Thermal Plasma Synthesis of Different Alloys and Intermetallics from Ball Milled Al-Mo and Al-Ni Powder Systems
Abstract
:1. Introduction
2. Materials, Machines Used, and Characterization Methods
2.1. Characterization of the Initial Powders
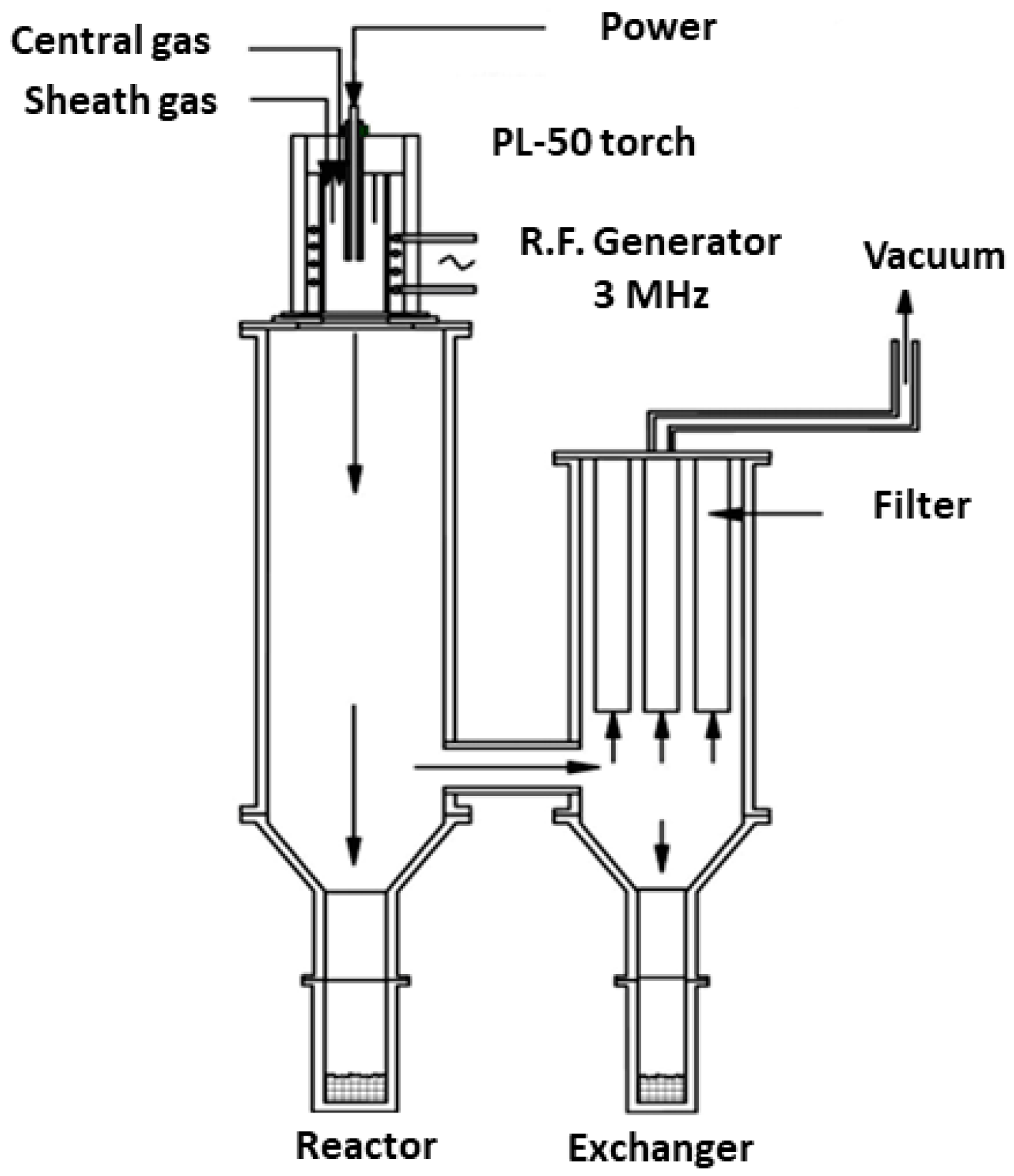
2.2. Mechanical Agglomeration of the Powders and Characteristics of the Plasma Spraying Machine
2.3. Separation of the Obtained Powder Particles
