First-Principles Computational Study of the Modification Mechanism of Graphene/Graphene Oxide on Hydroxyapatite
Abstract
:1. Introduction
2. Calculation Method and Model
2.1. Calculation Method
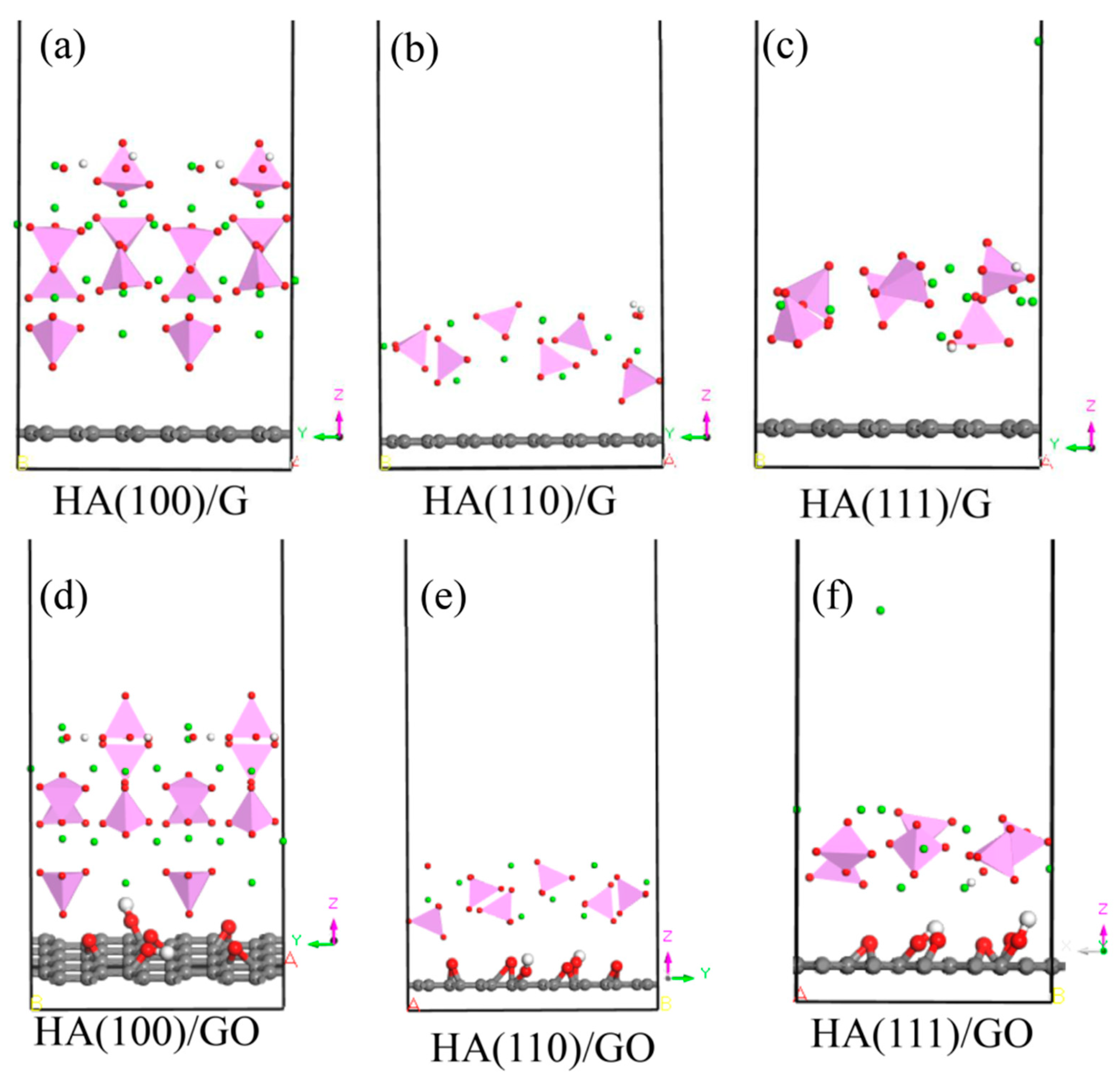
2.2. Establishment of Model
3. Discussion and Analysis of Results
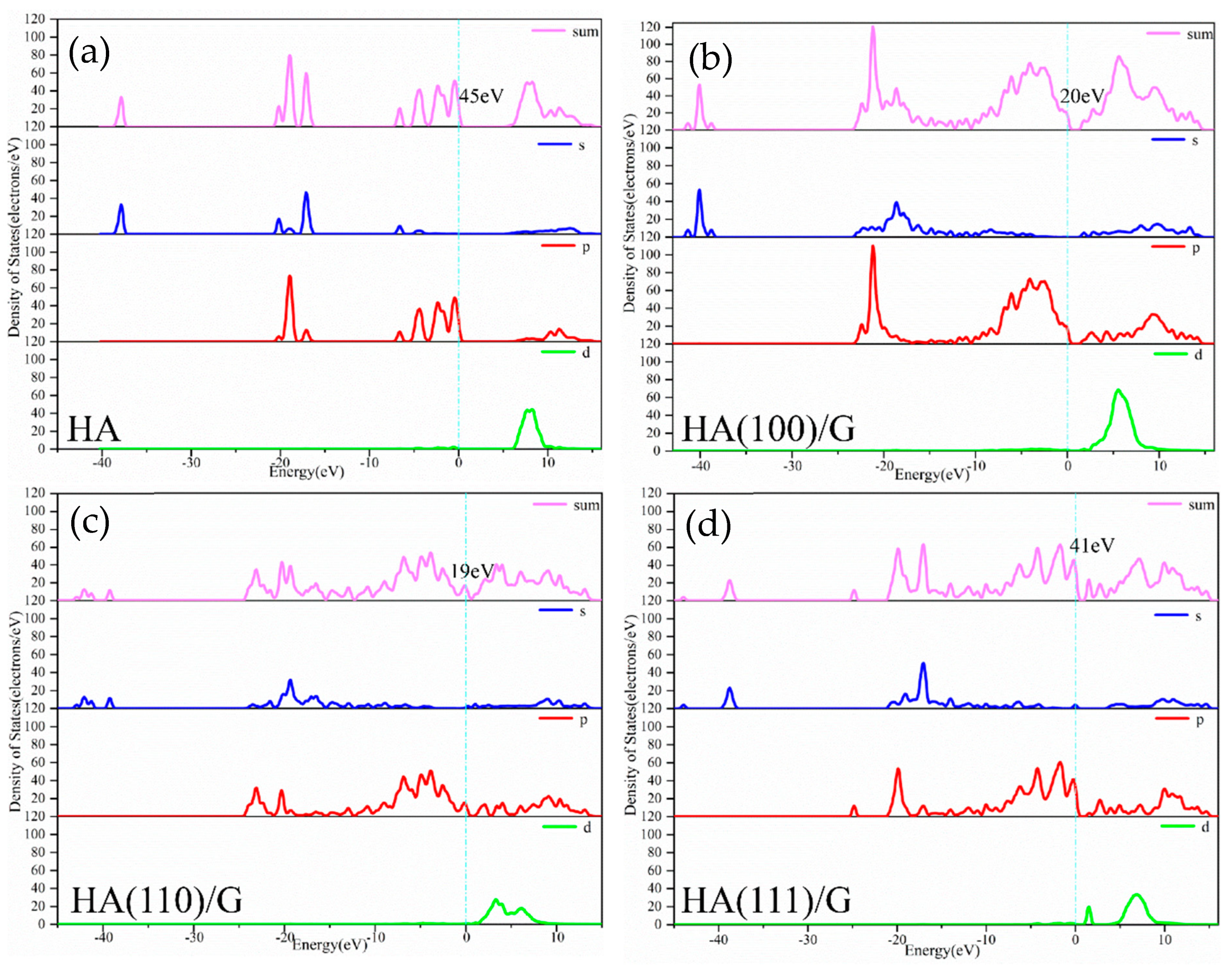
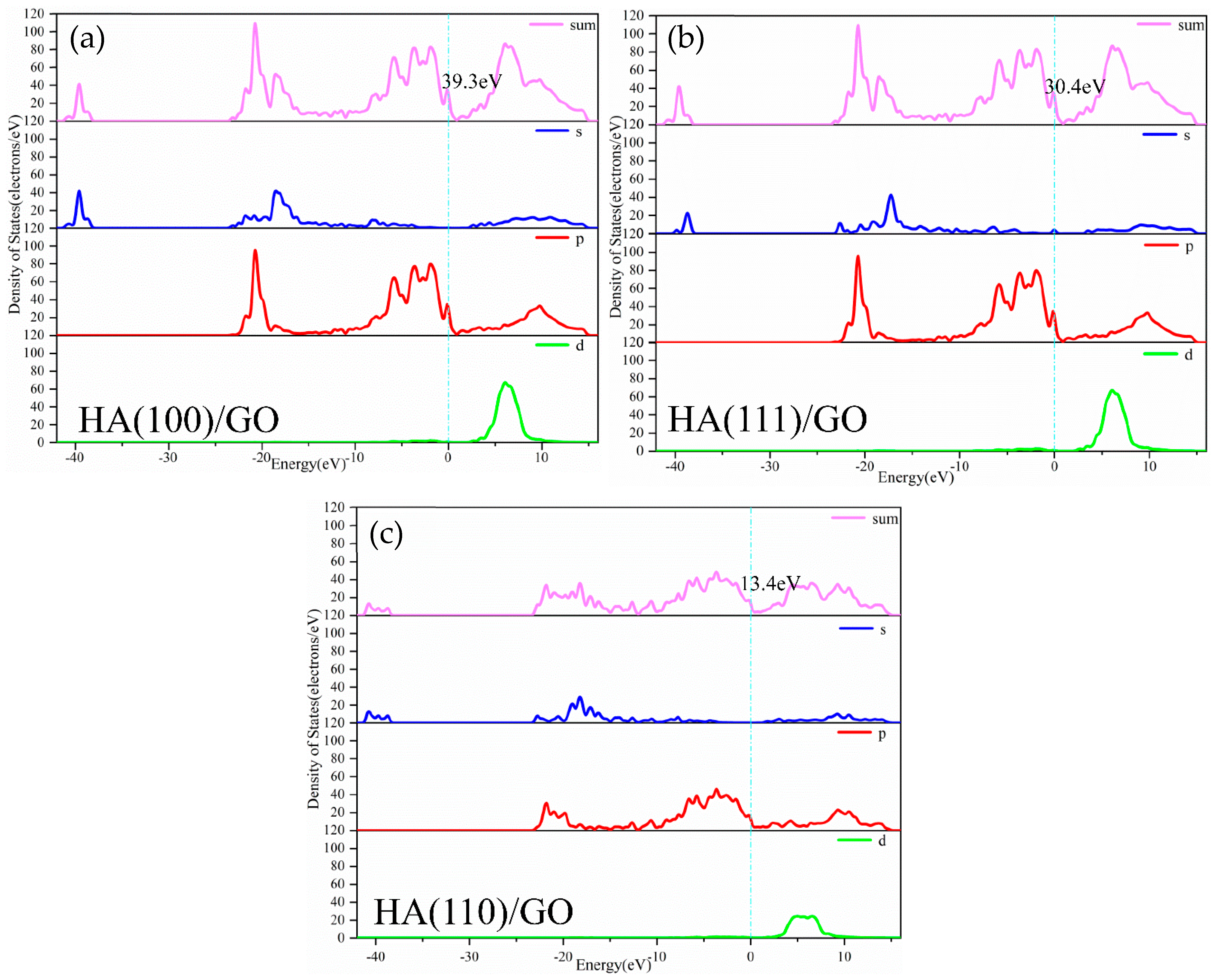
3.1. Density of States
3.2. Differential Charge Density
3.3. Interface Adhesion Work
3.4. Elastic Modulus
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Yin, D.G.; Gou, J.H.; Yuan, Q.; Zhang, Z.L. Mechanisms of Large Tensile Stiffness and High Fracture Strength of Bone. J. Comput. Theor. Nanosci. 2022, 6, 1425–1428. [Google Scholar] [CrossRef]
- Bunz, O.; Steegmann, M.C.; Benz, K.; Testrich, H.; Quade, A.; Naumova, E.A.; Arnold, W.H.; Fricke, K.; Piwowarczyk, A.; Dittmar, T. Human Gingival Fibroblast Adhesion and Proliferation on Hydroxyapatite-Coated Zirconia Abutment Surfaces. Materials 2022, 10, 3625. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xi, X.; Fang, J.J.; Liu, P.; Cai, K.Y. Influences of magnetized hydroxyapatite on the growth behaviors of osteoblasts and the mechanism from molecular dynamics simulation. Mater. Sci. Eng. C 2013, 33, 3753–3759. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.W.; Xie, J.; Li, L.J.; Wang, X.C.; Zhang, T. First-principles study of electronic structure of fluorapatite, chlorapatite and hydroxyapatite. Min. Metall. Eng. 2017, 37, 84–90. [Google Scholar]
- Zhao, J.H.; Lei, Y.L.; Chu, T.; Hu, Q.; Li, H.K.; Xu, H. Effect of surface modification of polyethylene glycol on the properties of nanocomposite insulating paper. Trans. China Pulp Pap. 2022, 37, 49–56. [Google Scholar]
- Chauhan, I.; Aggrawal, S.; Chandravati Mohanty, P. Metal oxide nanostructures incorporated/immobilized paper matrices and their applications: A review. RSC Adv. 2015, 5, 83036–83055. [Google Scholar] [CrossRef]
