Energetic and Protective Coating via Chemical and Physical Synergism for High Water-Reactive Aluminum Powder
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Samples Preparation
2.3. Methodology
3. Results and Discussion
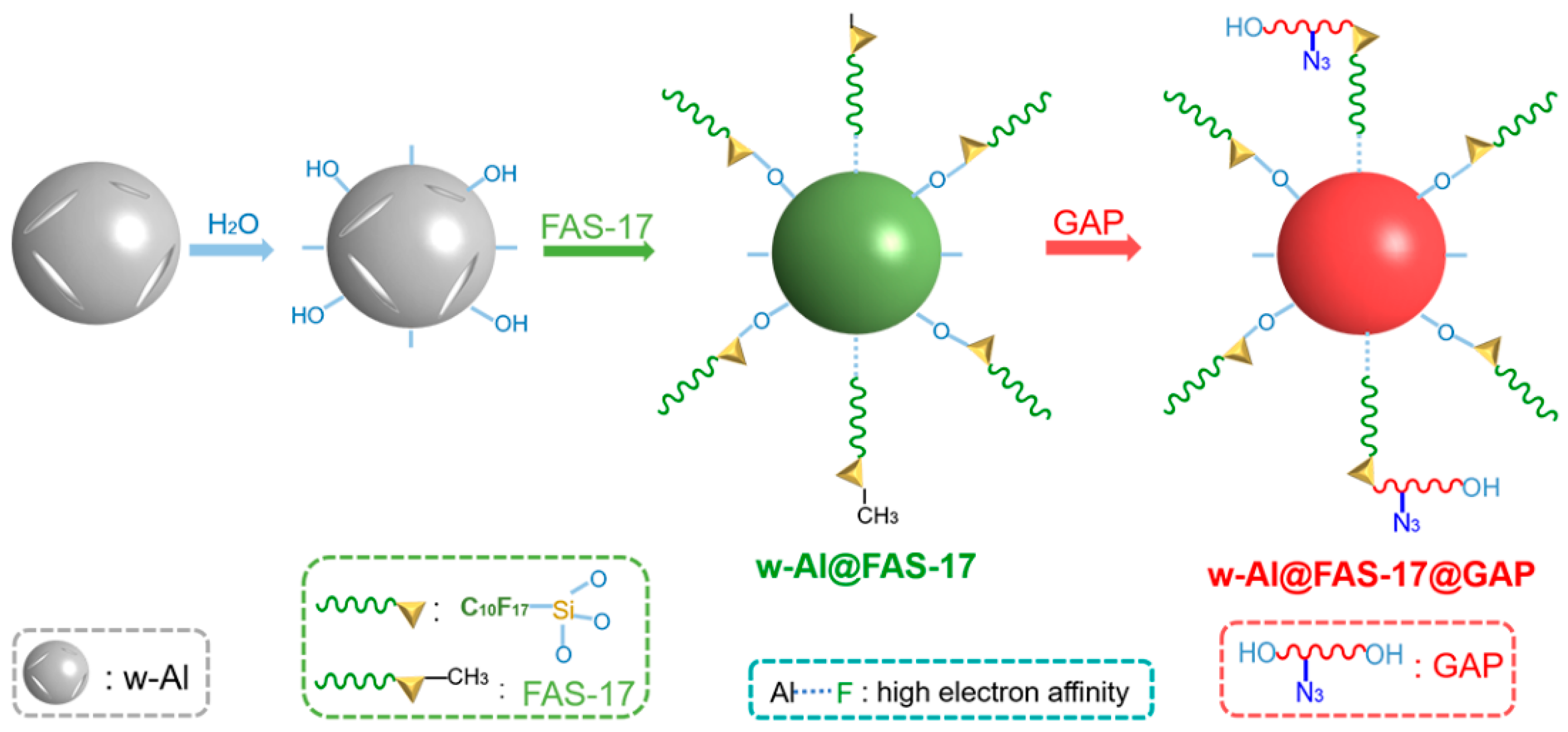
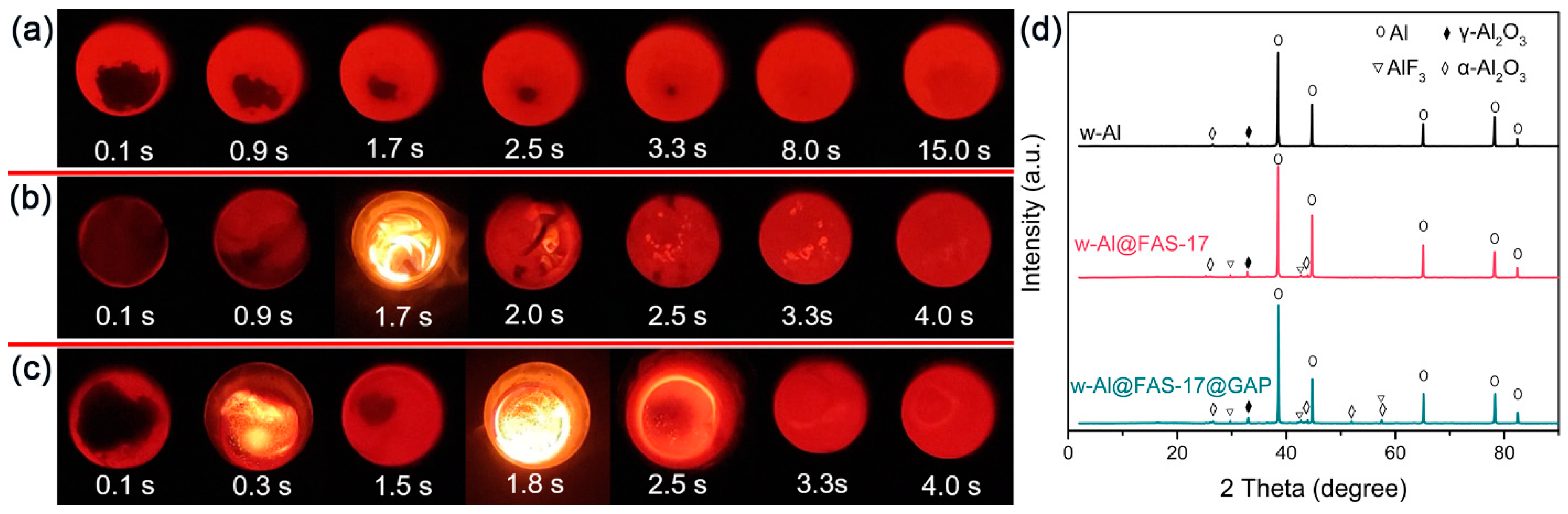
3.1. Morphologies and Composition
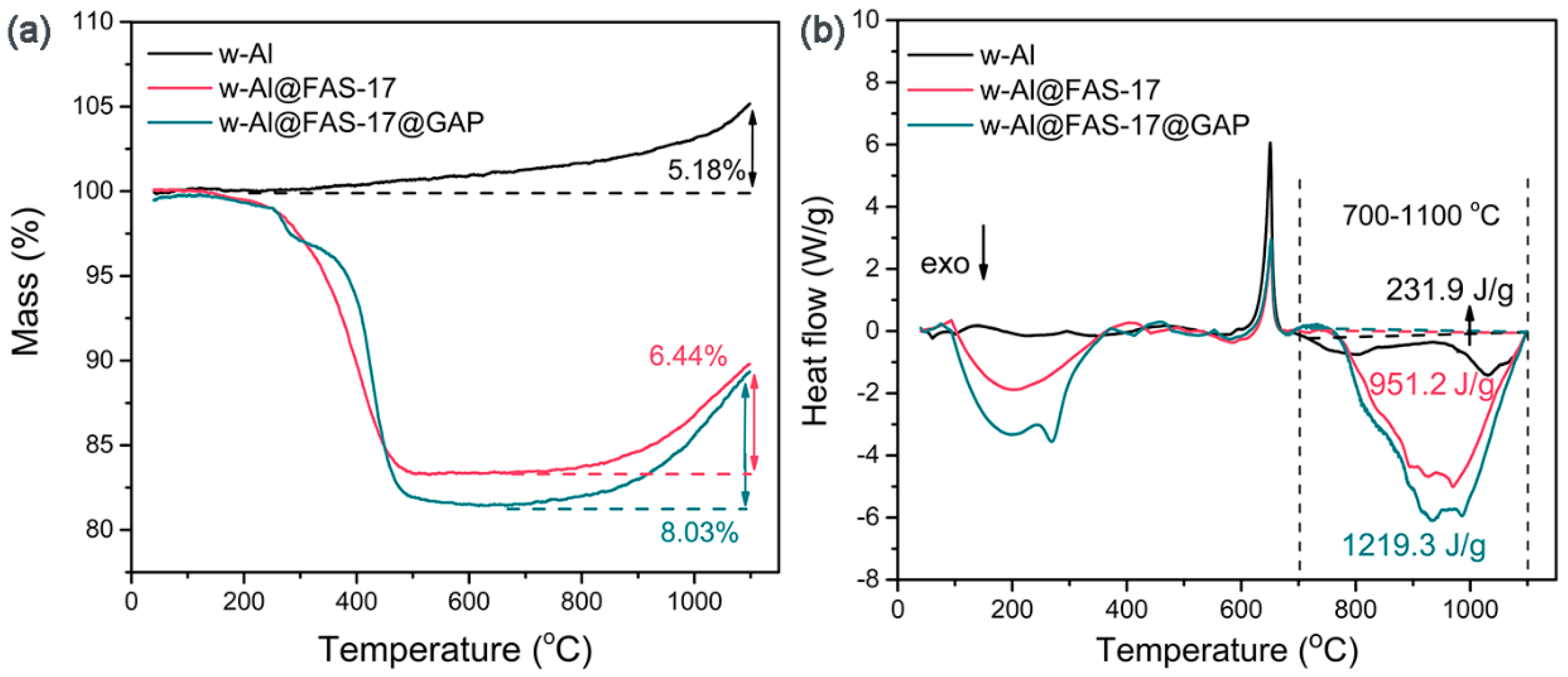
3.2. Thermal Reaction Properties
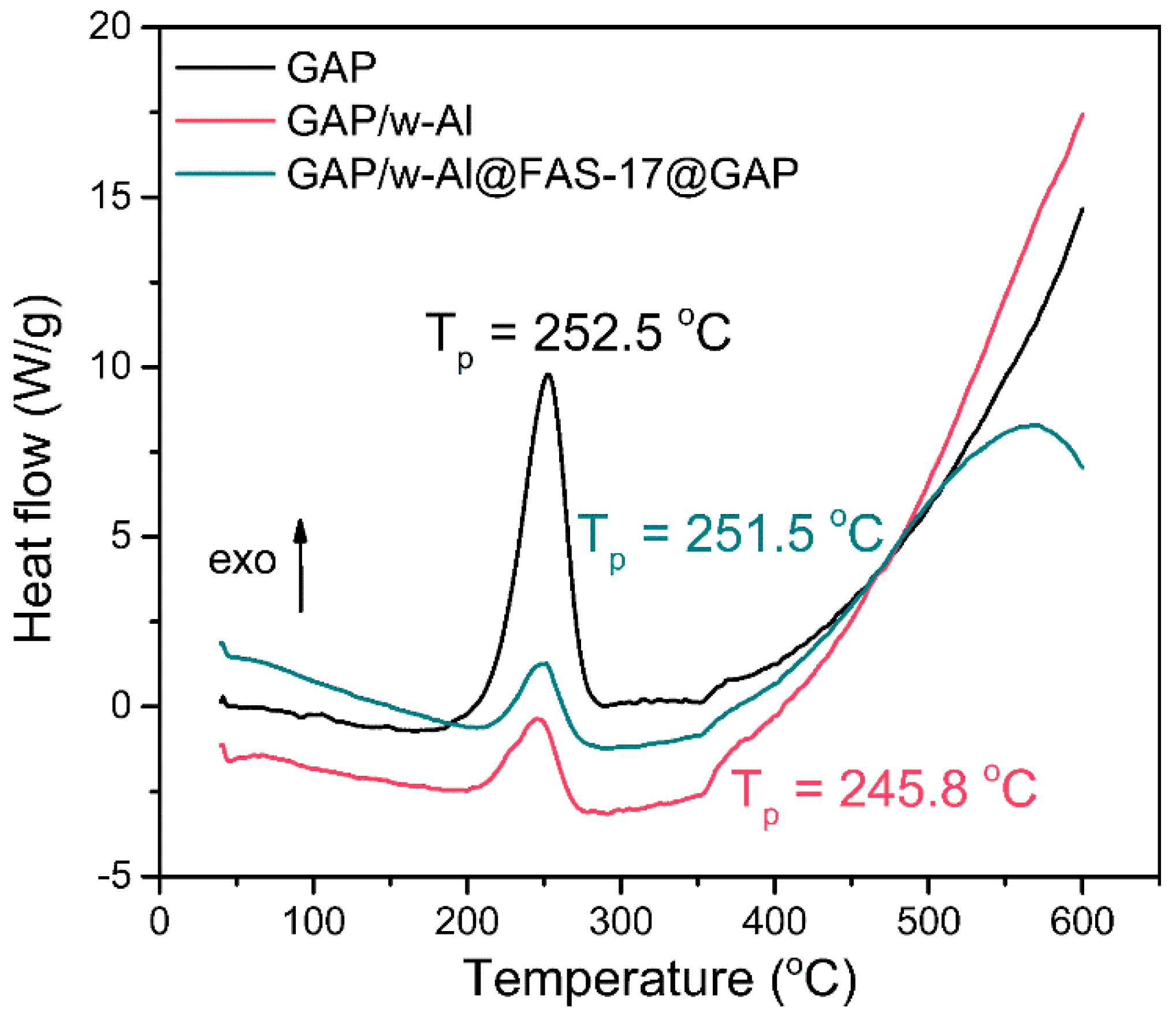
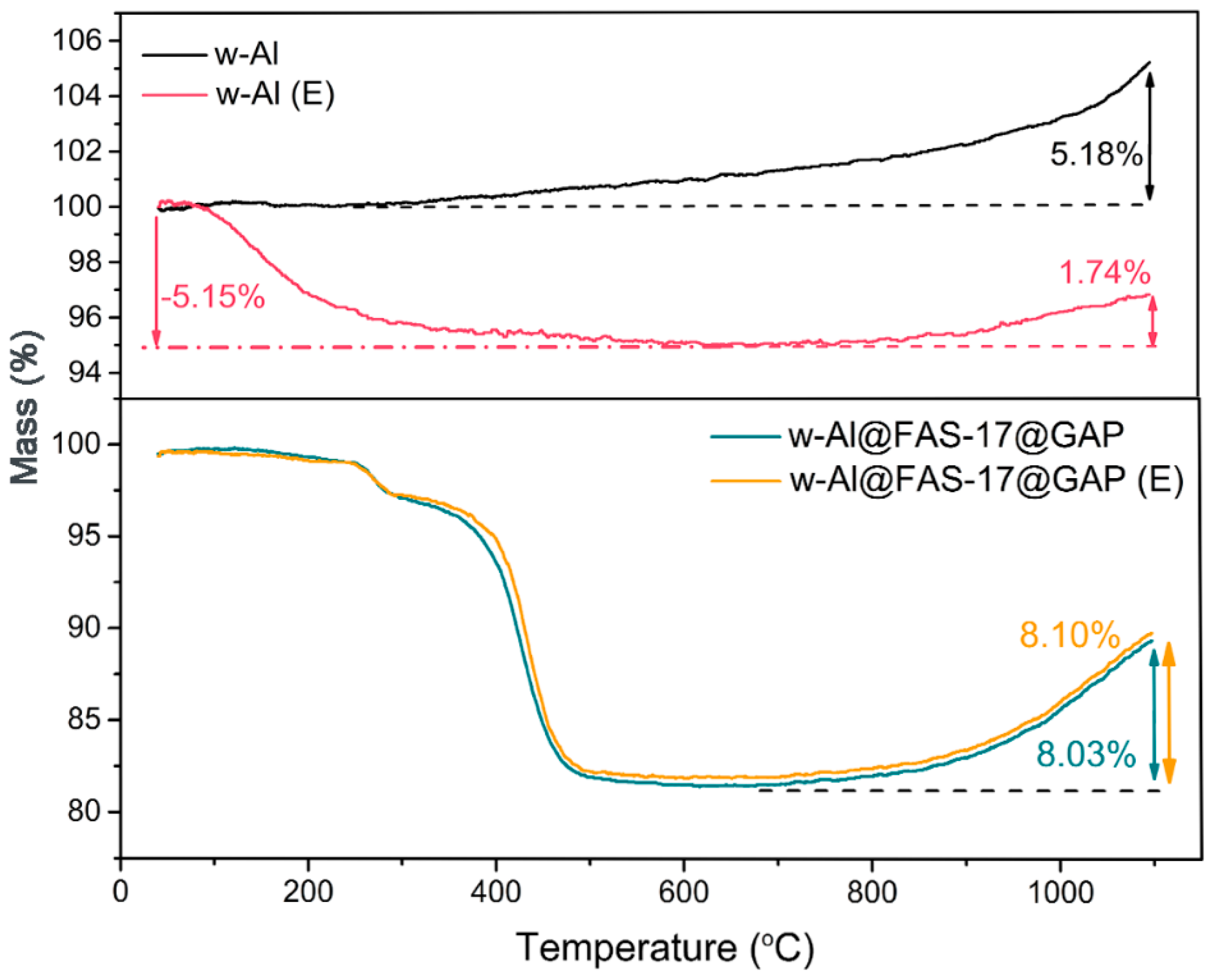
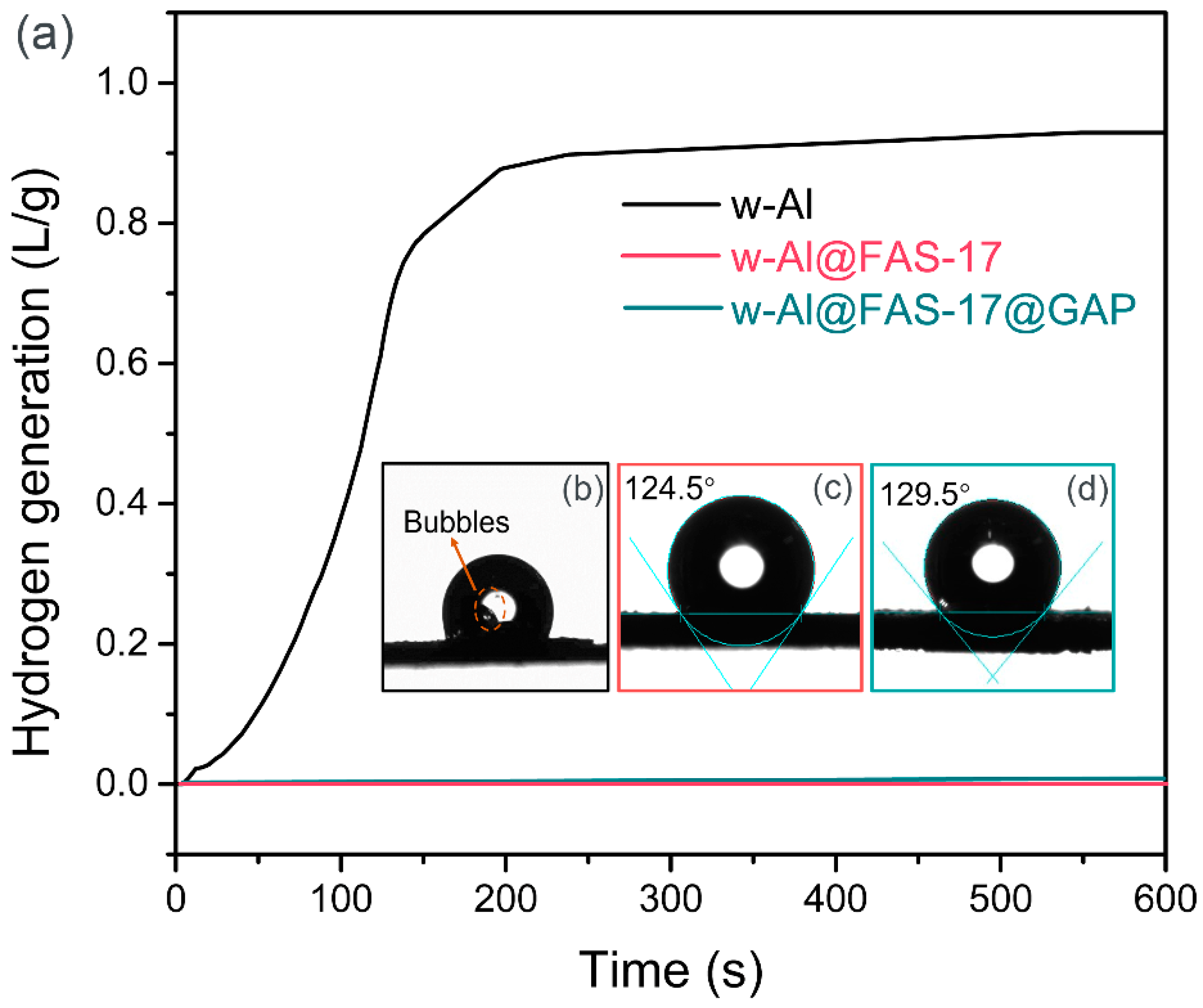
3.3. Compatibility and Environmental Adaptability
3.4. Water Reactivity
3.5. The Ignition and Combustion Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Hao, D.; Tao, B.; Wang, F.; Wang, D.; Fan, R.; Xia, D.; Wang, P.; Yang, Y.; Pang, A.; et al. Core-shell nAl@Fc-Fx nanocomposites with dual function: Combustion and anti-migration performance. Chem. Eng. J. 2020, 394, 124884. [Google Scholar] [CrossRef]
- Zhang, L.; Su, X.; Wang, S.; Li, X.; Zou, M. In situ preparation of Al@3-Perfluorohexyl-1, 2-epoxypropane@glycidyl azide polymer (Al@PFHP@GAP) high-energy material. Chem. Eng. J. 2022, 450, 137118. [Google Scholar] [CrossRef]
- Tang, D.-Y.; Lyu, J.; He, W.; Chen, J.; Yang, G.; Liu, P.-J.; Yan, Q.-L. Metastable intermixed Core-shell Al@M(IO3)x nanocomposites with improved combustion efficiency by using tannic acid as a functional interfacial layer. Chem. Eng. J. 2020, 384, 123369. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Song, C.; Sun, Y.; Han, Y.; Zhao, Y.; Wang, J. Fabrication and Characterization of Viton@FOX-7@Al Spherical Composite with Improved Thermal Decomposition Property and Safety Performance. Materials 2021, 14, 1093. [Google Scholar] [CrossRef]
- Wang, H.Z.; Leung, D.Y.C.; Leung, M.K.H.; Ni, M. A review on hydrogen production using aluminum and aluminum alloys. Renew. Sustain. Energy Rev. 2009, 13, 845–853. [Google Scholar] [CrossRef]
- Wang, H.; Chang, Y.; Dong, S.; Lei, Z.; Zhu, Q.; Luo, P.; Xie, Z. Investigation on hydrogen production using multicomponent aluminum alloys at mild conditions and its mechanism. Int. J. Hydrogen Energy 2013, 38, 1236–1243. [Google Scholar] [CrossRef]
- Bolt, A.; Dincer, I.; Agelin-Chaab, M. A Review of Unique Aluminum—Water Based Hydrogen Production Options. Energy Fuels 2021, 35, 1024–1040. [Google Scholar] [CrossRef]
- Sheng, P.; Zhang, S.; Yang, J.; Guan, C.; Li, J.; Liu, M.; Pan, W.; Wang, Y. Investigation on the Al/low-melting-point metals/salt composites for hydrogen generation. Int. J. Hydrogen Energy 2021, 45, 9627–9637. [Google Scholar] [CrossRef]
- Guan, X.; Zhou, Z.; Luo, P.; Wu, F.; Dong, S. Hydrogen generation from the reaction of Al-based composites activated by low-melting-point metals/oxides/salts with water. Energy 2019, 188, 116107. [Google Scholar] [CrossRef]