2.4. Characterization of the Obtained Powder Particles
3. Results and Discussion
3.1. Characterization of the Initial Powders
3.2. Mechanical Agglomeration of the Powders
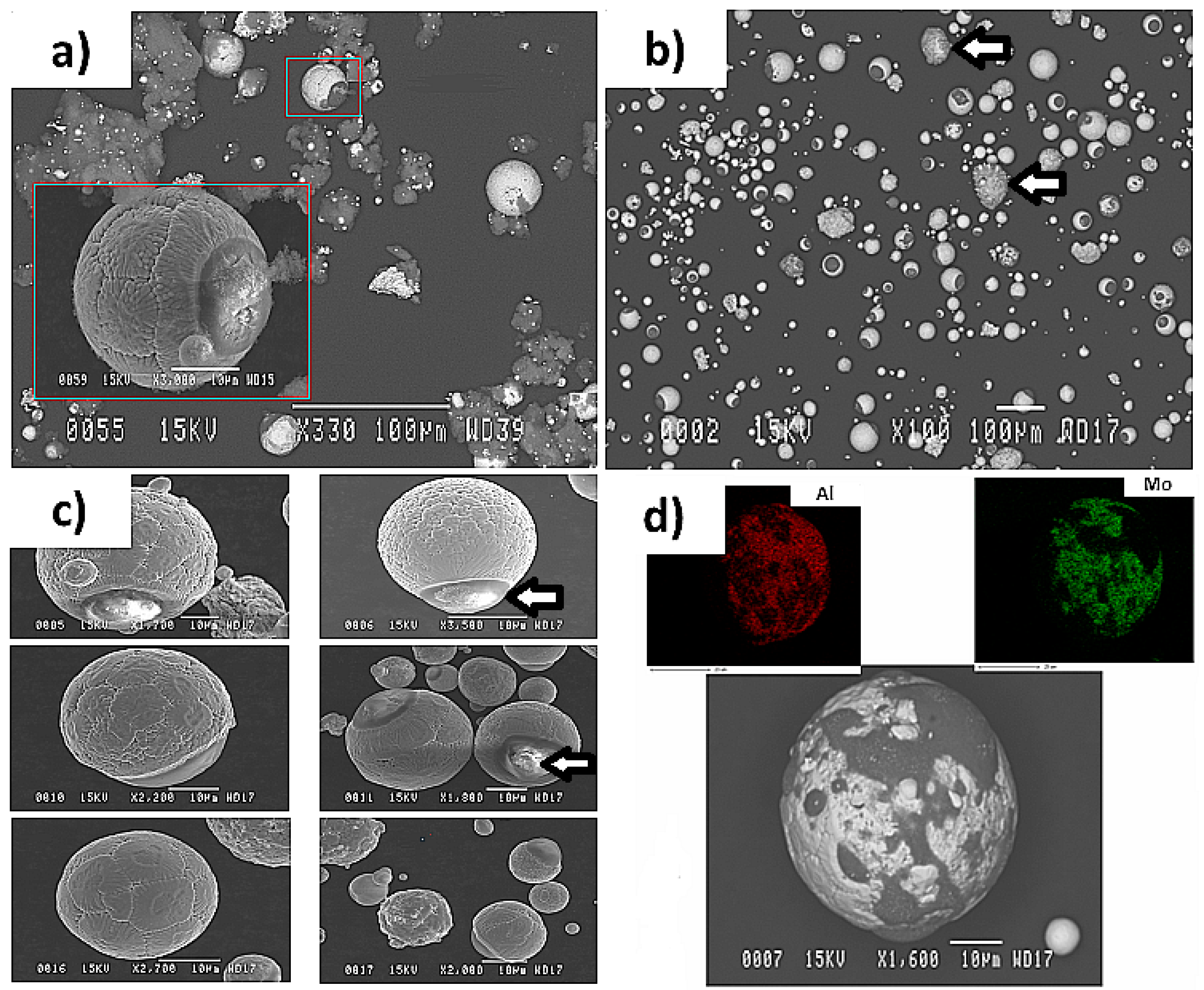
3.3. Plasma Deposition Results
4. Conclusions
- The variation of 3 h of milling time was found to be the optimum time to agglomerate and form non-alloyed Al-Ni and Al-Mo powder systems having a homogeneous distribution of elements in their particles.