- Li, H.; Zhu, Y.J. Nanowires: Synthesis and Energy/Environmental Applications. Energy Environ. Mater. 2021, 4, 544–561. [Google Scholar] [CrossRef]
- Oprea, M.; Voicu, S.I. Recent Advances in Applications of Cellulose Derivatives-Based Composite Membranes with Hydroxyapatite. Materials 2020, 13, 2481. [Google Scholar] [CrossRef]
- Halim NA, A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials-Upconverted Hydroxyapatite for Bone Tissue Engineering and a Platform for Drug Delivery. Int. J. Nanomed. 2021, 16, 6477–6496. [Google Scholar] [CrossRef]
- Huang, J.Z.; Songfeng, E.; Si, L.M.; Li, J.Y.; Tian, Z.J.; Lu, Z.Q. Composite films of hydroxyethyl cellulose and hydroxyapatite nanowires with high mechanical strength and electrical insulation property. J. Wood Chem. Technol. 2022, 42, 15–25. [Google Scholar] [CrossRef]
- Dasgupta, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Effect of grain size on mechanical, surface and biological properties of microwave sintered hydroxyapatite. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2846–2854. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Zhao, X.; Ni, S.L.; Lu, J.J.; Yang, Y.L. Research Progress of Composite or Modified Hydroxyapatite Microspheres as Scaffold Material. Med. Recapitul. 2020, 26, 4168–4172. [Google Scholar]
- Knupp, W.G.; Ribeiro, M.S.; Mir, M.; Camps, I. Dynamics of hydroxyapatite and carbon nanotubes interaction. Appl. Surf. Sci. 2019, 495, 30. [Google Scholar] [CrossRef]
- Li, C.Y.; Yan, T.T.; Lou, Z.K.; Jiang, Z.M.; Shi, Z.; Chen, Q.H.; Gong, Z.Q.; Wang, B. Characterization and in vitro assessment of three-dimensional extrusion Mg-Sr codoped SiO2-complexed porous microhydroxyapatite whisker scaffolds for biomedical engineering. Biomed. Eng. OnLine 2021, 20, 116. [Google Scholar] [CrossRef]