- Zhu, L.; Zou, M.; Zhang, X.; Zhang, L.; Wang, X.; Song, T.; Wang, S.; Li, X. Enhanced Hydrogen Generation Performance of Al-Rich Alloys by a Melting-Mechanical Crushing-Ball Milling Method. Materials 2021, 14, 7889. [Google Scholar] [CrossRef]
- Hao, J.; Ding, A.; Huang, J.; Zhang, Y.; Qian, K.; Ji, S. Cladding Assembly and Characterization of HTPB-TDI Coated High Activity Nano-Aluminum Powder HTPB-TDI. Chin. J. Rare Met. 2018, 42, 168–174. [Google Scholar]
- Cao, Z.; Hu, A.; Xia, B.; Pang, A.-M.; Cai, S. Preparation and Characterization of Aluminum-Lithium Alloy Powder Coated by In-situ Polymerization of Styrene. Propellants Explos. Pyrotech. 2020, 45, 1141–1152. [Google Scholar] [CrossRef]
- Tran, V.T.; Kim, J.H.; Jeong, K.-J.; Kwon, J.; Kim, S.H.; Lee, J. Highly stable functionalized aluminum nanoparticles for magneto-energetic composite fabrication. Combust. Flame 2018, 187, 96–104. [Google Scholar] [CrossRef]
- DeLisio, J.B.; Hu, X.; Wu, T.; Egan, G.C.; Young, G.; Zachariah, M.R. Probing the Reaction Mechanism of Aluminum/Poly(vinylidene fluoride) Composites. J. Phys. Chem. B 2016, 120, 5534–5542. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Wang, B.; Ma, H.; Han, Z. An approach to the induced reaction mechanism of the combustion of the nano-Al/PVDF composite particles. Surf. Coat. Technol. 2022, 429, 127912. [Google Scholar] [CrossRef]
