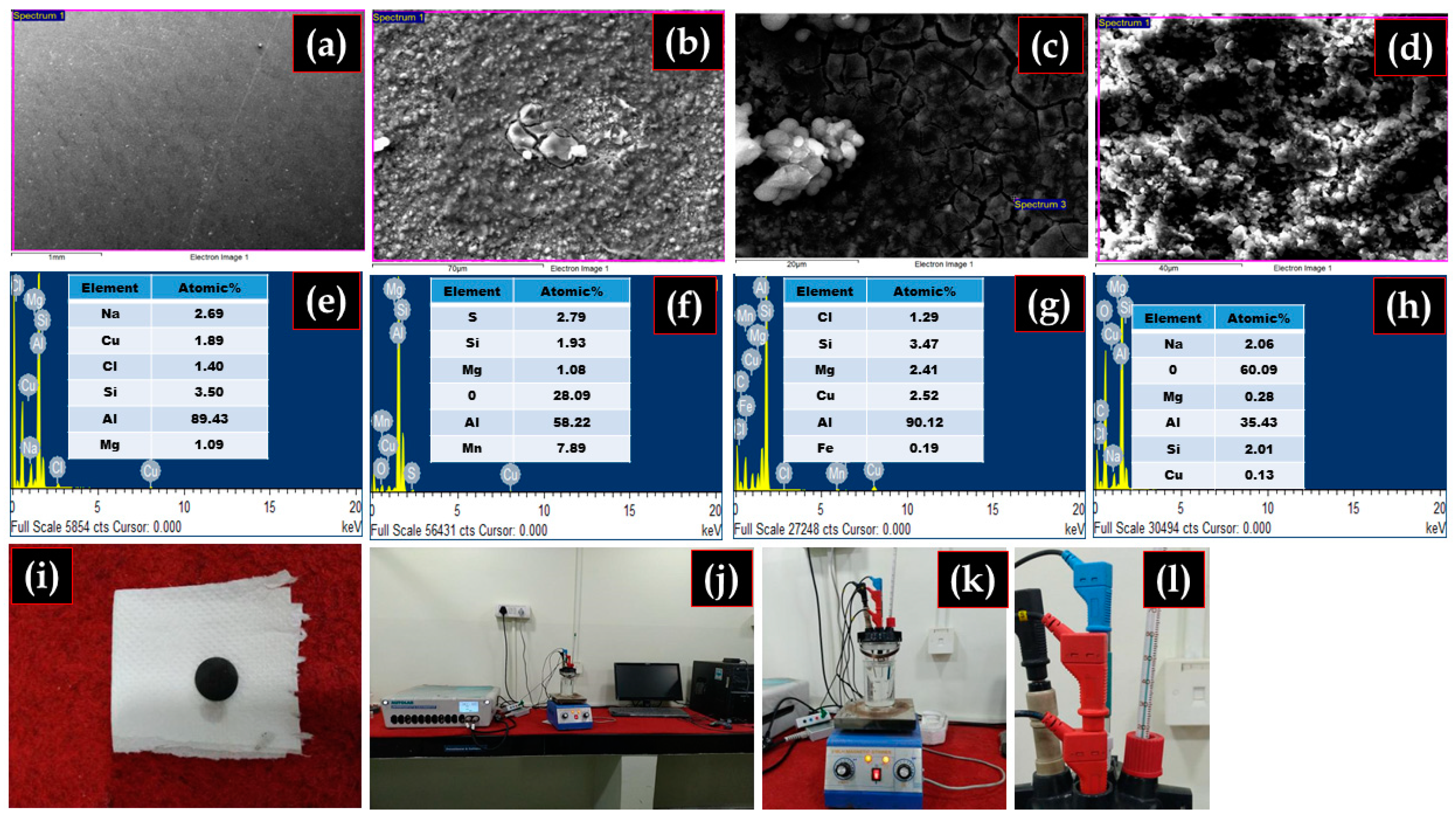
3.1. Composite Fabrication and Morphology
3.2. Hardness and Density Measurements
3.3. OCP Measurements
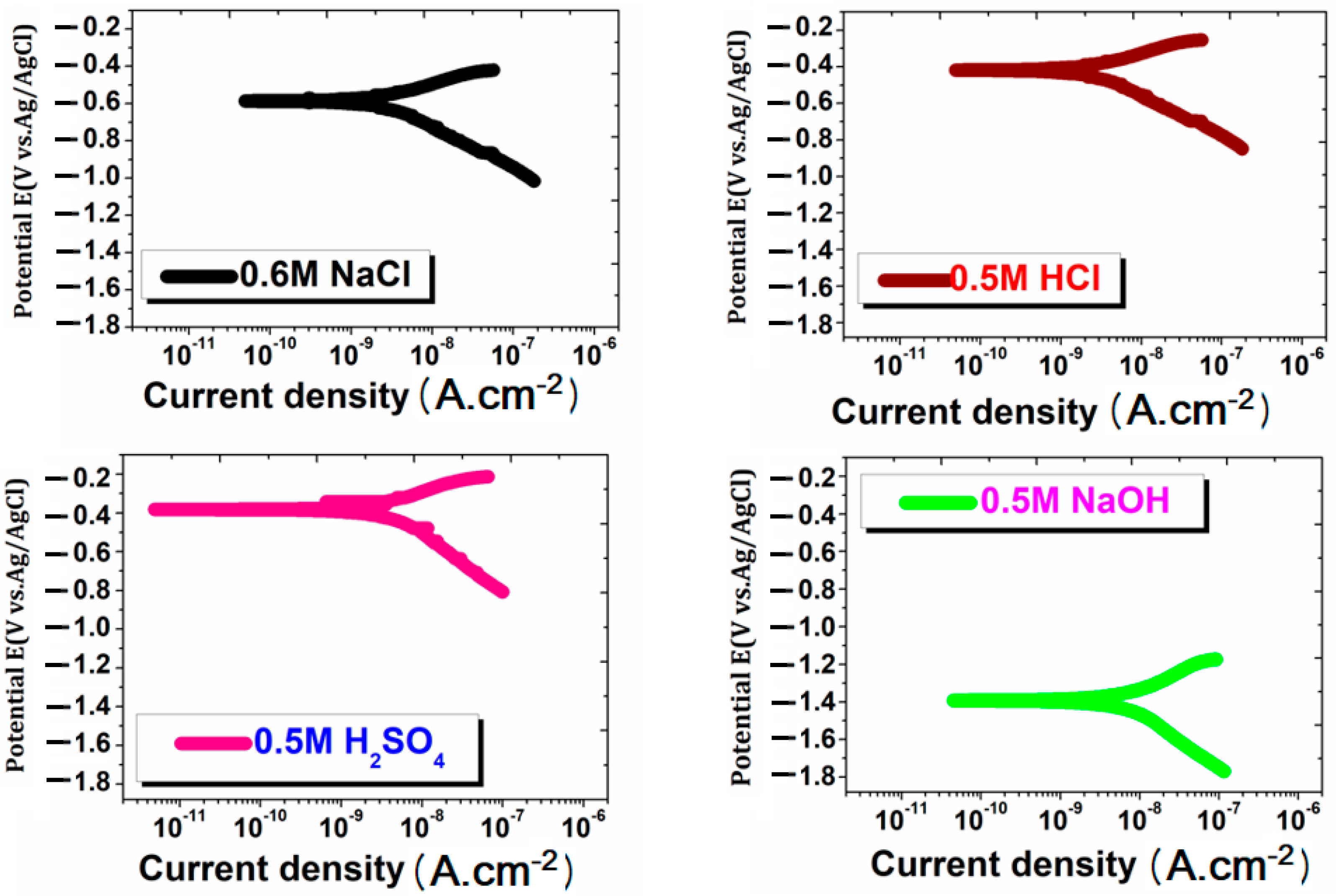
3.4. Potentiodynamic Polarization Plots
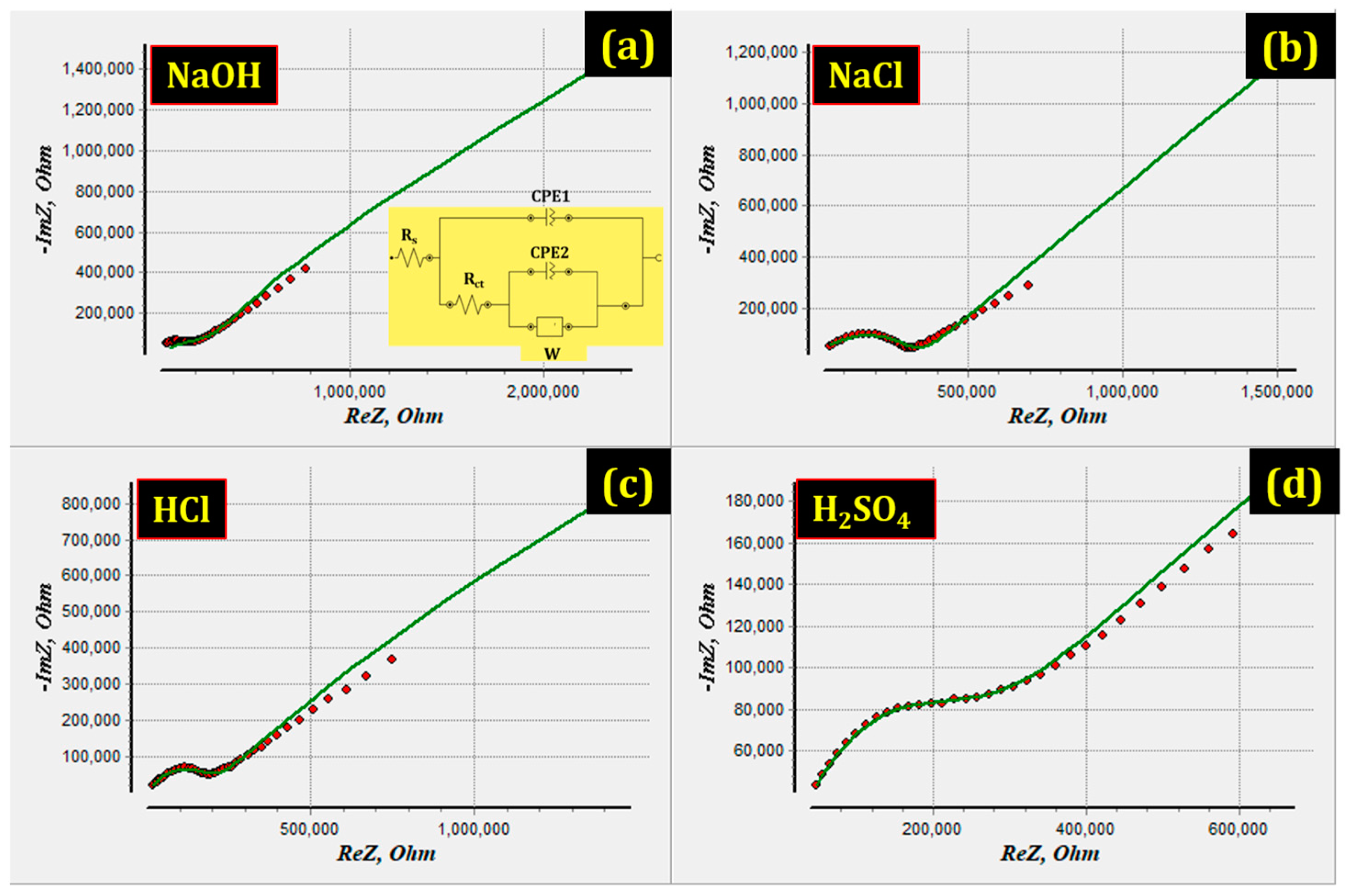
3.5. Electrochemical Impedance Spectroscopy
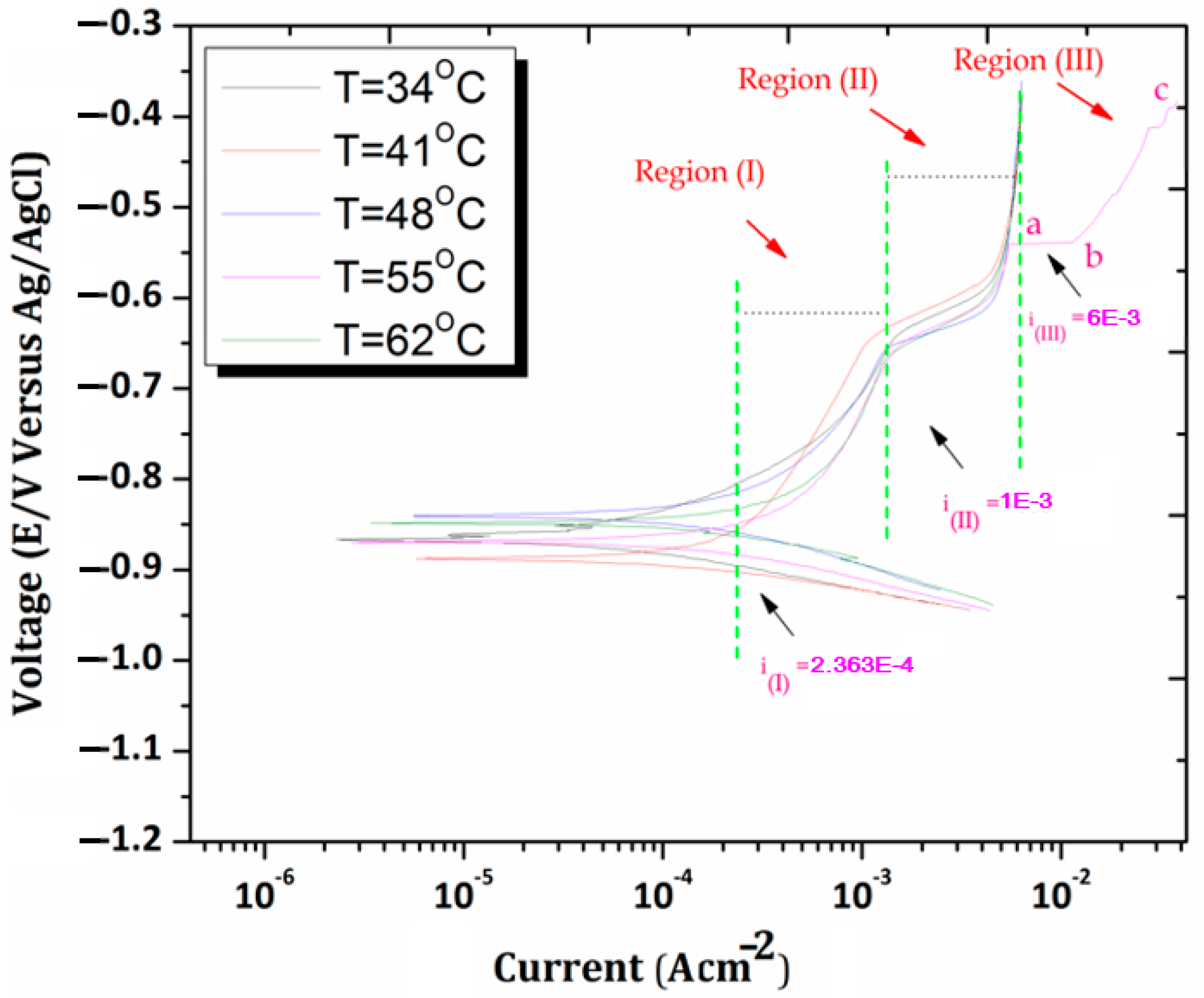
3.6. Effect of Temperature on Corrosion Behavior in Cl-Environment
- Region 1: Tafel activity region
- Region 2: Maximum current density region
- Region 3: Abrupt current region
3.7. Analysis of Corroded Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mavhungu, S.; Akinlabi, E.; Onitiri, M.; Varachia, F. Aluminum Matrix Composites for Industrial Use: Advances and Trends. Procedia Manuf. 2017, 7, 178–182. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Hu, X.; Wang, C.-A.; Huang, Y. Characterization of a powder metallurgy SiC/Cu–Al composite. J. Mater. Process. Technol. 2008, 197, 43–48. [Google Scholar] [CrossRef]
- Manoj, M.K.; Gadpale, V. Synthesis, Characterization and Dry Sliding Wear Behaviour of Al7075–MoSi2 Composites Prepared by Stir Casting Technique. Trans. Indian Inst. Metals 2019, 72, 3153–3169. [Google Scholar] [CrossRef]
- De Salazar, J.M.; Urena, A.; Manzanedo, S.; Barrena, M.I. Corrosion behaviour of AA6061 and AA7005 reinforced with Al2O3 particles in aerated 3.5% chloride solutions: Potentiodynamic measurements and microstructure evaluation. Corros. Sci. 1998, 41, 529–545. [Google Scholar] [CrossRef]
- Karunanithi, R.; Bera, S.; Ghosh, K. Electrochemical behaviour of TiO2 reinforced Al7075 composite. Mater. Sci. Eng. B 2014, 190, 133–143. [Google Scholar] [CrossRef]