- Wu, Y.H.; Cao, Q.L.; Niu, M.Y.; Feng, C.; Li, X.F.; Zhu, X.D.; Zhang, X.D. Comparative studies on micromechanical properties and biological performances in hydroxyapatite ceramics with micro/nanocrystalline. J. Am. Ceram. Soc. 2021, 105, 742–756. [Google Scholar] [CrossRef]
- Shang, S.C.; Zhao, Q.; Zhang, D.Q.; Sun, R.X.; Tang, Y.Z. Molecular dynamics simulation of the adsorption behavior of two different drugs on hydroxyapatite and Zn-doped hydroxyapatite. Mater. Sci. Eng. 2019, 105, 110017.1–110017.5. [Google Scholar] [CrossRef]
- Yuan, Q.H.; Chen, Z.H.; Wan, L.; Shi, X.; Lin, S.X.; Zhang, Z.Q.; Xu, A.P.; Deng, L.B.; Yi, Z. Preparation and characterization of Sr-doped hydroxyapatite-graphene composites. J. Shenzhen Univ. (Sci. Technol.) 2020, 37, 298–304. [Google Scholar] [CrossRef]
- Duong, H.M.; Myint, S.M.; Tran, T.Q.; Le, D.K. Chapter 6—Post-spinning treatments to carbon nanotube fibers. In Carbon Nanotube Fibers and Yarns; The Textile Institute Book, Series; Miao, M., Ed.; Woodhead Publishing: Sawston, UK, 2020; pp. 103–134. [Google Scholar]
- Duong, H.M.; Tran, T.Q.; Kopp, R.; Myint, S.M.; Peng, L. Chapter 1—Direct Spinning of Horizontally Aligned Carbon Nanotube Fibers and Films From the Floating Catalyst Method. In Nanotube Superfiber Materials, 2nd ed.; Micro and Nano, Technologies; Schulz Mark, J., Shanov, V., Yin, Z., Cahay, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 3–29. [Google Scholar]
- Ming, S.F.; Song, H.Y.; An, M.R. Mechanical behavior of graphene magnesium matrix composites based on molecular dynamics simulation. J. Phys. 2022, 71, 266–274. [Google Scholar] [CrossRef]
- Liang, F.X.; Yang, K.L.; Li, Y.L.; Lu, W.T. Electronic properties of holey graphene nanosheets. J. At. Mol. Phys. 2023, 40, 79–82. [Google Scholar]