- Hao, D.; Hu, Y.; Wang, F.; Xia, D.; Wang, D.; Fan, R.; Yang, Y.; Lin, K. Core-shell structured nAl@F-x nanocomposite: Preparation and their improved combustion performances. J. Energetic Mater. 2020, 40, 61–81. [Google Scholar] [CrossRef]
- Ou, Y.; Jiao, Q.; Li, N.; Yan, S.; Yang, R. Pyrolysis of ammonium perfluorooctanoate (APFO) and its interaction with nano-aluminum. Chem. Eng. J. 2021, 403, 126367. [Google Scholar] [CrossRef]
- Wang, J.; Mao, Y.; Li, G.; Yin, H.-M. Bio-inspired nacre-like fluorographene/Al energetic paper with superior chemical reactivity and mechanical properties. Chem. Eng. J. 2022, 441, 136014. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, X.; Li, G.; Gong, F.; Liu, Y.; Yan, Q.; Yang, Z.; Nie, F. Surface fluorination of n-Al particles with improved combustion performance and adjustable reaction kinetics. Chem. Eng. J. 2021, 425, 131619. [Google Scholar] [CrossRef]
- Osborne, D.T.; Pantoya, M.L. Effect of Al Particle Size on the Thermal Degradation of Al/Teflon Mixtures. Combust. Sci. Technol. 2007, 179, 1467–1480. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Baek, J.; Wang, H.; Gottfried, J.L.; Wu, C.-C.; Shi, X.; Zachariah, M.R.; Zheng, X. Ignition and combustion of Perfluoroalkyl-functionalized aluminum nanoparticles and nanothermite. Combust. Flame 2022, 242, 112170. [Google Scholar] [CrossRef]
