Development of Natural Plant Extracts as Sustainable Inhibitors for Efficient Protection of Mild Steel: Experimental and First-Principles Multi-Level Computational Methods
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Plant Extracts
2.2. Metal Specimens and Test Solutions
2.3. WL Measurements
2.4. Evaluation of Electrochemical Corrosion
2.5. Surface Characterization
2.6. Theoretical Details and Models
3. Results and Discussion
3.1. Assessment of Inhibitors Concentration Effect
3.1.1. Weight Loss Measurements
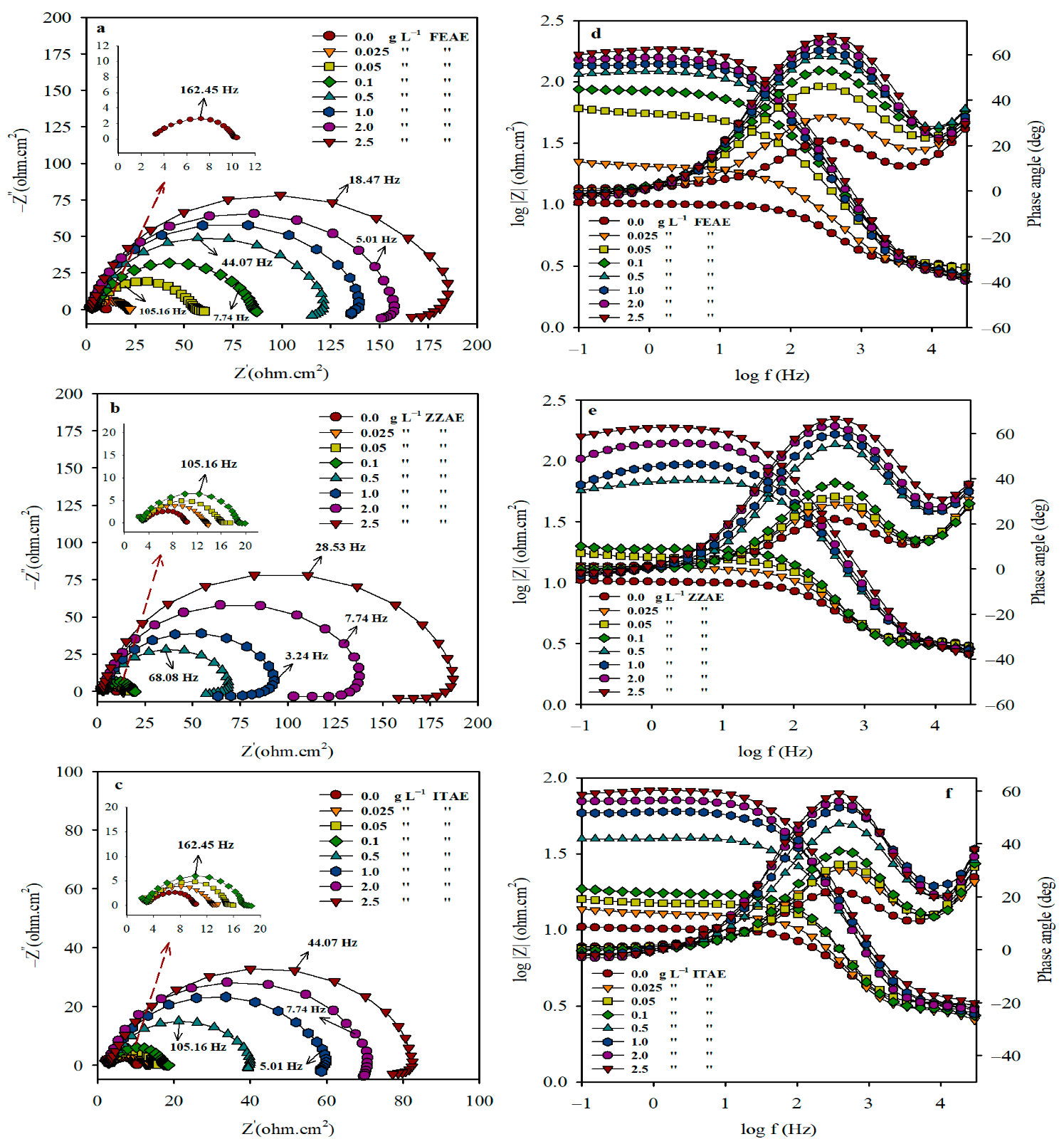
3.1.2. EIS Coupled with Equivalent Circuit Model
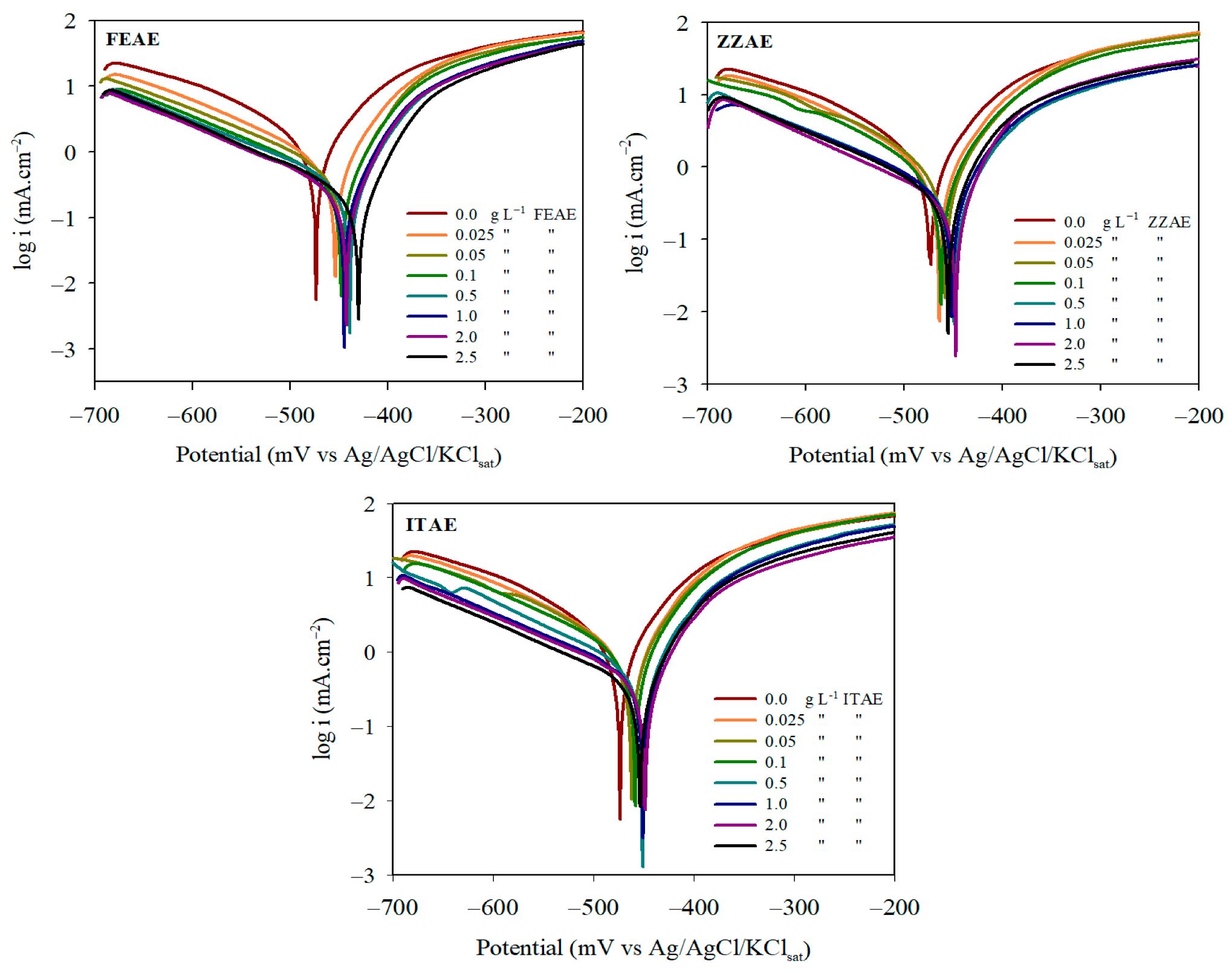
3.1.3. Polarization Behavior
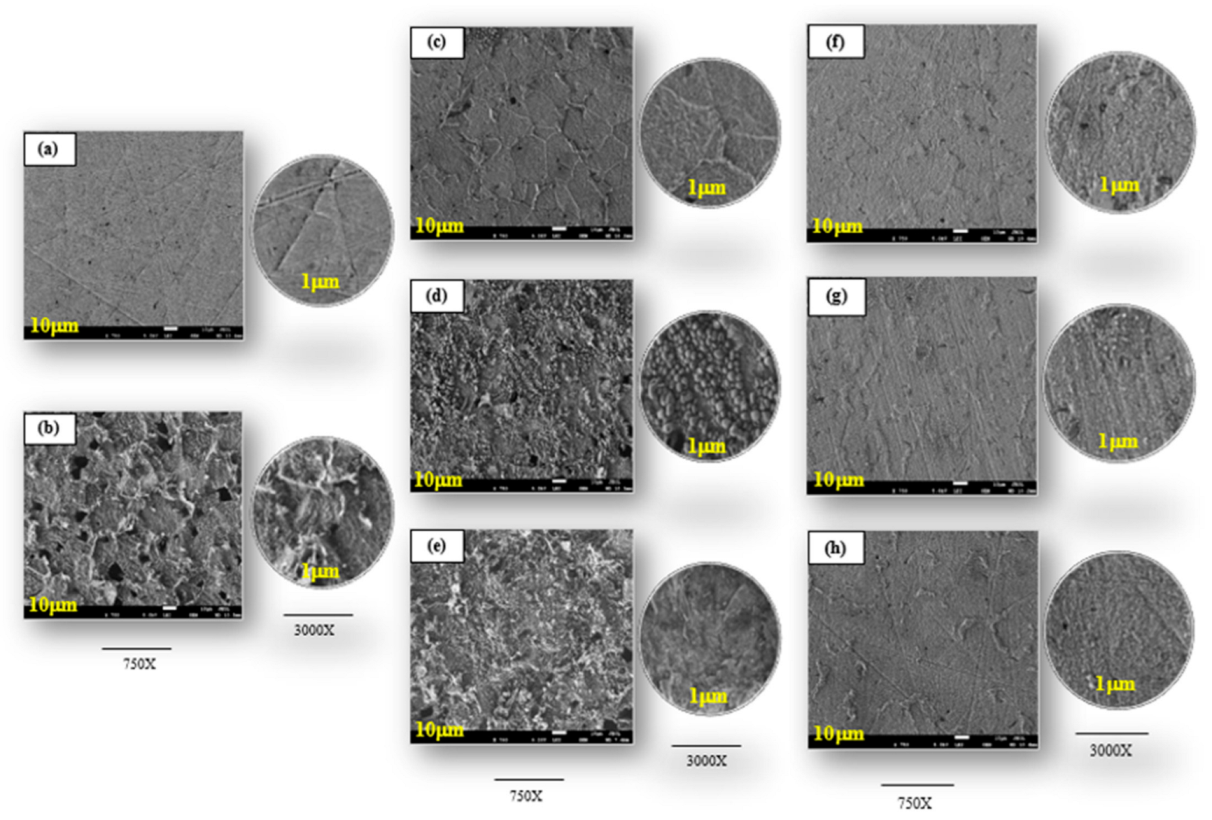
3.2. Morphological Analysis
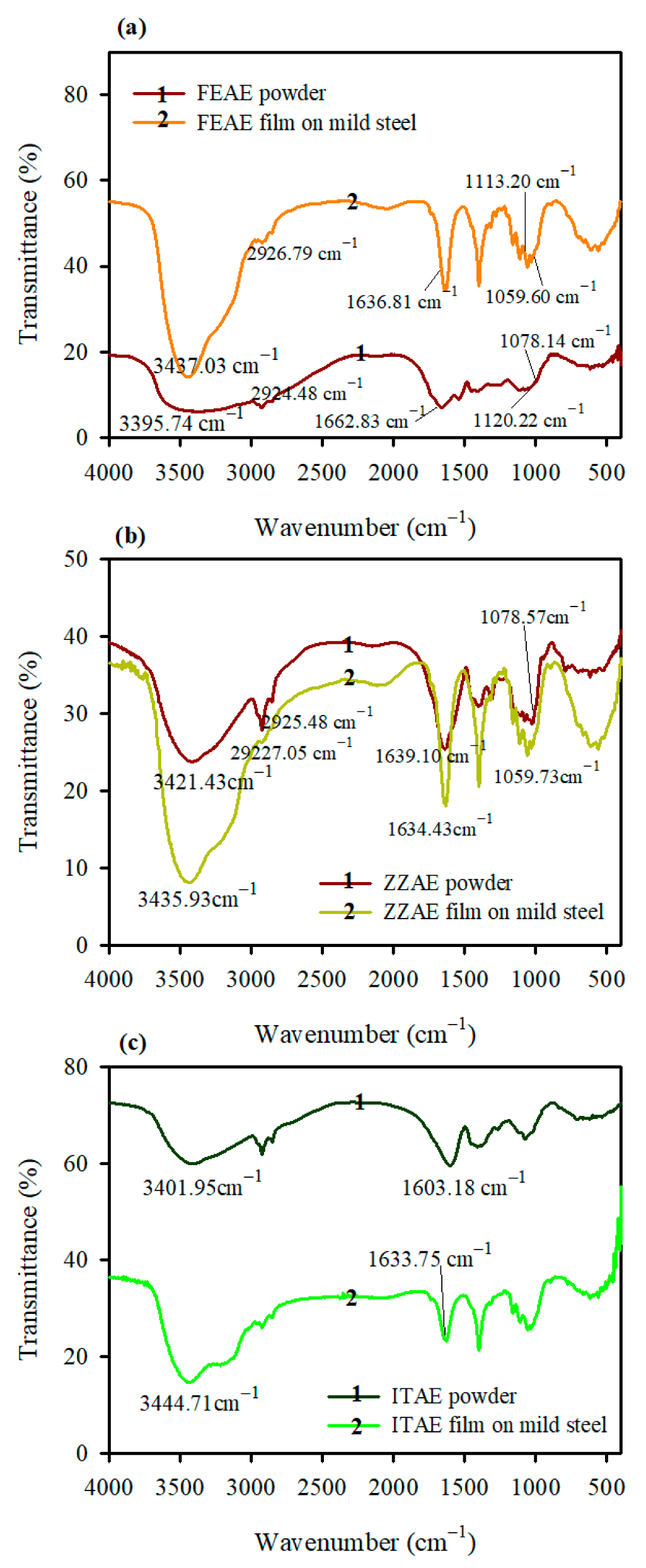
3.3. FT-IR Analysis
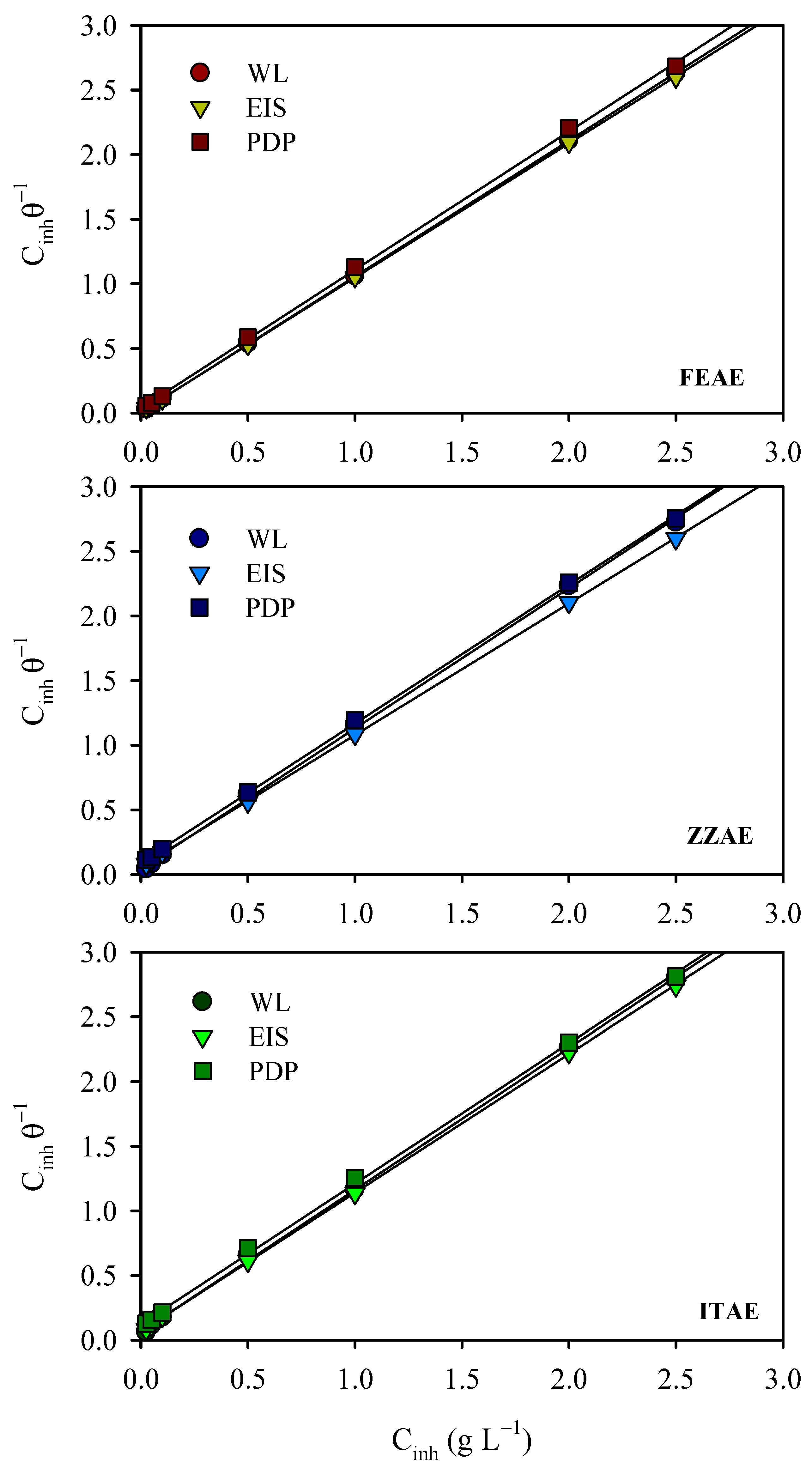
3.4. Adsorption Isotherms
3.5. Effect of Temperature on Electrochemical Stability and Thermodynamic Behavior
3.6. Computational Approaches
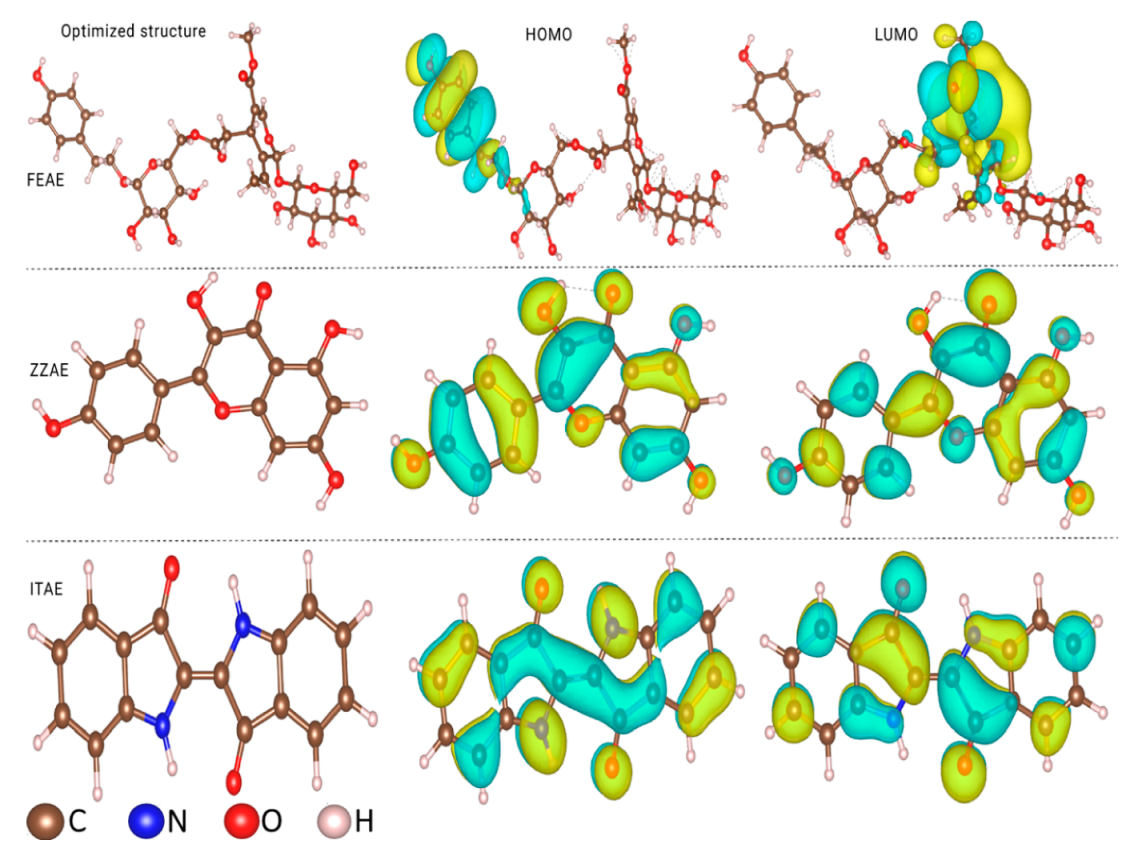
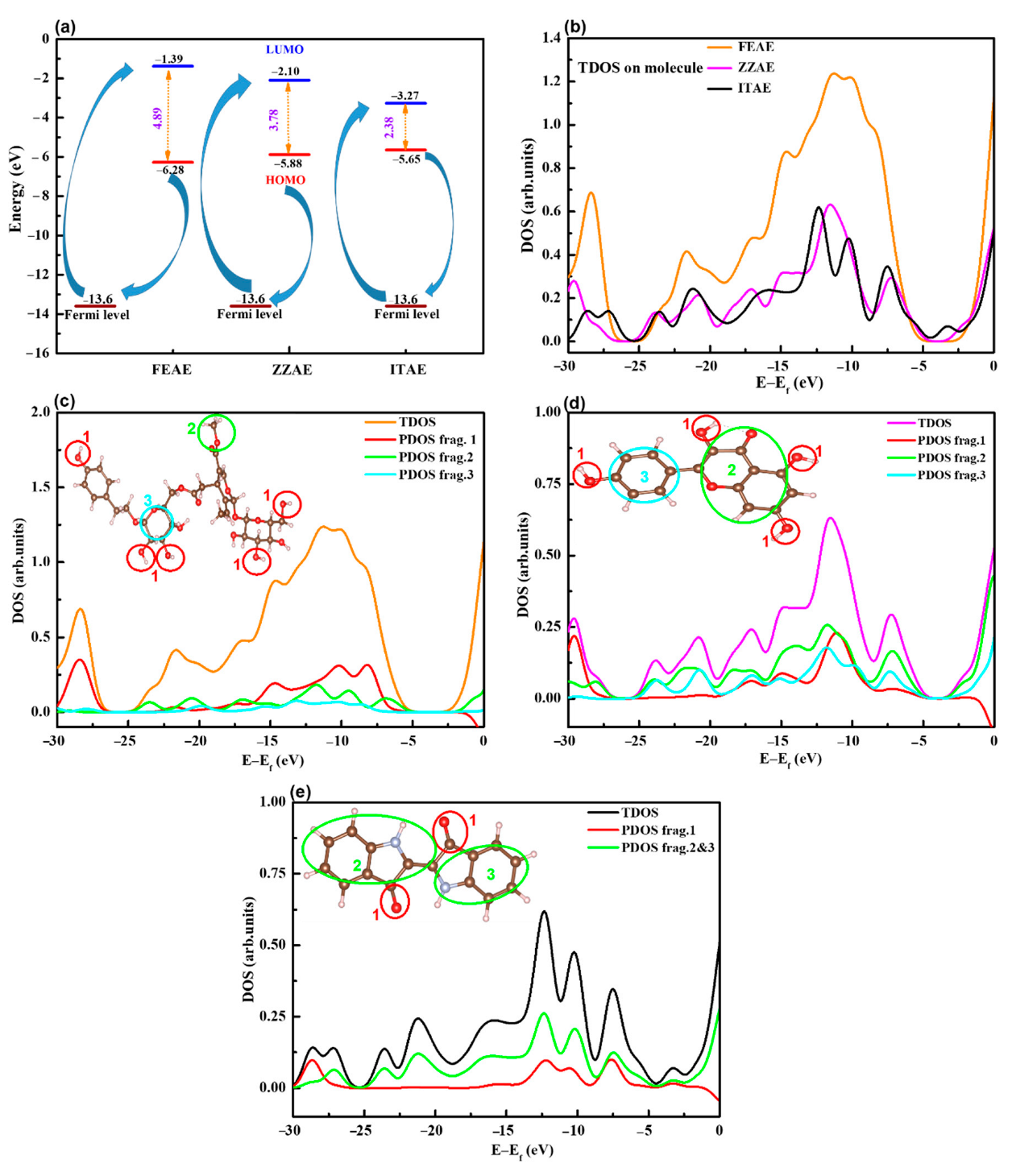
3.6.1. Molecular Electronic Properties of Isolated Molecules
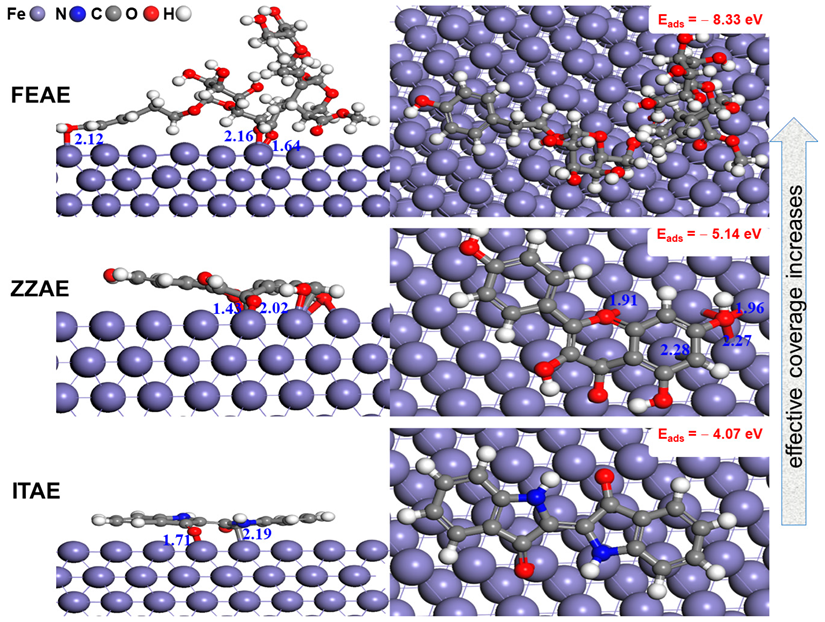
3.6.2. Adsorption of Inhibitors on Fe(110) Surface Based on First-Principles DFT Calculations
3.6.3. Molecular Dynamics Simulations
3.7. Comparison with Other Plants Used as Inhibitors for Steels in H3PO4 Acid
4. Conclusions
- Regardless of the technique used, the inhibition efficiency for mild steel corrosion in 2 M H3PO4 increased with the inhibitor concentration.
- At 2.5 g L−1, the inhibition ability of the plant extracts followed the order: FEAE (95.1 %) > ZZAE (91.7 %) > ITAE (89.4 %), which was confirmed by surface analyses based on SEM and electrochemical measurements.
- The plant extracts act as mixed-type inhibitors by simply blocking the anodic and cathodic active sites without changing the corrosion mechanism.
- The Cdl value decreases significantly with the addition of the extracts, indicating that the thickness of the film adsorbed on the steel surface depends on the concentration of the extracts.
- The SEM and FT-IR techniques confirmed the formation of the adsorbed film. The adsorbed layers exhibited effective anti-corrosion behavior.
- The adsorption of the extracts obeyed the Langmuir adsorption isotherm, and the free energy of adsorption indicated a mixed type of adsorption for the inhibitor species on the metal surface.
- The adsorption of the extracts increased considerably in the parallel mode with the increase in the number of lone-pair electrons (reactive sites) in the molecular structures.
