Study of the Structure and Properties of Electrical Sand Concrete under Prolonged Exposure to Sulfate Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Process
2.1.1. Exposure
2.1.2. Test Methods and Preparation of Specimens
Specimens
Testing Methods
3. Results
3.1. Corrosion Resistance
3.2. Electrical Properties
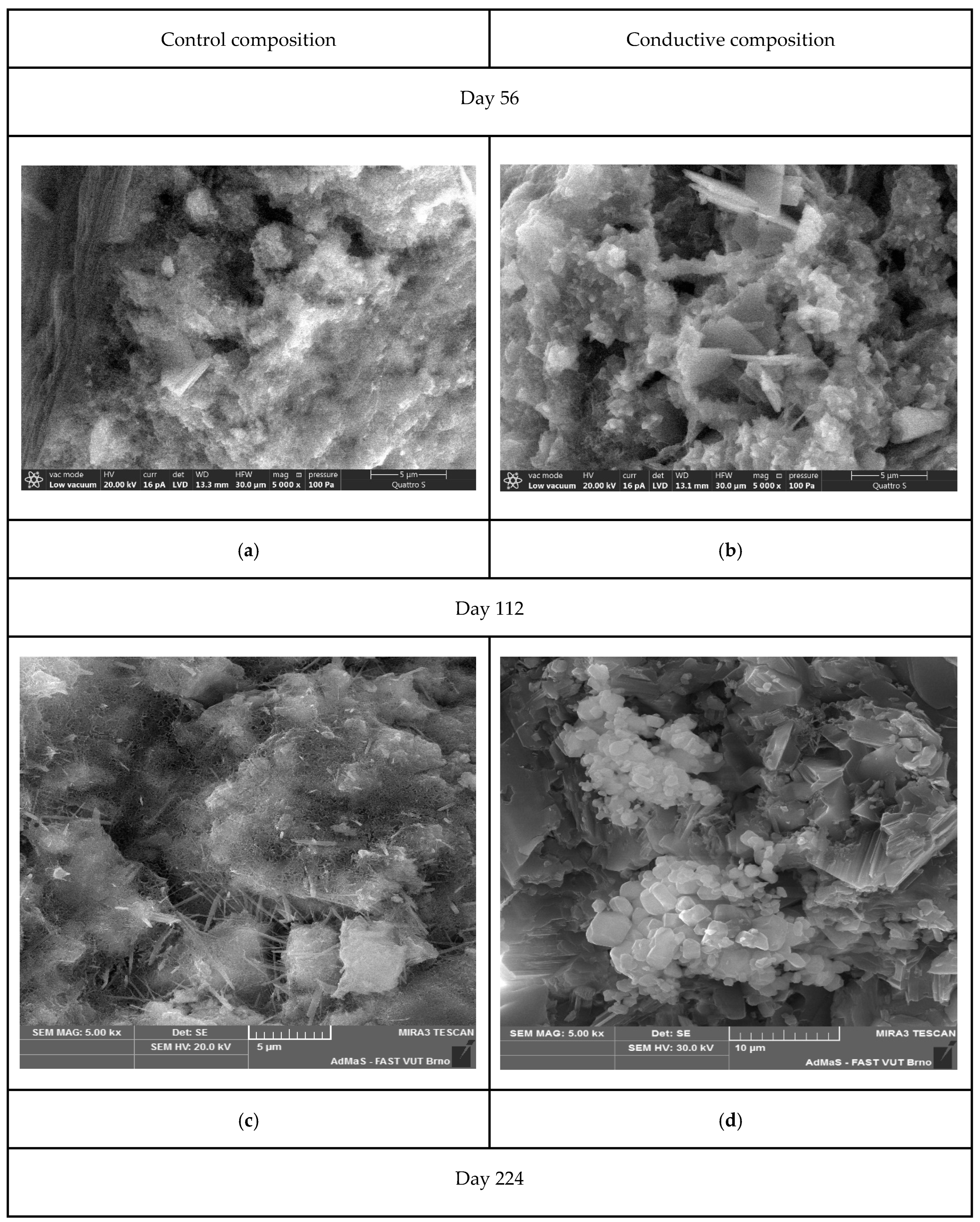
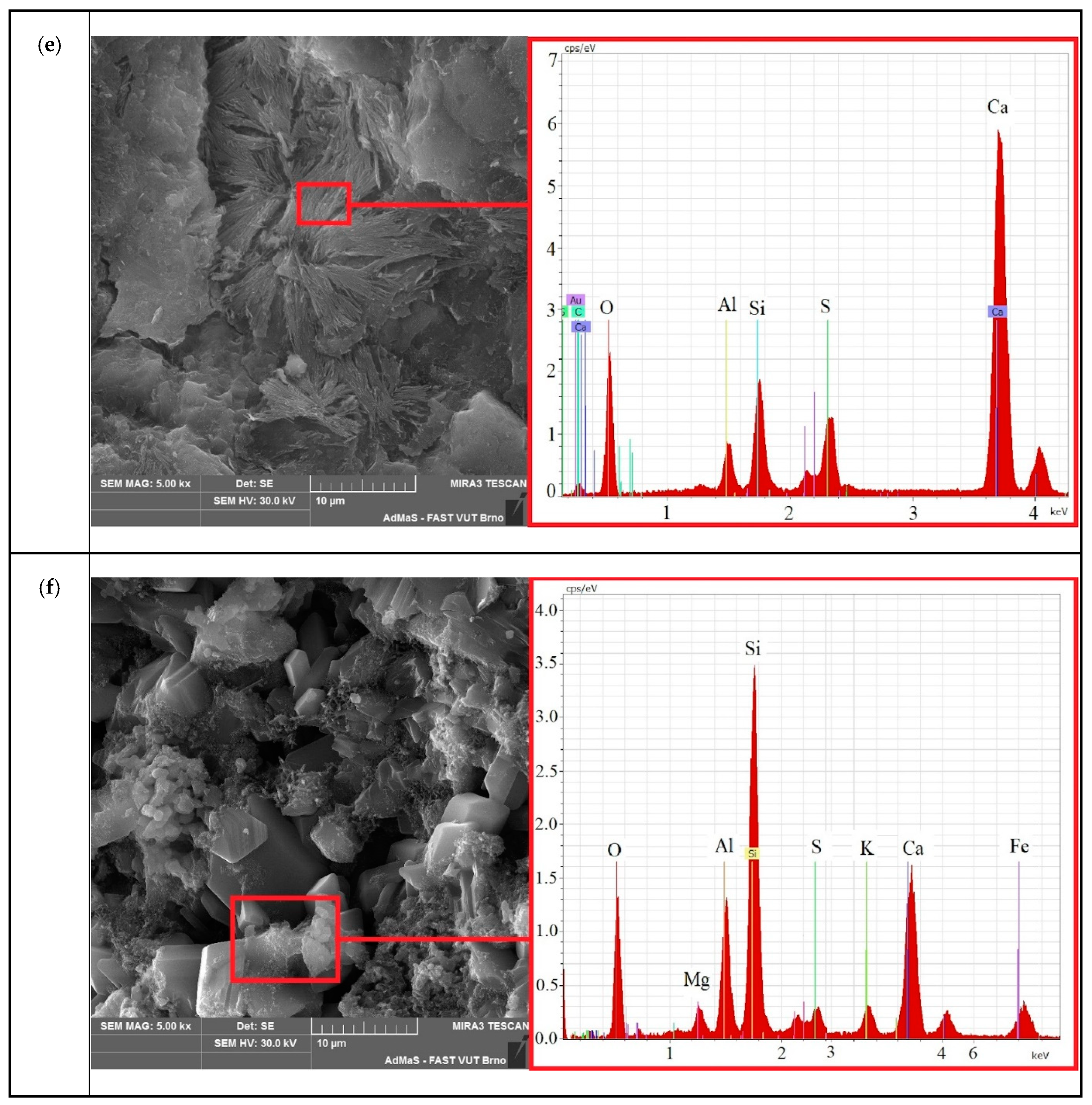
3.3. Microstructural Morphology by SEM
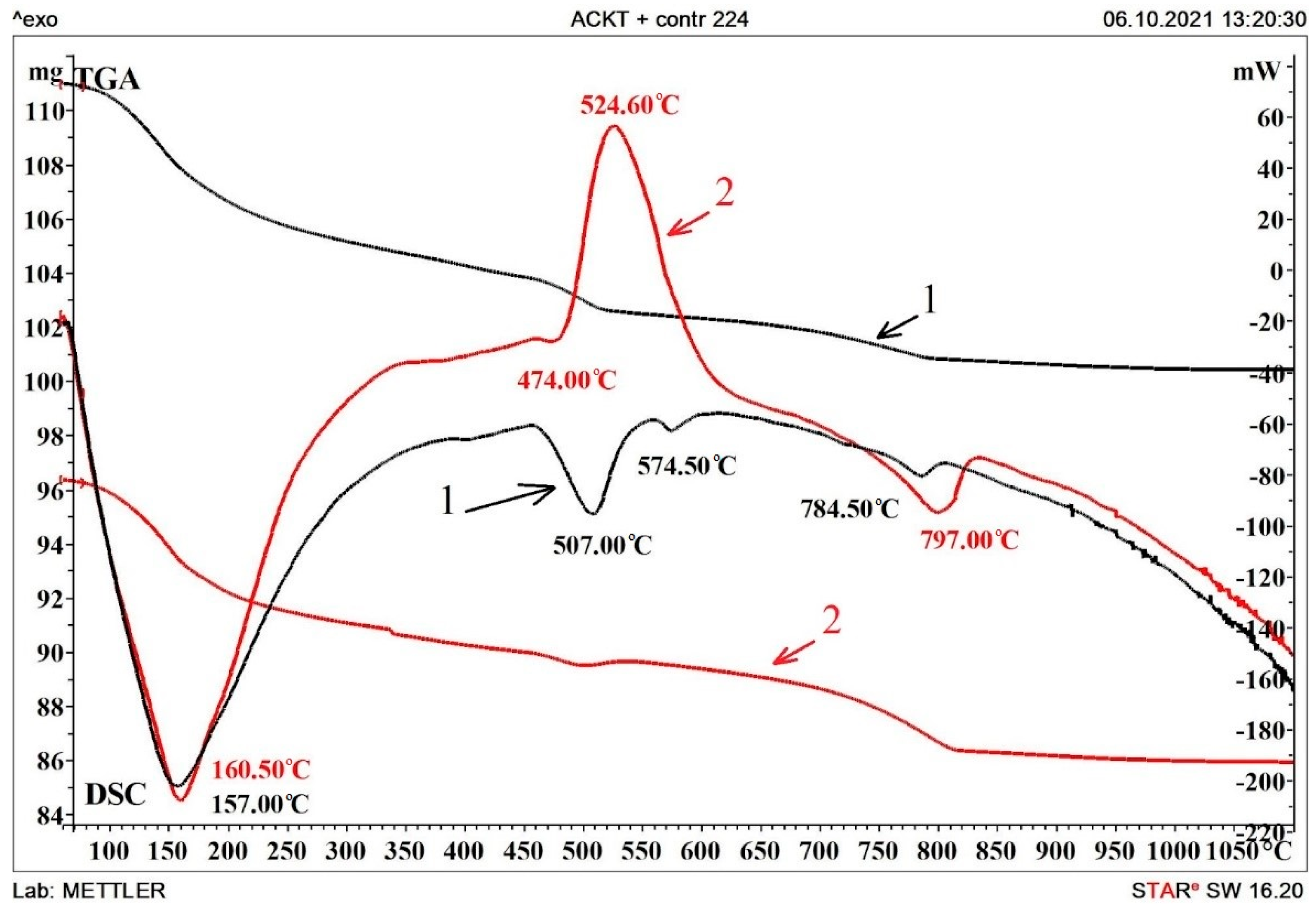
3.4. Differential Thermal Analysis of Compositions
4. Conclusions
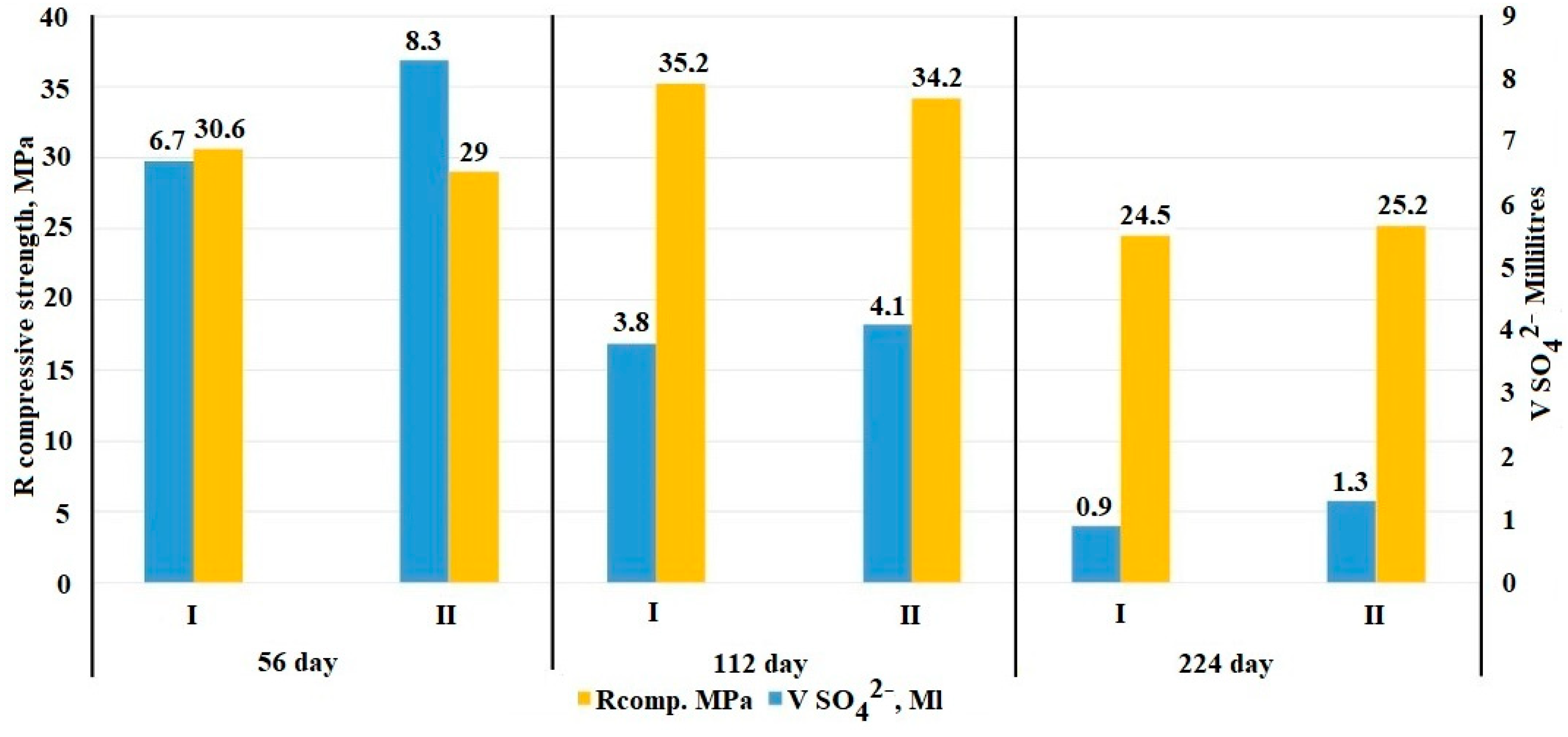
- The mode of constant exposure to an N sodium sulfate solution at the later stages of the experiment showed that in the period from 28 to 224 days, the absorption of sulfate ions slows down and averages 26% for the control and 29% for the electrically conductive compositions of the total volume of absorbed sulfates.
- Monitoring of the physico-mechanical parameters of the samples during the entire time of exposure to a 1 N sodium sulfate solution showed an increase in the density of the samples, for the control composition—by 6%, for the electrically conductive one—by 6.5%. In turn, the cyclic change in the mechanical strength, on average by 15% for the control and electrically conductive samples, in the period from 56 to 224 days, is a relaxing release of internal stress, followed by compaction of the formed microcracks.
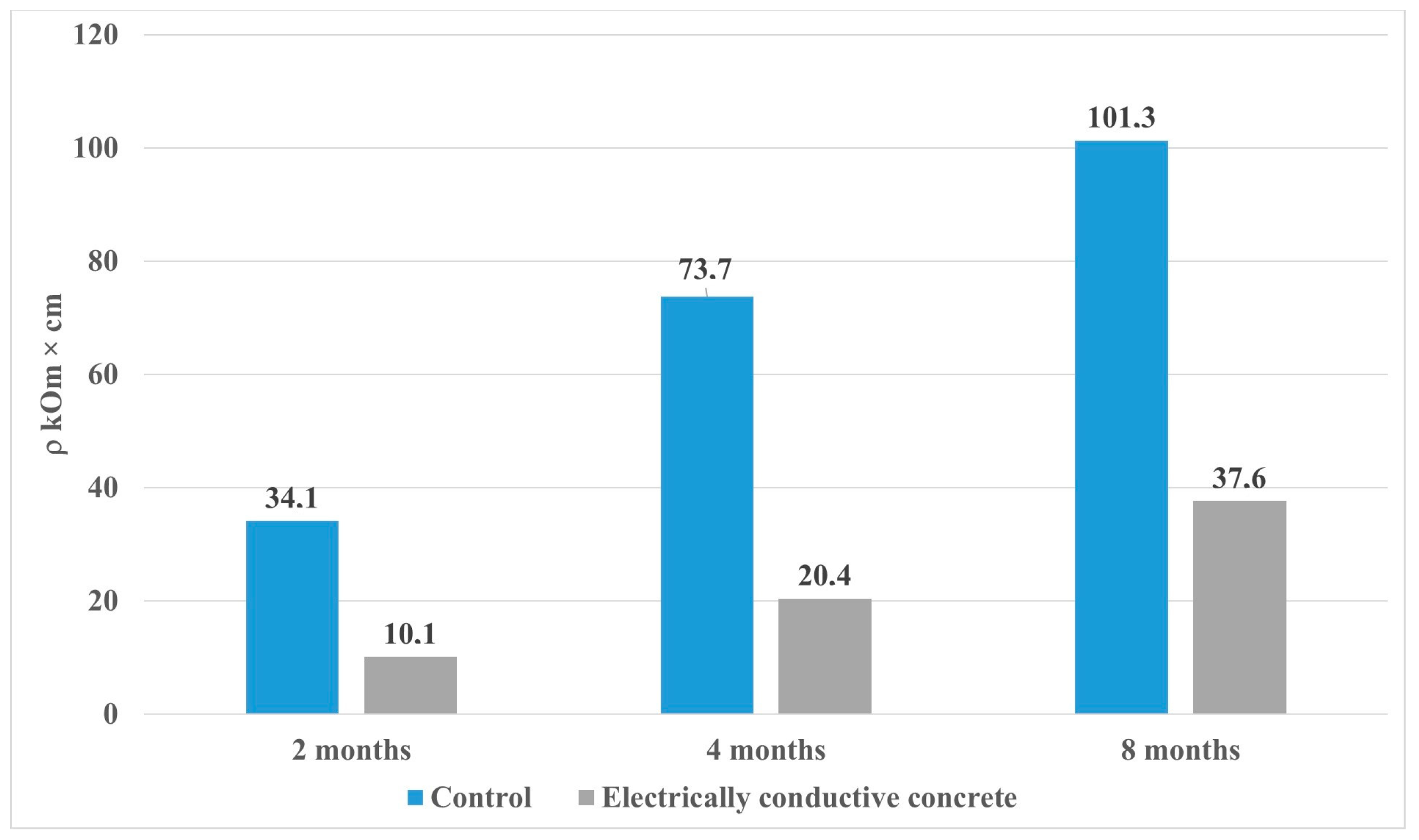
- The electrical resistivity of the samples has a steady growth trend during the entire experiment, while the growth in the control and electrically conductive compositions has a different character. Thus, the increase in the resistivity of the control composition for the period from 28 to 224 days was 75%, for the electrically conductive composition of 74%, while the final value is 101.3 kOhm cm for the control sample, and for the electrically conductive composition is 37.6 kOhm cm, which indicates a difference in the physicochemical properties of the phase composition of mineral matrices.