- Lin, J.; Cai, X.; Liu, Z.; Liu, N.; Xie, M.; Zhou, B.; Wang, H.; Guo, Z. Anti-liquid-Interfering and Bacterially Antiadhesive Strategy for Highly Stretchable and Ultrasensitive Strain Sensors Based on Cassie-Baxter Wetting State. Adv. Funct. Mater. 2020, 30, 2000398. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Wang, C.; Li, J. A facile method to fabricate superhydrophobic cotton fabrics. Appl. Surf. Sci. 2012, 261, 561–566. [Google Scholar] [CrossRef]
- Liu, J.; Wang, M.; Gu, C.; Zhang, L.; Yan, Y. Fabrication of a superhydrophobic surface by straightforward immersion for copperplate artwork protection. RSC Adv. 2021, 11, 32038–32046. [Google Scholar] [CrossRef]
- Zeng, C.; Yang, Z.; Wen, Y.; He, W.; Zhang, J.; Wang, J.; Huang, C.; Gong, F. Performance optimization of core-shell HMX@(Al@GAP) aluminized explosives. Chem. Eng. J. 2021, 407, 126360. [Google Scholar] [CrossRef]
- Zeng, C.; Wang, J.; He, G.; Huang, C.; Yang, Z.; Liu, S.; Gong, F. Enhanced water resistance and energy performance of core–shell aluminum nanoparticles via in situ grafting of energetic glycidyl azide polymer. J. Mater. Sci. 2018, 53, 12091–12102. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Ye, S.; Jiang, B.; Guo, Z.; Mumtaz, Y.; Yi, W.B. General Access to N-CF3 Secondary Amines and Their Transformation to N-CF3 Sulfonamides. Angew. Chem. Int. Ed. Engl. 2022. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, K.T.; Min, T.S.; Kim, K.J.; Kim, S.H. Improved Energetic-Behaviors of Spontaneously Surface-Mediated Al Particles. Sci. Rep. 2017, 7, 4659. [Google Scholar] [CrossRef] [Green Version]