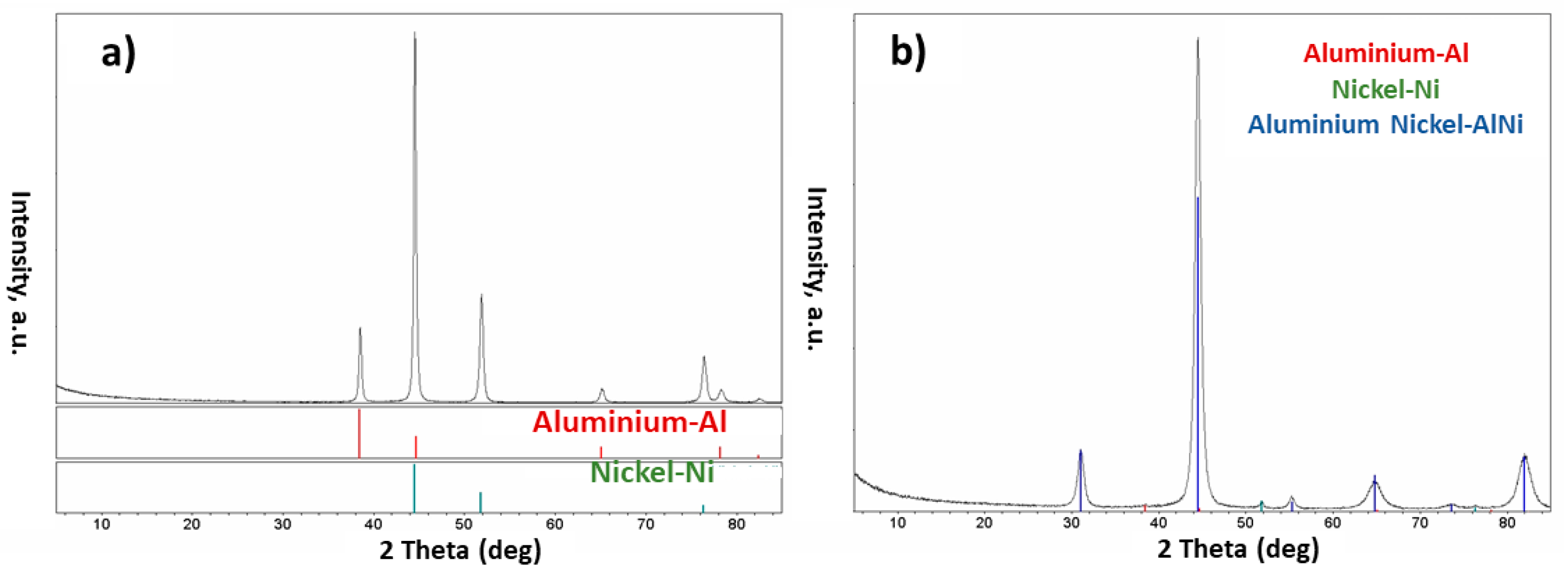
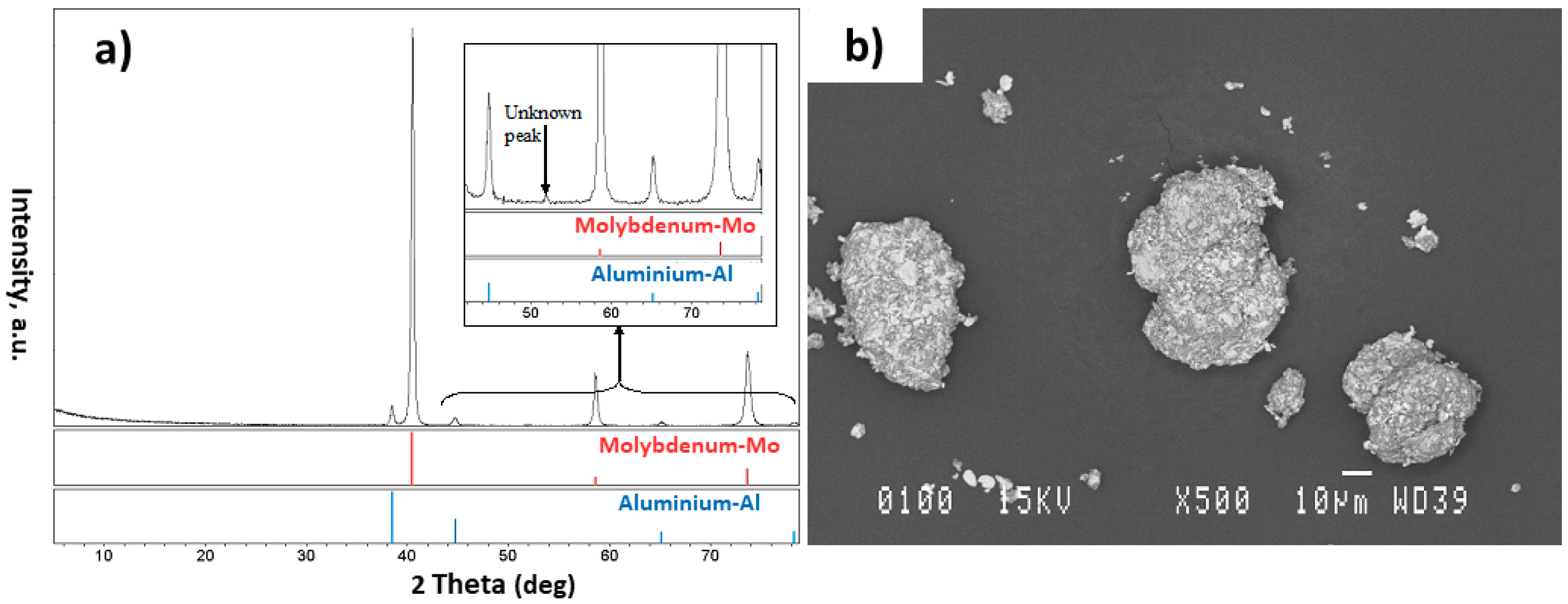
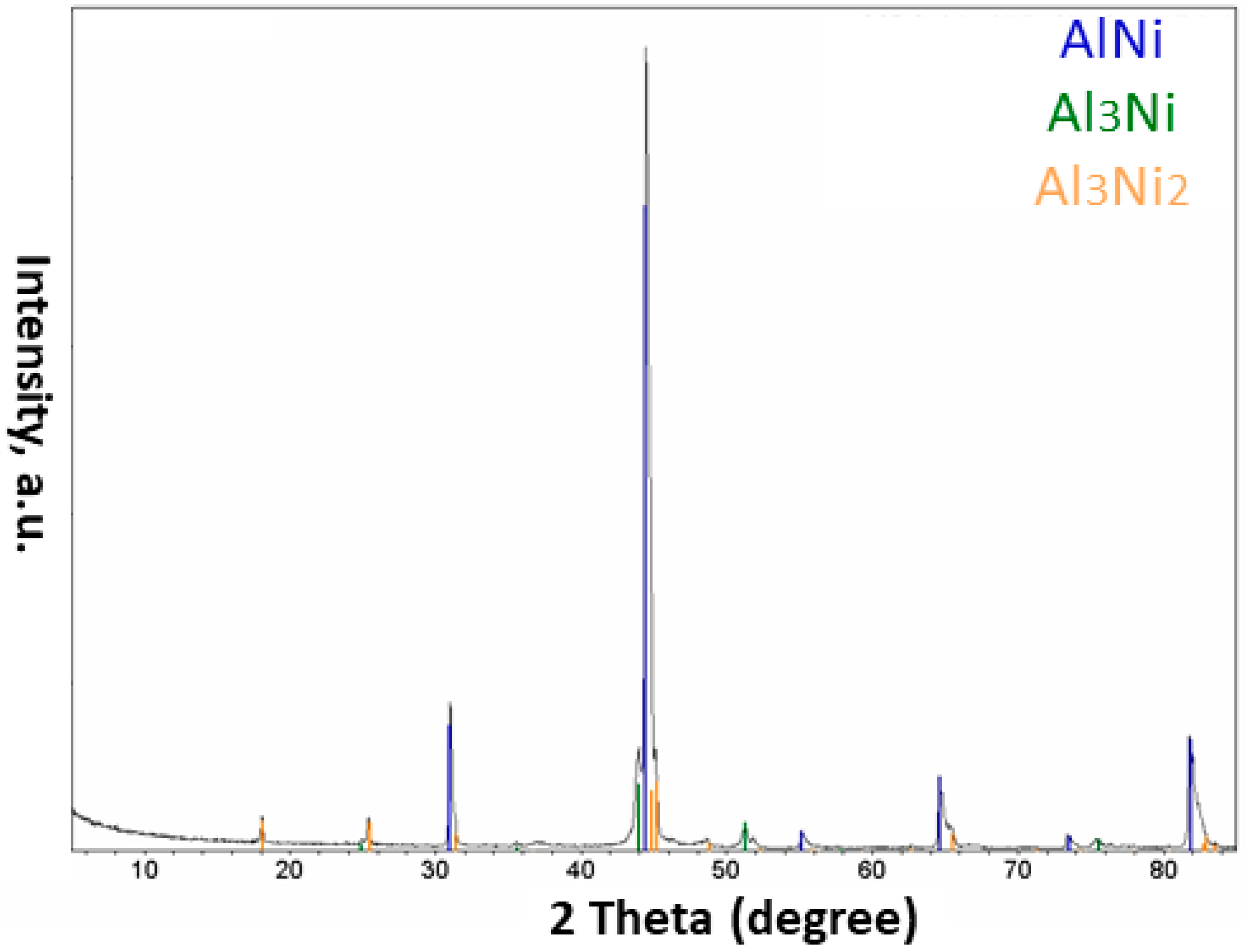
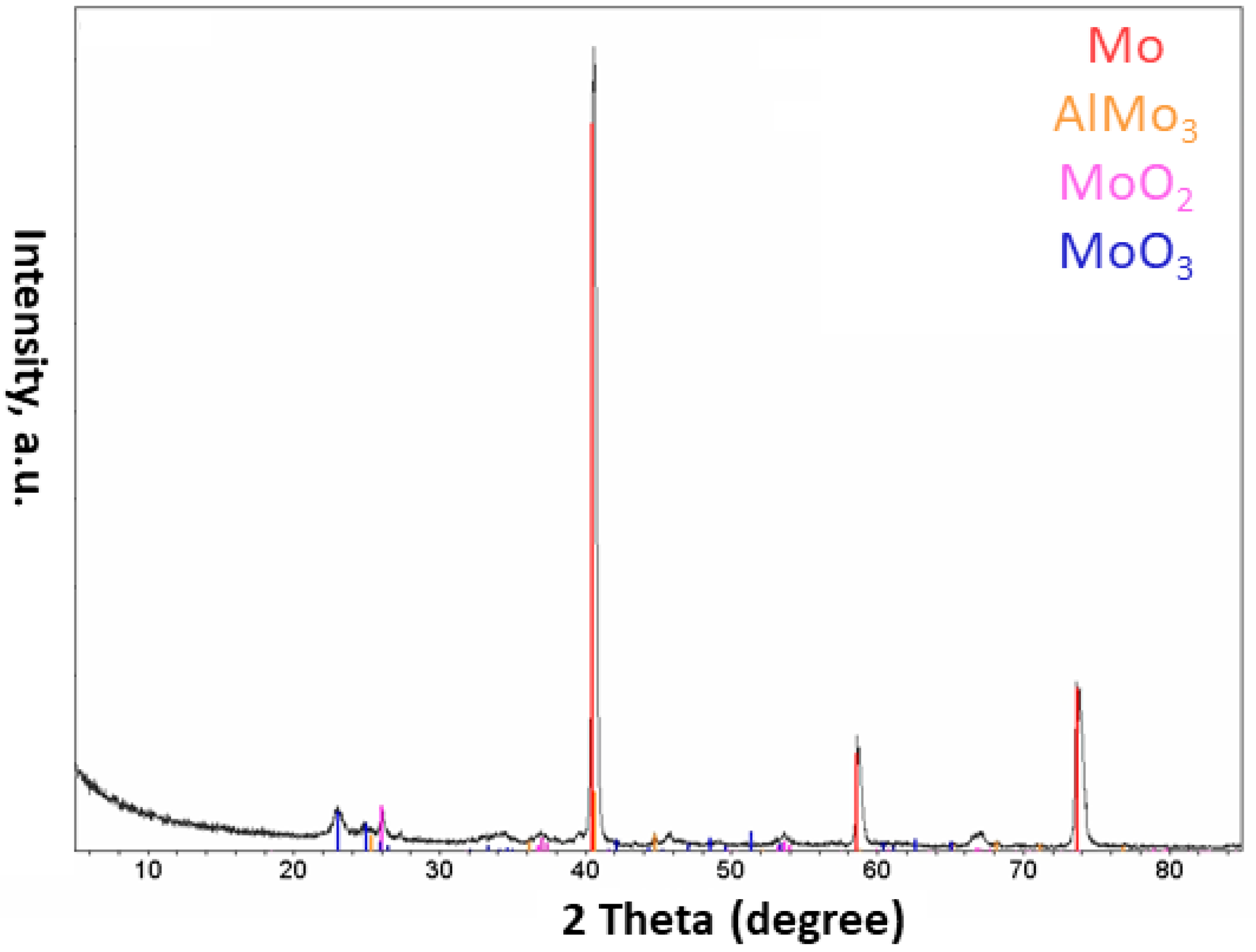
- The injection of each of these composite powders into the plasma system resulted in the formation of different phases in the obtained particles. Different phases such as Al, AlNi3, Al3Ni2, and AlNi were detected in the particles of the Al-Ni powder system, and Al, Mo, AlMo3, MoO3, and MoO2 in the Al-Mo powder system
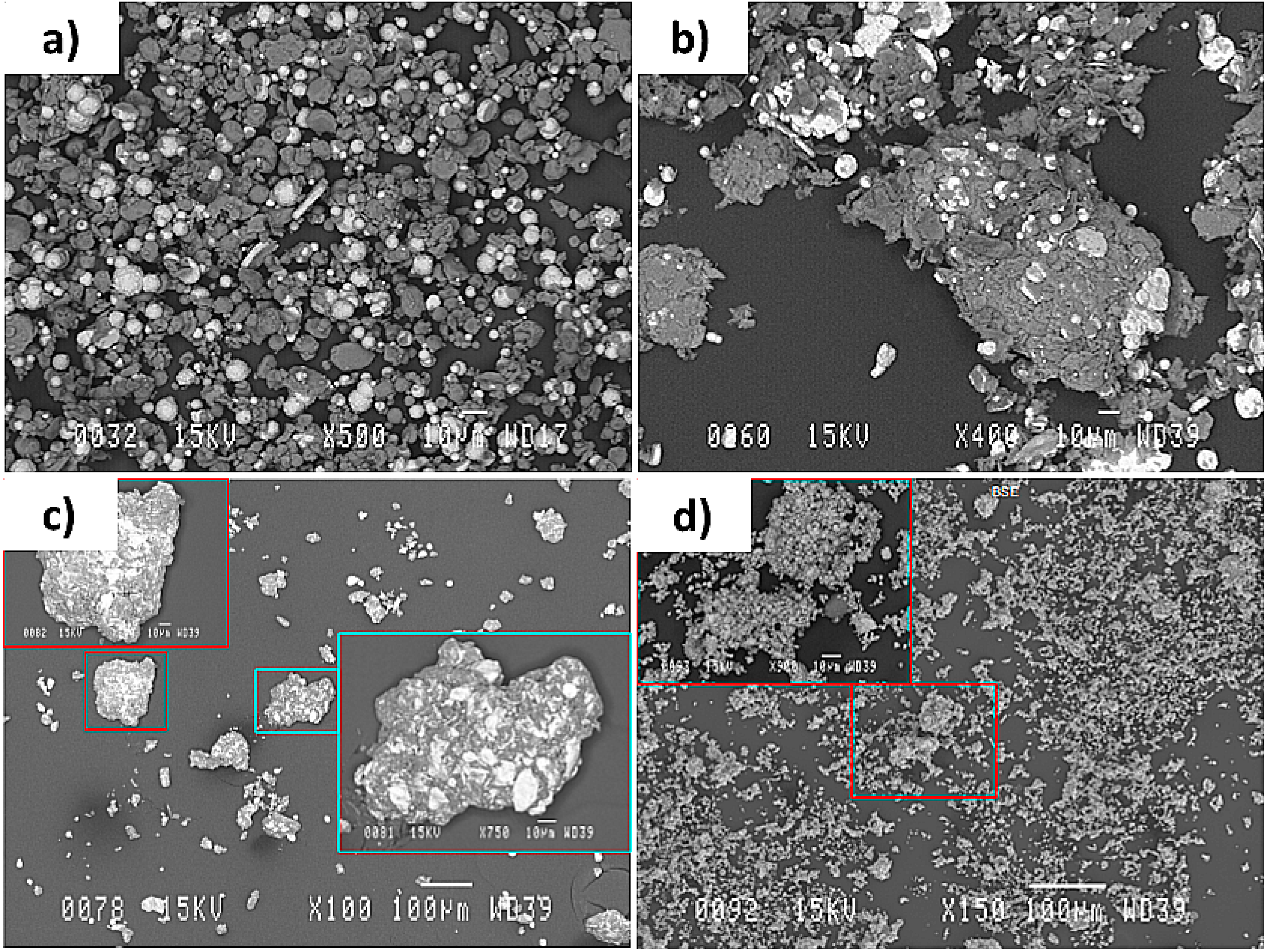
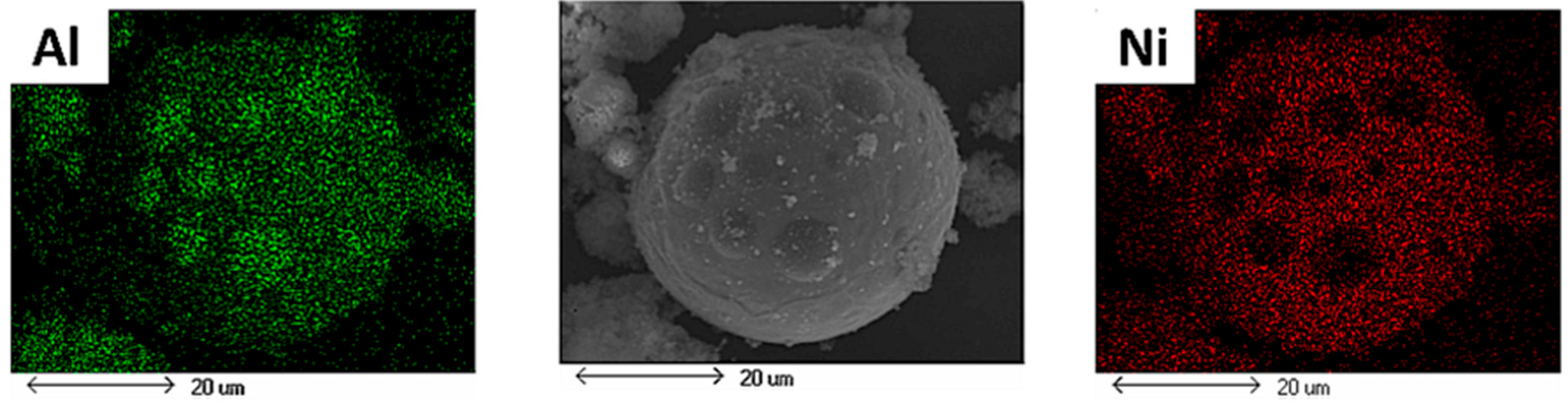
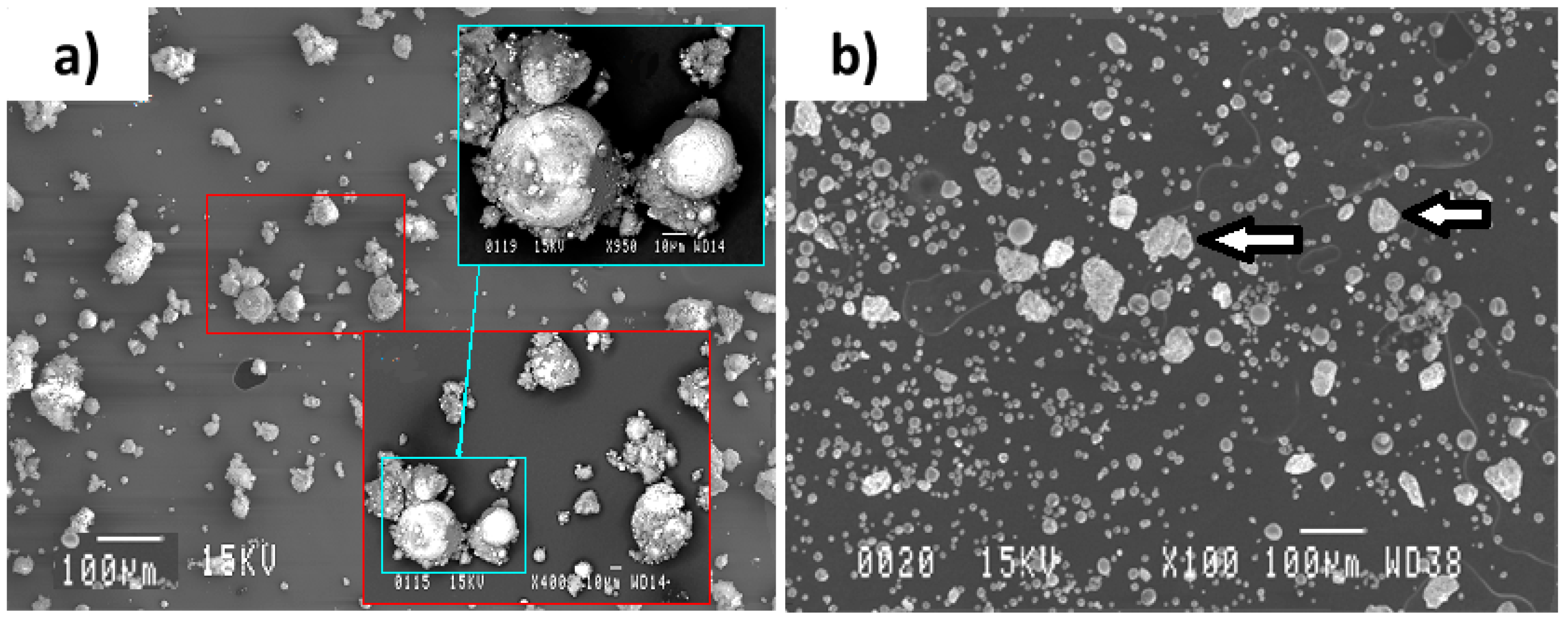
- The powders collected from the reactor wall consisted of large particles being covered within a cloud of very fine particles. The fine particles are thought to be condensed from the vapor phase and the reacted large particles were mostly seen to have Al spots on their surfaces. This shows the diffusion of molten Al into the molten Mo or Ni and was interpreted as a sign of immiscibility of Al into these molten elements
- Particles appearing similar to their initial agglomerated non-treated condition, being absent in the Al-Ni particles, were also seen amongst the large particles of the Al-Mo powder system. This indicates that the level of reaction between Al and Mo was less than that of Al and Ni during the plasma processes.