- Omoniyi, F.O.; Alabi, A.B.; Salawu, M.A.; Buba, A.D.A. Synthesis and Characterization of Graphene-Cu Composite Via Hydrothermal Method. J. Sustain. Mater. Process. Manag. 2022, 2, 30–40. [Google Scholar]
- Kakanakova-Georgieva, A.; Giannazzo, F.; Nicotra, G.; Cora, I.; Péczet, B. Material Proposal for 2D Indium Oxide. Appl. Surf. Sci. 2021, 548, 149275. [Google Scholar] [CrossRef]
- Lundgren, C.; Kakanakovageorgieva, A.; Gueorguiev, G.K. A perspective on thermal stability and mechanical properties of 2D Indium Bismide from ab initio molecular dynamics. Nanotechnology 2022, 33, 335706. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Chen, B.; Chen, J.; Dong, Y.; Liu, J.H.; Xu, L. Effect of graphene oxide on the function of erythrocytes. Chin. J. Biotech 2021, 37, 4047–4055. [Google Scholar]
- Dong, S.N.; Sun, Y.Y.; Gao, B.; Shi, X.Q.; Xu, H.X.; Wu, J.F.; Wu, J.C. Retention and transport of graphene oxide in water-saturated limestone media. Chemosphere 2017, 180, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Sarraf-Mamoory, R.; Perez, M.C.; Le, D.Q.S.; Emameh, R.Z.; Bünger, C.E. Characteristics of hydroxyapatite-reduced graphene oxide composite powders synthesized via hydrothermal method in the absence and presence of diethylene glycol. Open Ceram. 2021, 5, 100067. [Google Scholar] [CrossRef]
- Zhang, L. Mechanical and Biocompatibility Properties of a Graphene Nanosheet Toughened Hydroxyapatite Ceramic Composite; Suzhou University: Suzhou, China, 2014. (In Chinese) [Google Scholar]
- Liu, Y.; Huang, J.; Niinomi, M.; Li, H. Inhibited grain growth in hydroxyapatite–graphene nanocomposites during high temperature treatment and their enhanced mechanical properties. Ceram. Int. 2016, 42, 11248–11255. [Google Scholar] [CrossRef]
- Fan, Z.J.; Wang, J.Q.; Wang, Z.F.; Ran, H.Q.; Li, Y.; Niu, L.Y.; Gong, P.W.; Liu, B.; Yang, S.R. One-pot synthesis of graphene/hydroxyapatite nanorod composite for tissue engineering. Carbon 2014, 66, 407–416. [Google Scholar] [CrossRef]