- Beach, N.E.; Canfield, V.K. Compatibility of Explosives with Polymers III. Plast. Rep. 1971, 40, 73–76. [Google Scholar]
- Pei, J.F.; Zhao, F.Q.; Lu, H.L.; Song, X.D.; Zhou, R.; Yuan, Z.F.; Zhang, J.; Chen, J.B. Compatibility study of BAMO–GAP copolymer with some energetic materials. J. Therm. Anal. Calorim. 2016, 124, 1301–1307. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, L.; Zhang, L.; Zhang, X.; Wang, X.; Ge, M.; Li, X.; Zou, M. Preparation of Al-3Ga-3In-3Sn Alloy Powder by Coupling Alloying and Ball Milling and Its Application on High-Rate Hydrogen Generation at Room Temperature. Metals 2021, 11, 1704. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Liu, Y.; Ren, M.; Xiao, H.; Liu, X. Characterization of Conformation and Locations of C-F Bonds in Graphene Derivative by Polarized ATR-FTIR. Anal. Chem. 2016, 88, 3926–3934. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singla, P.; Sahoo, S.C.; Soni, P.K. Compatibility and thermal decomposition behavior of an epoxy resin with some energetic compounds. J. Energetic Mater. 2020, 38, 432–444. [Google Scholar] [CrossRef]
- Li, J.S.; Chen, J.J.; Hwang, C.C.; Lu, K.T.; Yeh, T.F. Study on Thermal Characteristics of TNT Based Melt-Cast Explosives. Propellants Explos. Pyrotech. 2019, 44, 1270–1281. [Google Scholar] [CrossRef]