- It was noted that using a mixture of He and Ar gases, instead of solely using the Ar gas, could prevent the formation of oxides in the Al-Mo plasma treated powder system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czerwinski, F. Current Trends in Automotive Lightweighting Strategies and Materials. Materials 2021, 14, 6631. [Google Scholar] [CrossRef] [PubMed]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A Review on Electric Vehicles: Technologies and Challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Tisza, M.; Czinege, I. Comparative study of the application of steels and aluminium in lightweight production of automotive parts. Int. J. Lightweight Mater. Manuf. 2018, 1, 229–238. [Google Scholar] [CrossRef]
- Deb, A.; Mahendrakumar, M.S.; Chavan, C.; Karve, J.; Blankenburg, D.; Storen, S. Design of an aluminium-based vehicle platform for front impact safety. Int. J. Impact Eng. 2004, 30, 1055–1079. [Google Scholar] [CrossRef]
- Schuster, J.C.; Ipser, H. The Al-Al8Mo3 Section of the Binary System Aluminum-Molybdenum. Metall. Mater. Trans. A 1991, 22, 1729. [Google Scholar] [CrossRef]
- Okamoto, H.J. Al-Ni (aluminum-nickel). Phase Equilibria 1993, 14, 257–259. [Google Scholar] [CrossRef]
- Koinuma, H.; Takeuchi, I. Combinatorial solid-state chemistry of inorganic materials. Nat. Mater. 2004, 3, 429–438. [Google Scholar] [CrossRef]
- Xiang, X.D.; Takeuchi, I. Combinatorial Materials Synthesis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Xiang, X.D.; Sun, X.; Briceno, G.; Lou, Y.; Wang, K.A.; Chang, H. A Combinatorial Approach to Materials Discovery. Science 1995, 268, 1738–1740. [Google Scholar] [CrossRef]
- Xiang, X.D. Combinatorial Materials Synthesis and Screening: An Integrated Materials Chip Approach to Discovery and Optimization of Functional Materials. Annu. Rev. Mater. Sci. 1999, 29, 149–171. [Google Scholar] [CrossRef]
- Zhao, J.C. A combinatorial approach for structural materials. Adv. Eng. Mater. 2001, 3, 143–147. [Google Scholar] [CrossRef]
- Robert, F. High-speed materials design. Science 1997, 277, 474–475. [Google Scholar]