- An analysis of the microstructure of the samples showed that in the control composition, at the later stages of the experiment (from 56 to 224 days), ettringite splits with the formation of “two-leaves”. At the same time, the formation of calcite spherulites and calcium hydroaluminates is observed in the electrically conductive composition, which leads to an increase in the amount of the solid phase, to a cyclic increase in crystallization pressure, and causes a negative effect in the form of a decrease in strength.
- Differential thermal analysis confirmed the differences between the features of the interaction of compositions with an aggressive environment and a decrease in strength characteristics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dehghanpour, H.; Yilmaz, K. Heat behavior of electrically conductive concretes with and without rebar reinforcement. Mater. Sci. 2020, 26, 4. [Google Scholar] [CrossRef]
- Rahman, L.; Malakooti, A.; Ceylan, H.; Kim, S.; Taylor, P.C. A review of electrically conductive concrete heated pavement system technology: From the laboratory to the full-scale implementation. Constr. Build. Mater. 2022, 329, 127139. [Google Scholar] [CrossRef]
- Sassani, A.; Ceylan, H.; Kim, S.; Arabzadeh, A.; Taylor, P.C.; Gopalakrishnan, K. Development of carbon fiber-modified electrically conductive concrete for implementation in Des Moines International Airport. Case Stud. Constr. Mater. 2018, 8, 277–291. [Google Scholar] [CrossRef]