- Han, Y.-M.; Chen, X.-G. Electrochemical Behavior of Al-B4C Metal Matrix Composites in NaCl Solution. Materials 2015, 8, 6455–6470. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.C.; Zitoun, E. Tensile properties and fracture behavior of 6061/Al2O3 metal matrix composites fabricated by low pressure die casting process. Int. J. Mater. Sci. 2011, 6, 147–157. [Google Scholar]
- Shabani, M.O.; Baghani, A.; Khorram, A.; Heydari, F. Evaluation of Fracture Mechanisms in Al-Si Metal Matrix Nanocomposites Produced by Three Methods of Gravity Sand Casting, Squeeze Casting and Compo Casting in Semi-Solid State. Silicon 2020, 12, 2977–2987. [Google Scholar] [CrossRef]
- Zakaria, H.M. Microstructural and corrosion behavior of Al/SiC metal matrix composites. Ain Shams Eng. J. 2014, 5, 831–838. [Google Scholar] [CrossRef]
- Ceschini, L.; Boromei, I.; Minak, G.; Morri, A.; Tarterini, F. Effect of friction stir welding on microstructure, tensile and fatigue properties of the AA7005/10 vol.% Al2O3p composite. Compos. Sci. Technol. 2007, 67, 605–615. [Google Scholar] [CrossRef]
- Bhandare, R.G.; Sonawane, P.M. Preparation of aluminium matrix composite by using stir casting method. Int. J. Eng. Adv. Technol. (IJEAT) 2013, 3, 61–65. [Google Scholar]
- Vani, V.V.; Chak, S.K. The effect of process parameters in aluminum metal matrix composites with powder metallurgy. Manuf. Rev. 2018, 5, 7. [Google Scholar] [CrossRef]
- Rao, S.R.; Padmanabhan, G. Fabrication and mechanical properties of aluminium-boron carbide composites. Int. J. Mater. Biomater. Appl. 2012, 2, 15–18. [Google Scholar]