- Direct chemisorptive interactions through the π-current of the aromatic rings and functional groups played a leading role in the stability of the parallel arrangement of the studied inhibitors.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tzaneva, B.; Loukaycheva, M.; Fachikov, L. Effect of Phosphoric Acid Concentration on Corrosion of Cr–Mn–N and Cr–Ni Stainless Steels. Corros. Eng. Sci. Technol. 2014, 49, 219–227. [Google Scholar] [CrossRef]
- Nigam, A.; Tripathi, R.; Dhoot, K. The Effect of Phosphoric Acid on Rust Studied by Mössbauer Spectroscopy. Corros. Sci. 1990, 30, 799–803. [Google Scholar] [CrossRef]
- Yang, H.-M. Role of Organic and Eco-Friendly Inhibitors on the Corrosion Mitigation of Steel in Acidic Environments—A State-of-Art Review. Molecules 2021, 26, 3473. [Google Scholar] [CrossRef] [PubMed]
- Avdeev, Y.G. Protection of Metals in Phosphoric Acid Solutions by Corrosion Inhibitors. A Review. Int. J. Corros. Scale Inhib. 2019, 8, 760–798. [Google Scholar]
- Li, X.; Deng, S.; Fu, H. Allyl Thiourea as a Corrosion Inhibitor for Cold Rolled Steel in H3PO4 Solution. Corros. Sci. 2012, 55, 280–288. [Google Scholar] [CrossRef]
- Benabdellah, M.; Aouniti, A.; Dafali, A.; Hammouti, B.; Benkaddour, M.; Yahyi, A.; Ettouhami, A. Investigation of the Inhibitive Effect of Triphenyltin 2-Thiophene Carboxylate on Corrosion of Steel in 2 M H3PO4 Solutions. Appl. Surf. Sci. 2006, 252, 8341–8347. [Google Scholar] [CrossRef]
- Noor, E.A. The Inhibition of Mild Steel Corrosion in Phosphoric Acid Solutions by Some N-Heterocyclic Compounds in the Salt Form. Corros. Sci. 2005, 47, 33–55. [Google Scholar] [CrossRef]
- Zarrok, H.; Zarrouk, A.; Salghi, R.; Hammouti, B.; Elbakri, M.; Ebn Touhami, M.; Bentiss, F.; Oudda, H. Study of a Cysteine Derivative as a Corrosion Inhibitor for Carbon Steel in Phosphoric Acid Solution. Res. Chem. Intermed. 2014, 40, 801–815. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A.; Kumar, R. Experimental and Theoretical Studies of Ficus Religiosa as Green Corrosion Inhibitor for Mild Steel in 0.5 M H2SO4 Solution. Sustain. Chem. Pharm. 2018, 9, 95–105. [Google Scholar] [CrossRef]
- Kairi, N.I.; Kassim, J. The Effect of Temperature on the Corrosion Inhibition of Mild Steel in 1 M HCl Solution by Curcuma Longa Extract. Int. J. Electrochem. Sci. 2013, 8, 7138–7155. [Google Scholar]
- Zakeri, A.; Bahmani, E.; Aghdam, A.S.R. Plant Extracts as Sustainable and Green Corrosion Inhibitors for Protection of Ferrous Metals in Corrosive Media: A Mini Review. Corros. Commun. 2022, 5, 25–38. [Google Scholar] [CrossRef]