- Dehghanpour, H.; Yilmaz, K.; Ipek, M. Evaluation of recycled nano carbon black and waste erosion wires in electrically conductive concretes. Constr. Build. Mater. 2019, 221, 109–121. [Google Scholar] [CrossRef]
- Makul, N. Advanced smart concrete-A review of current progress, benefits and challenges. J. Clean. Prod. 2020, 274, 122899. [Google Scholar] [CrossRef]
- Nalon, G.H.; Santos, R.F.; de Lima, G.E.S.; Andrade, I.K.R.; Pedroti, L.G.; Ribeiro, J.C.L.; de Carvalho, J.M.F. Recycling waste materials to produce self-sensing concretes for smart and sustainable structures: A review. Constr. Build. Mater. 2022, 325, 126658. [Google Scholar] [CrossRef]
- Bernatsky, A.F. Electrical Insulating Concrete (Technology, Properties, Constructions); EDN: Novosibirsk, Russia, 2016; 186p, ZTIVHF. [Google Scholar]
- Hong, S.-H.; Choi, J.-S.; Yuan, T.-F.; Yoon, Y.-S. Mechanical and Electrical Characteristics of Lightweight Aggregate Concrete Reinforced with Steel Fibers. Materials 2021, 14, 6505. [Google Scholar] [CrossRef]
- Cordon, H.C.F.; Tadini, F.B.; Akiyama, G.A.; De Andrade, V.O.; Da Silva, R.C. Development of electrically conductive concrete. Cerâmica 2020, 66, 88–92. [Google Scholar] [CrossRef]
- Fiala, L.; Pommer, V.; Böhm, M.; Scheinherrová, L.; Černý, R. Self-heating alkali activated materials: Microstructure and its effect on electrical, thermal and mechanical properties. Constr. Build. Mater. 2022, 335, 127527. [Google Scholar] [CrossRef]
- Liu, X.; Qu, M.; Nguyen, A.P.T.; Dilley, N.R.; Yazawa, K. Characteristics of new cement-based thermoelectric composites for low-temperature applications. Constr. Build. Mater. 2021, 304, 124635. [Google Scholar] [CrossRef]
- Urkhanova, L.A.; Buyantuev, S.L.; Urkhanova, A.A.; Lkhasaranov, S.A.; Ardashova, G.R.; Fediuk, R.S.; Svintsov, A.P.; Ivanov, I.A. Mechanical and electrical properties of concrete modified by carbon nanoparticles. Mag. Civ. Eng. 2019, 92, 163–172. [Google Scholar] [CrossRef]