- Daagani, R. Materials a la combi. Chem. Eng. News 2000, 78, 66–68. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Sakhnevich, V.N.; Buravlev, I.Y.; Lembikov, A.O.; Buravleva, A.A.; Azon, S.A.; Yarusova, S.B.; Danilova, S.N.; Fedorets, A.N.; Belov, A.A.; et al. Synthesis of Ti-Cu Multiphase Alloy by Spark Plasma Sintering: Mechanical and Corrosion Properties. Metals 2022, 12, 1089. [Google Scholar] [CrossRef]
- Zhang, T.; Huamg, Z.; Yang, T.; Kong, H.; Luan, J.; Wang, A.; Wang, D.; Kuo, W.; Wang, Y.; Liu, C. In situ design of advanced titanium alloy with concentration modulations by additive manufacturing. Science 2021, 374, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Khanlari, K.; Shi, Q.; Hu, K.; Yan, Z.; Kelly, P.; Cao, P.; Liu, X. A study on the possibility to process dense 60NiTi from elementally blended Ni and Ti powders. Vacuum 2021, 192, 110500. [Google Scholar] [CrossRef]
- Khanlari, K.; Shi, Q.; Li, K.; Hu, K.; Tan, C.; Zhang, W.; Cao, P.; Achouri, I.E.; Liu, X. Fabrication of Ni-Rich 58NiTi and 60NiTi from Elementally Blended Ni and Ti Powders by a Laser Powder Bed Fusion Technique: Their Printing, Homogenization and Densification. Int. J. Mol. Sci. 2022, 23, 9495. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Parvez, M.M.; Liou, F. A Review on Metallic Alloys Fabrication Using Elemental Powder Blends by Laser Powder Directed Energy Deposition Process. Materials 2020, 13, 3562. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Xiao, M.; Mao, X.; Khanlari, K.; Shi, Q.; Liu, X. Fabrication of spherical WC-Co powders by radio frequency inductively coupled plasma and a consequent heat treatment. Powder Technol. 2021, 385, 160–169. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, Y.; Tan, C.; Mao, X.; Khanlari, K.; Liu, X. Preparation of Ni–Ti composite powder using radio frequency plasma spheroidization and its laser powder bed fusion densification. Intermetallics 2021, 136, 107–273. [Google Scholar] [CrossRef]
- Iwao, M.; Okuno, M.; Koyano, M.; Katayama, S.I. Structural changes of SiO2 glass by mechanical milling. J. Mineral. Petrol. Sci. 2010, 105, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Calka, A.; Wexler, D. A Study of the Evolution of Particle Size and Geometry During Ball Milling. J. Metastable Nanocrystalline Mater. 2001, 10, 301–310. [Google Scholar] [CrossRef]


| Powder | O (wt.%) | C (wt.%) |
|---|---|---|
| Al | 0.05 | 0.06 |
| Mo | 0.003 | 0.002 |
| Ni | 0.04 | 0.05 |