- Fekri, M.H.; Omidali, F.; Alemnezhad, M.M.; Ghaffarinejad, A. Turnip Peel Extract as Green Corrosion Bio-Inhibitor for Copper in 3.5% NaCl Solution. Mater. Chem. Phys. 2022, 286, 126150. [Google Scholar] [CrossRef]
- Deyab, M.; Osman, M.; Elkholy, A.; Heakal, F.E.-T. Green Approach towards Corrosion Inhibition of Carbon Steel in Produced Oilfield Water Using Lemongrass Extract. RSC Adv. 2017, 7, 45241–45251. [Google Scholar] [CrossRef] [Green Version]
- Gunasekaran, G.; Chauhan, L. Eco Friendly Inhibitor for Corrosion Inhibition of Mild Steel in Phosphoric Acid Medium. Electrochim. Acta 2004, 49, 4387–4395. [Google Scholar] [CrossRef]
- Bendahou, M.; Benabdellah, M.; Hammouti, B. A Study of Rosemary Oil as a Green Corrosion Inhibitor for Steel in 2 M H3PO4. Pigment Resin Technol. 2006, 35, 95–100. [Google Scholar] [CrossRef]
- Benabdellah, M.; Benkaddour, M.; Hammouti, B.; Bendahhou, M.; Aouniti, A. Inhibition of Steel Corrosion in 2 M H3PO4 by Artemisia Oil. Appl. Surf. Sci. 2006, 252, 6212–6217. [Google Scholar] [CrossRef]
- Yaro, A.S.; Khadom, A.A.; Wael, R.K. Apricot Juice as Green Corrosion Inhibitor of Mild Steel in Phosphoric Acid. Alex. Eng. J. 2013, 52, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Verma, D.K.; Khan, F. Corrosion Inhibition of High Carbon Steel in Phosphoric Acid Solution by Extract of Black Tea. Adv. Res. 2015, 5, 1. [Google Scholar] [CrossRef]
- Victoria, S.N.; Prasad, R.; Manivannan, R. Psidium Guajava Leaf Extract as Green Corrosion Inhibitor for Mild Steel in Phosphoric Acid. Int. J. Electrochem. Sci. 2015, 10, 2220–2238. [Google Scholar]
- Messali, M.; Lgaz, H.; Dassanayake, R.; Salghi, R.; Jodeh, S.; Abidi, N.; Hamed, O. Guar Gum as Efficient Non-Toxic Inhibitor of Carbon Steel Corrosion in Phosphoric Acid Medium: Electrochemical, Surface, DFT and MD Simulations Studies. J. Mol. Struct. 2017, 1145, 43–54. [Google Scholar] [CrossRef]
- Visen, P.; Saraswat, B.; Visen, A.; Roller, M.; Bily, A.; Mermet, C.; He, K.; Bai, N.; Lemaire, B.; Lafay, S. Acute Effects of Fraxinus excelsior L. Seed Extract on Postprandial Glycemia and Insulin Secretion on Healthy Volunteers. J. Ethnopharmacol. 2009, 126, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, A.; Bai, N.; He, K.; Bily, A.; Cases, J.; Roller, M.; Sang, S. Fraxinus Excelsior Seed Extract FraxiPureTM Limits Weight Gains and Hyperglycemia in High-Fat Diet-Induced Obese Mice. Phytomedicine 2011, 18, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Singh, V.R.; Goswami, P.; Singh, S.; Verma, S.K.; Luqman, S.; Chanotiya, C.S.; Darokar, M.P. Zingiber zerumbet (L.) Roscoe Ex Sm. from Northern India: Potential Source of Zerumbone Rich Essential Oil for Antiproliferative and Antibacterial Applications. Ind. Crops Prod. 2018, 112, 749–754. [Google Scholar] [CrossRef]