- Yan, H.; Xiaolong, L.U.; Chunrui, W.U.; Gao, Q.; Wang, X.; Jia, Y.; Chen, H. Super-hydrophobic modification of polyvinylidene fluoride hollow fiber membranes viaaparticle coating process. Membr. Sci. Technol. 2017, 37, 38–44. [Google Scholar]
- Zhou, X.-Y.; Xiao, F.; Yang, R.-J.; Huang, F.-L.; Li, J.-M. Investigation of the ignition and combustion of compressed aluminum/polytetrafluoroethylene bulk composites. J. Therm. Anal. Calorim. 2019, 139, 3013–3021. [Google Scholar] [CrossRef]
- Wang, C.; Zou, X.; Yin, S.; Wang, J.; Li, H.; Liu, Y.; Wang, N.; Shi, B. Improvement of ignition and combustion performance of micro-aluminum particles by double-shell nickel-phosphorus alloy coating. Chem. Eng. J. 2021, 433, 133585. [Google Scholar] [CrossRef]
- He, W.; Lyu, J.-Y.; Tang, D.-Y.; He, G.-Q.; Liu, P.-J.; Yan, Q.-L. Control the combustion behavior of solid propellants by using core-shell Al-based composites. Combust. Flame 2020, 221, 441–452. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, S.; Su, X.; Li, X.; Zou, M. Preparation and characterization of core-shell Al@PFHP with improving the combustion and ignition properties of aluminum powder. Particuology 2022. [Google Scholar] [CrossRef]