- Baradeswaran, A.; Perumal, A.E. Influence of B4C on the tribological and mechanical properties of Al 7075–B4C composites. Compos. Part B Eng. 2013, 54, 146–152. [Google Scholar] [CrossRef]
- Hu, H.; Lavernia, E.; Harrigan, W.; Kajuch, J.; Nutt, S. Microstructural investigation on B4C/Al-7093 composite. Mater. Sci. Eng. A 2001, 297, 94–104. [Google Scholar] [CrossRef]
- Li, S.; Hihara, L.H. In situ Raman Spectroscopic Study of NaCl Particle-Induced Marine Atmospheric Corrosion of Carbon Steel. J. Electrochem. Soc. 2012, 159, C147–C154. [Google Scholar] [CrossRef]
- Hihara, L.H.; Latanision, R.M. Corrosion of metal matrix composites. Int. Mater. Rev. 1994, 39, 245–264. [Google Scholar] [CrossRef]
- Hihara, L.H.; Kondepudi, P.K. Predicting Galvanic Corrosion Rates for SiC Monofilament/Magnesium Metal-Matrix Composites in Chloride, Sulfate, and Nitrate Solutions; Minerals, Metals and Materials Society: Warrendale, PA, USA, 1993. [Google Scholar]
- Murthy, H.C.A.; Raju, V.B.; Shivakumara, C. Effect of TiN particulate reinforcement on corrosive behaviour of aluminium 6061 composites in chloride medium. Bull. Mater. Sci. 2013, 36, 1057–1066. [Google Scholar] [CrossRef]
- Ding, H.; Hihara, L.H. Electrochemical Examinations on the Corrosion Behavior of Boron Carbide Reinforced Aluminum-Matrix Composites. J. Electrochem. Soc. 2011, 158, C118–C124. [Google Scholar] [CrossRef]
- Ding, H.; Hihara, L.H. Localized Corrosion of Silicon-Reinforced Aluminum Composites in Na2SO4 Solution. ECS Tran. 2007, 2, 99–110. [Google Scholar] [CrossRef]