| Sheath Gas (L/min) | Central Gas (L/min) | Carrier Gas (L/min) | Pressure (Torr) | Power (kW) |
|---|---|---|---|---|
| 80 | 25 | 6 | 300 | 20 |
| Sheath Gas (L/min) | Central Gas (L/min) | Carrier Gas (L/min) | Pressure (Torr) | Power (kW) |
|---|---|---|---|---|
| 80 | 25 | 6 | 300 | 10 |
| Sheath Gas (L/min) | Central Gas (L/min) | Carrier Gas (L/min) | Pressure (Torr) | Power (kW) |
|---|---|---|---|---|
| Ar: 15-He: 100 | Ar: 25 | He: 3 | 300 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanlari, K.; Achouri, I.E.; Gitzhofer, F. Thermal Plasma Synthesis of Different Alloys and Intermetallics from Ball Milled Al-Mo and Al-Ni Powder Systems. Materials 2022, 15, 8646. https://doi.org/10.3390/ma15238646
Khanlari K, Achouri IE, Gitzhofer F. Thermal Plasma Synthesis of Different Alloys and Intermetallics from Ball Milled Al-Mo and Al-Ni Powder Systems. Materials. 2022; 15(23):8646. https://doi.org/10.3390/ma15238646
Chicago/Turabian StyleKhanlari, Khashayar, Inès Esma Achouri, and Francois Gitzhofer. 2022. "Thermal Plasma Synthesis of Different Alloys and Intermetallics from Ball Milled Al-Mo and Al-Ni Powder Systems" Materials 15, no. 23: 8646. https://doi.org/10.3390/ma15238646