- Azelan, N.A.; Aziz, R.; Hasham, R. Optimisation of Essential Oil Yield and Zerumbone Content in Zingiber zerumbet Extract Using Hydrodistillation Process. Chem. Eng. Trans. 2018, 63, 595–600. [Google Scholar]
- Jang, D.S.; Seo, E.-K. Potentially Bioactive Two New Natural Sesquiterpenoids from the Rhizomes of Zingiber zerumbet. Arch. Pharm. Res. 2005, 28, 294–296. [Google Scholar] [CrossRef]
- Hamburger, M. Isatis Tinctoria–From the Rediscovery of an Ancient Medicinal Plant towards a Novel Anti-Inflammatory Phytopharmaceutical. Phytochem. Rev. 2002, 1, 333–344. [Google Scholar] [CrossRef]
- Brattström, A.; Schapowal, A.; Kamal, M.; Maillet, I.; Ryffel, B.; Moser, R. The Plant Extract Isatis tinctoria L. Extract (ITE) Inhibits Allergen-Induced Airway Inflammation and Hyperreactivity in Mice. Phytomedicine 2010, 17, 551–556. [Google Scholar] [CrossRef]
- Chaouiki, A.; Han, D.I.; Ko, Y.G. Computational Molecular-Level Prediction of Heterocyclic Compound–Metal Surface Interfacial Behavior. J. Colloid Interface Sci. 2022, 622, 452–468. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Salghi, R.; Chafiq, M.; Oudda, H.; Bhat, K.; Cretescu, I.; Ali, I.; Marzouki, R.; Chung, I. Assessing the Impact of Electron-Donating-Substituted Chalcones on Inhibition of Mild Steel Corrosion in HCl Solution: Experimental Results and Molecular-Level Insights. Colloids Surf. Physicochem. Eng. Asp. 2020, 588, 124366. [Google Scholar] [CrossRef]
- Chaouiki, A.; Lgaz, H.; Chung, I.-M.; Ali, I.; Gaonkar, S.L.; Bhat, K.; Salghi, R.; Oudda, H.; Khan, M. Understanding Corrosion Inhibition of Mild Steel in Acid Medium by New Benzonitriles: Insights from Experimental and Computational Studies. J. Mol. Liq. 2018, 266, 603–616. [Google Scholar] [CrossRef]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a Sparse Matrix-Based Implementation of the DFTB Method. J. Phys. Chem. A 2007, 111, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Witek, H.A.; Bobadova-Parvanova, P.; Irle, S.; Musaev, D.G.; Prabhakar, R.; Morokuma, K.; Lundberg, M.; Elstner, M.; Köhler, C. Parameter Calibration of Transition-Metal Elements for the Spin-Polarized Self-Consistent-Charge Density-Functional Tight-Binding (DFTB) Method: Sc, Ti, Fe, Co, and Ni. J. Chem. Theory Comput. 