- Tiwari, S.; Hihara, L.H. Development of a Corrosion Model to Correlate the Atmospheric Corrosion Rate of a Carbon-Fiber Reinforced Aluminum MMC to Weather and Environmental Parameters. J. Electrochem. Soc. 2014, 161, C382–C388. [Google Scholar] [CrossRef]
- Li, Y.-L.; Wang, W.-X.; Chen, H.-S.; Zhou, J.; Wu, Q.-C. Corrosion behavior of B4C/6061Al neutron absorber composite in dif ferent H3BO3 concentration solutions. Acta Metal. Sin. (Engl. Lett.) 2016, 29, 1037–1046. [Google Scholar] [CrossRef]
- Arab, M.; Azadi, M.; Mirzaee, O. Effects of manufacturing parameters on the corrosion behavior of Al–B4C nanocomposites. Mater. Chem. Phys. 2020, 253, 123259. [Google Scholar] [CrossRef]
- Zeng, Q.; Li, Q.; Liu, W. Research on the optimal design of surface treatment process for Al-B4C composite by the method of orthogonal experiment. In Proceedings of the 2016 3rd International Conference on Materials Engineering, Manufacturing Technology and Control, Taiyuan, China, 27–28 February 2016; Atlantis Press: Taiyuan, China, 2016. [Google Scholar] [CrossRef][Green Version]
- Ding, H.; Hihara, L.H. Galvanic Corrosion and Localized Degradation of Aluminum-Matrix Composites Reinforced with Silicon Particulates. J. Electrochem. Soc. 2008, 155, C226–C233. [Google Scholar] [CrossRef]
- Ding, H.; Hihara, L.H. Localized Corrosion Currents and pH Profile over B4C, SiC, and Al2O3 Reinforced 6092 Aluminum Composites: I. In Solution. J. Electrochem. Soc. 2005, 152, B161. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, A.; Singh, R.S.; Manoj, M.K. Study of B4C/Al–Mg–Si Composites as Highly Hard and Corrosion-Resistant Materials for Industrial Applications. Trans. Indian Inst. Met. 2019, 72, 2495–2501. [Google Scholar] [CrossRef]