- Brown, L.; Stephens, C.S.; Allison, P.G.; Sanchez, F. Effect of Carbon Nanofiber Clustering on the Micromechanical Properties of a Cement Paste. Nanomaterials 2022, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Qureshi, T.; Wang, G. Application of Graphene in Fiber-Reinforced Cementitious Composites: A Review. Energies 2021, 14, 4614. [Google Scholar] [CrossRef]
- Zaid, O.; Hashmi, S.R.Z.; Aslam, F.; Abedin, Z.U.; Ullah, A. Experimental study on the properties improvement of hybrid graphene oxide fiber-reinforced composite concrete. Diam. Relat. Mater. 2022, 124, 108883. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Zhu, P.; Ning, T. Snow Melting Performance of Graphene Composite Conductive Concrete in Severe Cold Environment. Materials 2021, 14, 6715. [Google Scholar] [CrossRef] [PubMed]
- Cleven, S.; Raupach, M.; Matschei, T. Electrical Resistivity of Steel Fibre-Reinforced Concrete—Influencing Parameters. Materials 2021, 14, 3408. [Google Scholar] [CrossRef] [PubMed]
- Dehghanpour, H.; Yilmaz, K.; Afshari, F.; Ipek, M. Electrically conductive concrete: A laboratory-based investigation and numerical analysis approach. Constr. Build. Mater. 2020, 260, 119948. [Google Scholar] [CrossRef]
- Plugin, A.A.; Pluhin, O.; Borziak, O.; Kaliuzhna, O. The Influence of Storage Conditions on the Electric Conductivity of Concrete. Mater. Sci. Forum 2019, 968, 50–60. [Google Scholar] [CrossRef]
- Skripkiūnas, G.; Kičaitė, A.; Justnes, H.; Pundienė, I. Effect of Calcium Nitrate on the Properties of Portland–Limestone Cement-Based Concrete Cured at Low Temperature. Materials 2021, 14, 1611. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Calcium Chloride on Concreate; Applied Science Publishers: Englewood, NJ, USA, 1972; p. 216. [Google Scholar]