2007, 3, 1349–1367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-Consistent-Charge Density-Functional Tight-Binding Method for Simulations of Complex Materials Properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Murmu, M.; Saha, S.K.; Murmu, N.C.; Banerjee, P. Effect of Stereochemical Conformation into the Corrosion Inhibitive Behaviour of Double Azomethine Based Schiff Bases on Mild Steel Surface in 1 Mol L−1 HCl Medium: An Experimental, Density Functional Theory and Molecular Dynamics Simulation Study. Corros. Sci. 2019, 146, 134–151. [Google Scholar] [CrossRef]
- Guo, L.; Obot, I.B.; Zheng, X.; Shen, X.; Qiang, Y.; Kaya, S.; Kaya, C. Theoretical Insight into an Empirical Rule about Organic Corrosion Inhibitors Containing Nitrogen, Oxygen, and Sulfur Atoms. Appl. Surf. Sci. 2017, 406, 301–306. [Google Scholar] [CrossRef]
- Guo, L.; Qi, C.; Zheng, X.; Zhang, R.; Shen, X.; Kaya, S. Toward Understanding the Adsorption Mechanism of Large Size Organic Corrosion Inhibitors on an Fe (110) Surface Using the DFTB Method. RSC Adv. 2017, 7, 29042–29050. [Google Scholar] [CrossRef] [Green Version]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A Theoretical and Experimental Study of Castor Oil-Based Inhibitor for Corrosion Inhibition of Mild Steel in Acidic Medium at Elevated Temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Klamt, A.; Schuurmann, G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. Chem. Soc. Perkin. Trans. 1993, 2, 799. [Google Scholar] [CrossRef]
- Saha, S.K.; Ghosh, P.; Hens, A.; Murmu, N.C.; Banerjee, P. Density Functional Theory and Molecular Dynamics Simulation Study on Corrosion Inhibition Performance of Mild Steel by Mercapto-Quinoline Schiff Base Corrosion Inhibitor. Phys. E Low-Dimens. Syst. Nanostructures 2015, 66, 332–341. [Google Scholar] [CrossRef]
- Baldridge, K.; Klamt, A. First principles implementation of solvent effects without outlying charge error. J. Chem. Phys. 1997, 106, 6622. [Google Scholar] [CrossRef]
- Alaoui, K.; El Kacimi, Y.; Galai, M.; Serrar, H.; Touir, R.; Kaya, S.; Kaya, C.; Ebn Touhami, M. New Triazepine Carboxylate Derivatives: Correlation between Corrosion Inhibition Property and Chemical Structure. Int. J. Ind. Chem. 2020, 11, 23–42. [Google Scholar] [CrossRef] [Green Version]
- Bozorg, M.; Shahrabi Farahani, T.; Neshati, J.; Chaghazardi, Z.; Mohammadi Ziarani, G. Myrtus Communis as Green Inhibitor of Copper Corrosion in Sulfuric Acid. Ind. Eng. Chem. Res. 2014, 53, 4295–4303. [Google Scholar] [CrossRef]
- Gadow, H.; Motawea, M. Investigation of the Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution by Using Ginger Roots Extract. RSC Adv. 2017, 7, 24576–24588. [Google Scholar] [CrossRef] [Green Version]