- Kumar, N.; Manoj, M.K. Influence of B4C on Dry Sliding Wear Behavior of B4C/Al–Mg–Si Composites Synthesized via Powder Metallurgy Route. Met. Mater. Int. 2020, 27, 4120–4131. [Google Scholar] [CrossRef]
- Pradhan, S.; Barman, T.K.; Sahoo, P.; Sahoo, S. Tribological behavior of Al-5% SiC metal matrix composite in sodium hydroxide environment. Mater. Today Proc. 2018, 5, 8057–8064. [Google Scholar] [CrossRef]
- Han, Y.-M.; Gallant, D.; Chen, X.-G. Galvanic corrosion associated with Al–B4C composites/SS304 and Al–B4C composites/AA6061 couples in NaCl and H3BO3 solutions. Electrochim. Acta 2013, 94, 134–142. [Google Scholar] [CrossRef]
- Gariba, A.M.M.; Islak, S. Corrosion properties of Ti-B4C/CNF functionally graded materials. Technol. Appl. Sci. 2020, 15, 41–49. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Effect of different electrolytes on the microstructure, corrosion and whisker growth of pulse plated tin coatings. Microelectron. Eng. 2017, 170, 59–68. [Google Scholar]
- Erek, H.; Asan, A.; Özyürek, D. Electrical Conductivity and Corrosion Performances of In Situ and Ex Situ AA7075 Aluminum Composites. Acta Phys. Pol. A 2017, 131, 153–155. [Google Scholar] [CrossRef]
- Kumar, N.; Dwivedi, D.; Sharma, A.; Ahn, B.; Manoj, M.K. Influence of various heat treatment procedures on the corrosion behavior of Al–Zn–Mg–Cu alloys. Mater. Res. Express 2019, 6, 126554. [Google Scholar] [CrossRef]