- Hornbostel, K.; Larsen, C.K.; Geiker, M.R. Relationship between concrete resistivity and corrosion rate—A literature review. Cem. Concr. Compos 2013, 39, 60–72. [Google Scholar] [CrossRef]
- Park, P.; Rew, Y.; Baranikumar, A. Controlling Conductivity of Asphalt Concrete with Graphite; Report Number: SWUTC/14/600451-00025-1; Texas A&M Transportation Institute College Station: Bryan, TX, USA, 2014. [Google Scholar]
- Malakooti, A.; Sadati, S.; Ceylan, H.; Kim, S.; Cetin, K.S.; Taylor, P.C.; Mina, M.; Cetin, B.; Theh, W.S. Self-Heating Electrically Conductive Concrete Demonstration Project; IHRB Project TR-724; Iowa State University: Ames, IA, USA, 2021. [Google Scholar]
- Wang, K.; Guo, J.; Yang, L.; Zhang, P.; Xu, H. Multiphysical damage characteristics of concrete exposed to external sulfate attack: Elucidating effect of drying–wetting cycles. Constr. Build. Mater. 2022, 329, 127143. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Bazanov, S.M. Sulfate Corrosion of Concrete-M; Publisher ASV: Moscow, Russia, 2003; 191p. [Google Scholar]
- Moskvin, V.M.; Ivanov, F.M.; Alekseev, S.N.; Guzeev, E.A. Corrosion of Concrete and Reinforced Concrete, Methods of Their Protection; Stroyizdat: Moscow, Russia, 1980; 520p. [Google Scholar]
- Stark, J.; Wicht, B. Concrete Durability/Translation from German. In RIA Quintet, 1st ed.; Weimar, Germany, 2004; p. 295. [Google Scholar]
- Tian, Y.; Yan, X.; Zhang, M.; Lu, D.; Yang, T.; Wang, Z.; Li, W. Internal transport and corrosion behaviors of sulfate corrosion media carried by recycled aggregate in concrete. Constr. Build. Mater. 2020, 260, 120480. [Google Scholar] [CrossRef]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.-M.; Roussel, N.; Divet, L.; de Lacaillerie, J.-B.D. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cem. Concr. Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Zhao, G.; Shi, M.; Guo, M.; Fan, H. Degradation Mechanism of Concrete Subjected to External Sulfate Attack: Comparison of Different Curing Conditions. Materials 2020, 13, 3179. [Google Scholar] [CrossRef]