- Yadav, D.K.; Quraishi, M.; Maiti, B. Inhibition Effect of Some Benzylidenes on Mild Steel in 1 M HCl: An Experimental and Theoretical Correlation. Corros. Sci. 2012, 55, 254–266. [Google Scholar] [CrossRef]
- El Faydy, M.; Lakhrissi, B.; Jama, C.; Zarrouk, A.; Olasunkanmi, L.O.; Ebenso, E.E.; Bentiss, F. Electrochemical, Surface and Computational Studies on the Inhibition Performance of Some Newly Synthesized 8-Hydroxyquinoline Derivatives Containing Benzimidazole Moiety against the Corrosion of Carbon Steel in Phosphoric Acid Environment. J. Mater. Res. Technol. 2020, 9, 727–748. [Google Scholar] [CrossRef]
- Kumar, S.; Vashisht, H.; Olasunkanmi, L.O.; Bahadur, I.; Verma, H.; Singh, G.; Obot, I.B.; Ebenso, E.E. Experimental and Theoretical Studies on Inhibition of Mild Steel Corrosion by Some Synthesized Polyurethane Tri-Block Co-Polymers. Sci. Rep. 2016, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- El Faydy, M.; Benhiba, F.; Berisha, A.; Kerroum, Y.; Jama, C.; Lakhrissi, B.; Guenbour, A.; Warad, I.; Zarrouk, A. An Experimental-Coupled Empirical Investigation on the Corrosion Inhibitory Action of 7-Alkyl-8-Hydroxyquinolines on C35E Steel in HCl Electrolyte. J. Mol. Liq. 2020, 317, 113973. [Google Scholar] [CrossRef]
- Singh, A.; Caihong, Y.; Yaocheng, Y.; Soni, N.; Wu, Y.; Lin, Y. Analyses of New Electrochemical Techniques to Study the Behavior of Some Corrosion Mitigating Polymers on N80 Tubing Steel. Acs Omega 2019, 4, 3420–3431. [Google Scholar] [CrossRef] [Green Version]
- Solmaz, R.; Kardaş, G.; Çulha, M.; Yazıcı, B.; Erbil, M. Investigation of Adsorption and Inhibitive Effect of 2-Mercaptothiazoline on Corrosion of Mild Steel in Hydrochloric Acid Media. Electrochim. Acta 2008, 53, 5941–5952. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Novel Schiff-Base Molecules as Efficient Corrosion Inhibitors for Mild Steel Surface in 1 M HCl Medium: Experimental and Theoretical Approach. Phys. Chem. Chem. Phys. 2016, 18, 17898–17911. [Google Scholar] [CrossRef]
- Abd El Rehim, S.S.; Hassan, H.H.; Amin, M.A. Corrosion Inhibition Study of Pure Al and Some of Its Alloys in 1.0 M HCl Solution by Impedance Technique. Corros. Sci. 2004, 46, 5–25. [Google Scholar] [CrossRef]
- Jawad, A.Q.; Zinad, D.S.; Dawood Salim, R.; Al-Amiery, A.A.; Sumer Gaaz, T.; Takriff, M.S.; Kadhum, A.H. Synthesis, Characterization, and Corrosion Inhibition Potential of Novel Thiosemicarbazone on Mild Steel in Sulfuric Acid Environment. Coatings 2019, 9, 729. [Google Scholar] [CrossRef] [Green Version]