- Yu, T. Study of Photoelectric Properties of Silicene on III-V Semiconductor Substrates; Beijing Jiaotong University: Beijing, China, 2021. (In Chinese) [Google Scholar]
- Wang, Y.Q.; Yu, M.R.; Wu, B.C.; Sun, Z.Y.; Yu, X.; Liu, Z.G.; Shang, T.Q.; Gu, H.W. Exploration of electronic structure and energy changes of cobalt-nickel on 1T-MoS2 surface. Surf. Innov. 2019, 7, 174–183. [Google Scholar] [CrossRef]
- Song, Q.; Gao, H.Q.; Wang, Y.X.; Li, L. Controllable synthesis of hydroxyapatite/graphene oxide(HAP/GO) and its application in the sustained release of ibuprofen. J. Hubei Univ. (Nat. Sci.) 2022, 44, 270–276. [Google Scholar]
- Zhao, D.L.; Lu, Y.W. Properties of interfacial structure between composite semiconductor graphene and gallium nitride. China Sci. Technol. Inf. 2017, 7, 74–76. [Google Scholar]
- Li, Q.W. Atomic-Scale Study on the Interface Properties and Mechanical Behavior of Graphene Reinforced Aluminium-Silicon Alloy Matrix Composites; Harbin Institute of Technology: Harbin, China, 2020. (In Chinese) [Google Scholar]
- Sun, S.Y.; Chi, Z.B.; Xu, P.P.; Ze-Yu, A.; Jun-Hao, Z.; Xin, T.; Yuan, R. First principles study on the formation and properties of diamond (111)/Al interface. Acta Phys. Sin. 2021, 70, 335–341. [Google Scholar] [CrossRef]
- Wu, J.B. Zn-Doped Hydroxyapatite Composite (Polylactic Acid or Graphene) Preparation and Simulation Research; Shenzhen University: Shenzhen, China, 2017. (In Chinese) [Google Scholar]
- Chen, F.; Ren, Y.Y.; He, L.; An, C.W.; Wen, S.F.; Shen, F.F. Molecular dynamics simulation of interface interaction and mechanical properties of PYX—Based polymer bonded explosives(PBX). J. At. Mol. Phys. 2022, 39, 167–172. [Google Scholar]