- Li, L.; Shi, J.; Kou, J. Experimental Study on Mechanical Properties of High-Ductility Concrete against Combined Sulfate Attack and Dry–Wet Cycles. Materials 2021, 14, 4035. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, Z.; Dai, L.; Wen, X.; Wang, R.; Zhang, D.; Song, X. Study on Corrosion Mechanism of Different Concentrations of Na2SO4 Solution on Early-Age Cast-In-Situ Concrete. Materials 2021, 14, 2018. [Google Scholar] [CrossRef]
- Hui Song, Jinwei Yao, Yuming Luo, FaliangGui A chemical-mechanics model for the mechanics deterioration of pervious concrete subjected to sulfate attack. Constr. Build. Mater. 2021, 312, 125383. [CrossRef]
- Sun, X.; Li, T.; Shi, F.; Liu, X.; Zong, Y.; Hou, B.; Tian, H. Sulphate Corrosion Mechanism of Ultra-High-Performance Concrete (UHPC) Prepared with Seawater and Sea Sand. Polymers 2022, 14, 971. [Google Scholar] [CrossRef]
- Yakovlev, G.; Polyanskikh, I.; Gordina, A.; Pudov, I.; Černý, V.; Gumenyuk, A.; Smirnova, O. Influence of Sulphate Attack on Properties of Modified Cement Composites. Appl. Sci. 2021, 11, 8509. [Google Scholar] [CrossRef]
- Peng, Y.; Meng, X.; Song, F.; Xu, G. Experimental study on the corrosion characteristics of concrete exposed to acid water containing aggressive carbon dioxide and sodium sulfate. Constr. Build. Mater. 2022, 321, 126397. [Google Scholar] [CrossRef]