- Loto, R.T.; Babalola, P. Corrosion polarization behavior and microstructural analysis of AA1070 aluminium silicon carbide matrix composites in acid chloride concentrations. Cogent Eng. 2017, 4, 1422229. [Google Scholar] [CrossRef]
- Qian, D.; Zhong, X.; Hashimoto, T.; Liu, Z. Effect of Reinforcements on the Corrosion Behavior of SiCp/AA2124 Metal Matrix Composites. Corrosion 2015, 71, 1083–1092. [Google Scholar] [CrossRef]
- Sharma, A.; Das, K.; Fecht, H.-J.; Das, S. Effect of various additives on morphological and structural characteristics ofpulse electrodeposited tin coatings from stannous sulfate electrolyte. Appl. Surf. Sci. 2014, 314, 516–522. [Google Scholar] [CrossRef]
- Sherif, E.S.; Mohammed, J.A.; Abdo, H.S.; Almajid, A.A. Corrosion behavior in highly concentrated sodium chloride solutions of nanocrystalline aluminum processed by high energy ball mill. Int. J. Electrochem. Sci. 2016, 11, 1355–1369. [Google Scholar]
- Sharma, A.; Oh, M.-C.; Kim, J.-T.; Srivastava, A.K.; Ahn, B. Investigation of electrochemical corrosion behavior of additive manufactured Ti–6Al–4V alloy for medical implants in different electrolytes. J. Alloys Compd. 2020, 830, 154620. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Electrochemical corrosion behavior of CeO2 nanoparticle reinforced Sn–Agbased lead free nanocomposite solders in 3.5 wt.% NaCl bath. Surf. Coat. Technol. 2015, 261, 235–243. [Google Scholar] [CrossRef]
- Noor, E.A. Potential of aqueous extract of Hibiscus sabdariffa leaves for inhibiting the corrosion of aluminum in alkaline solutions. J. Appl. Electrochem. 2009, 39, 1465–1475. [Google Scholar] [CrossRef]