- Ucar, N.; Olmez, M.; Kayaoglu, B.K.; Onen, A.; Yavuz, N.K.; Eksik, O. Structural properties of graphene oxide fibers: From graphene oxide dispersion until continuous graphene oxide fiber. J. Text. Inst. 2019, 109, 1642–1652. [Google Scholar]


| /eV | /eV | /eV | A/nm2 | Wad/J/m2 | |
|---|---|---|---|---|---|
| HA (100)/G | −45,683.534 | −75,52.622 | −53,239.145 | 1.289 | 0.371 |
| HA (110)/G | −22,551.869 | −7450.200 | −30,007.251 | 1.200 | 0.691 |
| HA (111)/G | −22,553.747 | −9310.013 | −31,865.209 | 1.479 | 0.157 |
| HA (100)/GO | −45,126.202 | −10,085.343 | −55,219.135 | 1.289 | 0.942 |
| HA (110)/GO | −22,556.637 | −8768.421 | −31,332.868 | 1.200 | 1.041 |
| HA (111)/GO | −22,548.922 | −11,948.078 | −34,502.087 | 1.479 | 0.678 |
| Bulk Modulus (B)/GPa | Shear Modulus (G)/GPa | Young’s Modulus (E)/GPa | |
|---|---|---|---|
| HA | 59.54 | 31.01 | 78.12 |
| HA (100)/G | 134.18 | 47.88 | 264.17 |
| HA (110)/G | 138.07 | 49.28 | 279.83 |
| HA (111)/G | 69.86 | 33.34 | 185.87 |
| HA (100)/GO | 57.20 | 31.88 | 130.58 |
| HA (110)/GO | 68.76 | 32.59 | 139.48 |
| HA (111)/GO | 56.37 | 30.49 | 134.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xie, M.; Zhou, Z.; Junaid, M.; Zong, W.; Du, S. First-Principles Computational Study of the Modification Mechanism of Graphene/Graphene Oxide on Hydroxyapatite. Materials 2022, 15, 8652. https://doi.org/10.3390/ma15238652
Wang Y, Xie M, Zhou Z, Junaid M, Zong W, Du S. First-Principles Computational Study of the Modification Mechanism of Graphene/Graphene Oxide on Hydroxyapatite. Materials. 2022; 15(23):8652. https://doi.org/10.3390/ma15238652
Chicago/Turabian StyleWang, Yanqing, Minghui Xie, Zheng Zhou, Muhammad Junaid, Weilin Zong, and Shengyang Du. 2022. "First-Principles Computational Study of the Modification Mechanism of Graphene/Graphene Oxide on Hydroxyapatite" Materials 15, no. 23: 8652. https://doi.org/10.3390/ma15238652