- Li, T.; Zhang, B. Stochastic Dynamic Model of Sulfate Corrosion Reactions in Concrete Materials considering the Effects of Colored Gaussian Noises. Complexity 2019, 6434718, 18. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, M.; Li, Z.; He, F. Microstructural insight into the deterioration mechanism of the mortar subject to the combined action of external sulfate attack and cyclic wetting–drying. Constr. Build. Mater. 2022, 317, 125484. [Google Scholar] [CrossRef]
- Gumeniuk, A.; Hela, R.; Polyanskikh, I.; Gordina, A.; Yakovlev, G. Durability of concrete with man-made thermoplastic sulfur additive. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; p. 032012. [Google Scholar] [CrossRef]
- Lialikov, U.S. Physico-Chemical Methods of Analysis, 5th ed.; Moscow, Russia, 1973; p. 536. [Google Scholar]
- Chaudhari, B.; Panda, B.; Šavija, B.; Chandra Paul, S. Microbiologically Induced Concrete Corrosion: A Concise Review of Assessment Methods, Effects, and Corrosion-Resistant Coating Materials. Materials 2022, 15, 4279. [Google Scholar] [CrossRef] [PubMed]
- Samchenko, S.V. Formirovanie and Genesis Struktury Cementnogo Kamny; MGSU, Ai Pi Er Media, EBS ASV: Moscow, Russia, 2016; 284p. [Google Scholar]
- Polyanskikh, I.S.; Gumenyuk, A.N.; Gordina, A.F.; Pudov, I.A.; Yakovlev, G.I. Resistance of Electrically Conductive Concrete to Sulphate Attack. Key Eng. Mater. 2022, 932, 197–203. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: London, UK, 2016; p. 508. [Google Scholar]
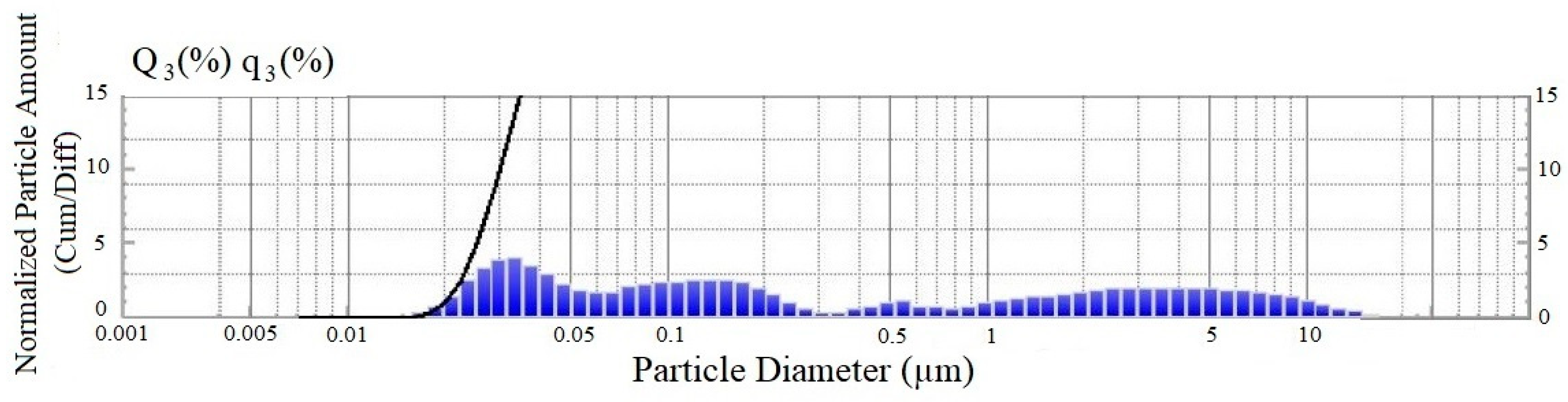
| Particle Size, Microns | Content, % |
|---|---|
| 0.014–0.091 | 34.8 |
| 0.1–1.05 | 28.3 |
| 1.05–20 | 36.9 |
| Mix | Portland Cement, CEM I 42.5 g | Quartz Sand, g | Industrial Soot, % | Calcium Nitrate % | Water-Cement Ratio [29] |
|---|---|---|---|---|---|
| Control | 800 | 1600 | - | - | 0.5 |
| Electrically conductive concrete | - | - | 7 | 3 |
| Curing Period, Days | 56 | 112 | 224 | |||
|---|---|---|---|---|---|---|
| Values | m | ρ | m | ρ | m | ρ |