- Han, Y.; Gallant, D.; Chen, X.G. Investigation on Corrosion Behavior of the Al-B4C Metal Matrix Composite in a Mildly Oxidizing Aqueous Environment. Corrosion 2011, 67, 115005. [Google Scholar] [CrossRef]
- Yeganeh, M.; Rezvani, M.H.; Laribaghal, S.M. Electrochemical behavior of additively manufactured 316L stainless steel in H2SO4 solution containing methionine as an amino acid. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127120. [Google Scholar] [CrossRef]
- Ahmad, Z.; Aleem, B.A. Degradation of aluminum metal matrix composites in salt water and its control. Mater. Des. 2002, 23, 173–180. [Google Scholar] [CrossRef]
- Kumari, P.R.; Nayak, J.; Shetty, A.N. Corrosion behavior of 6061/Al-15 vol. pct. SiC (p) composite and the base alloy in sodium hydroxide solution. Arab. J. Chem. 2016, 9, S1144–S1154. [Google Scholar] [CrossRef]
- Zhao, H.; Ding, J.; Liu, P.; Yu, H. Boron nitride-epoxy inverse “nacre-like” nanocomposite coatings with superior anticorrosion performance. Corros. Sci. 2021, 183, 109333. [Google Scholar] [CrossRef]


| Corrosive Environments | Medium | Molarity | pH |
|---|---|---|---|
| Alkaline | NaOH | 0.5 | 8.5 |
| Neutral | NaCl | 0.6 | 6.9 |
| Acidic | H2SO4 | 0.5 | 0.3 |
| Acidic | HCl | 0.5 | 1.0 |
| PDP Parameters | |||||||
|---|---|---|---|---|---|---|---|
| Environment | Ecorr (V) | icorr (nA/cm2) | OCP (V) | Mechanical Properties | |||
| NaCl | −0.605 ± 0.20 | 8.35 ± 0.0001 | −0.458 ± 0.016 | Experimental Density (g/cm3) | Hardness (HV) | ||
| H2SO4 | −0.397 ± 0.11 | 1.186 ± 0.0014 | −0.502 ± 0.014 | 2.6681 ± 0.021 | 98.0 ± 2.25 | ||