- Yan, T.; Ren, H.; Li, Y.; Wang, H.; Jiao, Q. Tailoring Structural Energetics for Enhanced Reactivity of Nano-Aluminum Particles Based Microspheres. Adv. Eng. Mater. 2019, 21, 1900176. [Google Scholar] [CrossRef]


| Sample | Oxygen Bomb Calorimeter Test | Ignition Test | ||
|---|---|---|---|---|
| Heat Release (kJ/g) | Minimum Ignition Temperature (°C) | tI* (s) | tB# (s) | |
| w-Al | 26.9 | -- | -- | -- |
| w-Al@FAS-17 | 24.8 | 790 | 1.7 | 2.3 |
| w-Al@FAS-17@GAP | 26.1 | 760 | 0.3 | 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, S.; Zhu, L.; Li, X.; Su, X.; Zou, M. Energetic and Protective Coating via Chemical and Physical Synergism for High Water-Reactive Aluminum Powder. Materials 2022, 15, 8554. https://doi.org/10.3390/ma15238554
Zhang L, Wang S, Zhu L, Li X, Su X, Zou M. Energetic and Protective Coating via Chemical and Physical Synergism for High Water-Reactive Aluminum Powder. Materials. 2022; 15(23):8554. https://doi.org/10.3390/ma15238554
Chicago/Turabian StyleZhang, Lichen, Shuo Wang, Lixiang Zhu, Xiaodong Li, Xing Su, and Meishuai Zou. 2022. "Energetic and Protective Coating via Chemical and Physical Synergism for High Water-Reactive Aluminum Powder" Materials 15, no. 23: 8554. https://doi.org/10.3390/ma15238554
APA StyleZhang, L., Wang, S., Zhu, L., Li, X., Su, X., & Zou, M. (2022). Energetic and Protective Coating via Chemical and Physical Synergism for High Water-Reactive Aluminum Powder. Materials, 15(23), 8554. https://doi.org/10.3390/ma15238554