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical Analysis of the Shape of Polarization Curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.; Awaji, H. 1-X-4-[4′-(-OCH 3)-Styryl] Pyridinium Iodides, Potent Inhibitors for Stainless Steel Corrosion in 2 M HCl Acid Solutions. Int. J. Corros. Scale Inhib. 2020, 9, 460–501. [Google Scholar]
- Chafiq, M.; Chaouiki, A.; Al-Hadeethi, M.R.; Salghi, R.; Chung, I.-M. A Joint Experimental and Theoretical Investigation of the Corrosion Inhibition Behavior and Mechanism of Hydrazone Derivatives for Mild Steel in HCl Solution. Colloids Surf. Physicochem. Eng. Asp. 2021, 610, 125744. [Google Scholar] [CrossRef]
- Ogunleye, O.; Arinkoola, A.; Eletta, O.; Agbede, O.; Osho, Y.; Morakinyo, A.; Hamed, J. Green Corrosion Inhibition and Adsorption Characteristics of Luffa Cylindrica Leaf Extract on Mild Steel in Hydrochloric Acid Environment. Heliyon 2020, 6, e03205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khamis, E. The Effect of Temperature on the Acidic Dissolution of Steel in the Presence of Inhibitors. Corrosion 1990, 46, 476–484. [Google Scholar] [CrossRef]
- Oral, A.; Menşur, E.; Aslan, M.; Başaran, E. The Preparation of Copper (II) Oxide Thin Films and the Study of Their Microstructures and Optical Properties. Mater. Chem. Phys. 2004, 83, 140–144. [Google Scholar] [CrossRef]
- Valek, L.; Martinez, S. Copper Corrosion Inhibition by Azadirachta Indica Leaves Extract in 0.5 M Sulphuric Acid. Mater. Lett. 2007, 61, 148–151. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Abd-El-Nabey, B.; Sidahmed, I.; El-Zayady, A.; Saadawy, M. Inhibitive Action of Some Plant Extracts on the Corrosion of Steel in Acidic Media. Corros. Sci. 2006, 48, 2765–2779. [Google Scholar] [CrossRef]
- Eddy, N.; Mamza, P. Inhibitive and Adsorption Properties of Ethanol Extract of Seeds and Leaves of Azadirachta Indica on the Corrosion of Mild Steel in H2SO4. Port. Electrochim. Acta 2009, 27, 443–456. [Google Scholar] [CrossRef]
- Ebenso, E.; Alemu, H.; Umoren, S.; Obot, I. Inhibition of Mild Steel Corrosion in Sulphuric Acid Using Alizarin Yellow GG Dye and Synergistic Iodide Additive. Int. J. Electrochem. Sci. 2008, 3, 1325–1339. [Google Scholar]
- Laabaissi, T.; Benhiba, F.; Missioui, M.; Rouifi, Z.; Rbaa, M.; Oudda, H.; Ramli, Y.; Guenbour, A.; Warad, I.; Zarrouk, A. Coupling of Chemical, Electrochemical and Theoretical Approach to Study the Corrosion Inhibition of Mild Steel by New Quinoxaline Compounds in 1 M HCl. Heliyon 2020, 6, e03939. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Feng, L.-J.; Wang, H.-Y.; Lu, Y.-B.; Lei, X.-W.; Bai, F.-L. Comparison of the Synergistic Effect of Counterions on the Inhibition of Mild Steel Corrosion in Acid Solution: Electrochemical, Gravimetric and Thermodynamic Studies. RSC Adv. 2015, 5, 4716–4726. [Google Scholar] [CrossRef]