| Control | +5% | +3% | +2% | +2% | +1% | +1% |
| Electrically conductive composition | +3% | +3% | +1.5% | +1.5% | +2% | +2% |
| Temperature Range | Effect Temperature/Mass Loss | Effect | |
|---|---|---|---|
| Control Composition (1) | Electrically Conductive Composition (2) | ||
| 100–300 °C | 157.0/−5.6% | 160.5/−5.65% | Dehydration of gel and crystalline set cement products, including calcium. monosulfoaluminate |
| 500–800 °C | - | 524.6/−1.6% | Oxidation of the carbon component. |
| 495–520 °C | 507.00; 574.5/−2.3% | - | Decomposition of elements of the ettringite system and recrystallization of silicon oxide. |
| 775–840 °C | 784.5/−1.6% | 797.0/−3.7% | Dehydration of ettringite, calcium carbonates, and hydroalumoferites. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordina, A.; Gumenyuk, A.; Polyanskikh, I.; Yakovlev, G.; Pudov, I. Study of the Structure and Properties of Electrical Sand Concrete under Prolonged Exposure to Sulfate Environment. Materials 2022, 15, 8542. https://doi.org/10.3390/ma15238542
Gordina A, Gumenyuk A, Polyanskikh I, Yakovlev G, Pudov I. Study of the Structure and Properties of Electrical Sand Concrete under Prolonged Exposure to Sulfate Environment. Materials. 2022; 15(23):8542. https://doi.org/10.3390/ma15238542
Chicago/Turabian StyleGordina, Anastasiya, Aleksandr Gumenyuk, Irina Polyanskikh, Grigory Yakovlev, and Igor Pudov. 2022. "Study of the Structure and Properties of Electrical Sand Concrete under Prolonged Exposure to Sulfate Environment" Materials 15, no. 23: 8542. https://doi.org/10.3390/ma15238542
APA StyleGordina, A., Gumenyuk, A., Polyanskikh, I., Yakovlev, G., & Pudov, I. (2022). Study of the Structure and Properties of Electrical Sand Concrete under Prolonged Exposure to Sulfate Environment. Materials, 15(23), 8542. https://doi.org/10.3390/ma15238542