| HCl | −0.415 ± 0.05 | 1.440 ± 0.0205 | −0.671 ± 0.017 | ||||
| NaOH | −1.378 ± 0.11 | 1.613 ± 0.0003 | −1.424 ± 0.007 | ||||
| EIS Measurement | |||||||
| Environments | Rs, Ω·cm2 | Rct (Ω·cm2) | CPE1 | CPE2 | W (Ohm·cm2) | ||
| p1 (F·cm−2) | n1 | p2 (F·cm−2) | n2 | ||||
| NaCl | 1.9732 | 3.3271 × 105 | 1.208 × 10−9 | 0.653 | 1.851 × 10−7 | 0.423 | 1.20 × 106 |
| H2SO4 | 1.8551 | 2.7787 × 105 | 1.870 × 10−9 | 0.642 | 2.343 × 10−7 | 0.362 | 3.62 × 106 |
| HCl | 1.8241 | 2.0610 × 105 | 2.150 × 10−9 | 0.676 | 2.603 × 10−7 | 0.412 | 6.69 × 106 |
| NaOH | 0.4417 | 2.0352 × 105 | 5.182 × 10−8 | 0.613 | 8.159 × 10−7 | 0.342 | 9.57 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, N.; Sharma, A.; Manoj, M.K. Influence of Different Aqueous Media on the Corrosion Behavior of B4C-Modified Lightweight Al-Mg-Si Matrix Composites. Materials 2022, 15, 8531. https://doi.org/10.3390/ma15238531
Kumar N, Sharma A, Manoj MK. Influence of Different Aqueous Media on the Corrosion Behavior of B4C-Modified Lightweight Al-Mg-Si Matrix Composites. Materials. 2022; 15(23):8531. https://doi.org/10.3390/ma15238531
Chicago/Turabian StyleKumar, Neeraj, Ashutosh Sharma, and Manoranjan Kumar Manoj. 2022. "Influence of Different Aqueous Media on the Corrosion Behavior of B4C-Modified Lightweight Al-Mg-Si Matrix Composites" Materials 15, no. 23: 8531. https://doi.org/10.3390/ma15238531
APA StyleKumar, N., Sharma, A., & Manoj, M. K. (2022). Influence of Different Aqueous Media on the Corrosion Behavior of B4C-Modified Lightweight Al-Mg-Si Matrix Composites. Materials, 15(23), 8531. https://doi.org/10.3390/ma15238531