- Akinbulumo, O.A.; Odejobi, O.J.; Odekanle, E.L. Thermodynamics and Adsorption Study of the Corrosion Inhibition of Mild Steel by Euphorbia Heterophylla L. Extract in 1.5 M HCl. Results Mater. 2020, 5, 100074. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.H. Thermodynamic Study of Metal Corrosion and Inhibitor Adsorption Processes in Mild Steel/1-Methyl-4 [4′(-X)-Styryl Pyridinium Iodides/Hydrochloric Acid Systems. Mater. Chem. Phys. 2008, 110, 145–154. [Google Scholar] [CrossRef]
- Ismail, A.S.; Farag, A.A. Experimental, Theoretical and Simulation Studies of Extracted Crab Waste Protein as a Green Polymer Inhibitor for Carbon Steel Corrosion in 2 M H3PO4. Surf. Interfaces 2020, 19, 100483. [Google Scholar] [CrossRef]
- Szauer, T.; Brandt, A. On the Role of Fatty Acid in Adsorption and Corrosion Inhibition of Iron by Amine—Fatty Acid Salts in Acidic Solution. Electrochim. Acta 1981, 26, 1257–1260. [Google Scholar] [CrossRef]
- Popova, A. Temperature Effect on Mild Steel Corrosion in Acid Media in Presence of Azoles. Corros. Sci. 2007, 49, 2144–2158. [Google Scholar] [CrossRef]
- Grigoryev, V.; Osipov, O. Influence of Polar Properties of Substituents in Inhibitor Molecules on the Kinetics of Hydrogen Evolution. In 3rd Europ. Symp. Corrosion Inhibitors; University of Ferrara: Ferrara, Italy, 1970; p. 473. [Google Scholar]
- Grigoryev, V.; Eklik, V. Synergistic Effect between 4-(2-Pyridylazo) Resorcin and Chloride Ion on the Corrosion of Cold Rolled Steel in 0.5 M Sulfuric Acid. Prot. Met. 1968, 4, 517–529. [Google Scholar]
- Gouron, A.; Le Mapihan, K.; Camperos, S.; Al Farra, A.; Lair, V.; Ringuedé, A.; Cassir, M.; Diawara, B. New Insights in Self-Assembled Monolayer of Imidazolines on Iron Oxide Investigated by DFT. Appl. Surf. Sci. 2018, 456, 437–444. [Google Scholar] [CrossRef]
- Al-Moubaraki, A.H. Corrosion Protection of Mild Steel in Acid Solutions Using Red Cabbage Dye. Chem. Eng. Commun. 2015, 202, 1069–1080. [Google Scholar] [CrossRef]
- Noor, E.A.; Al-Moubaraki, A.; Al-Zhrani, A.H.; Hubani, M.H. Testing and Comparing the Inhibitory Action of Red Onion Seeds and Peels Extracts on the Corrosion of Steel in Phosphoric Acid. Int. J. Electrochem. Sci. 2016, 11, 6523–6539. [Google Scholar] [CrossRef]
- Rashid, K.; Khadom, A. Evaluation of Environmentally Friendly Inhibitor for Corrosion of Mild Steel in Phosphoric Acid Solution: Unconventional Approach. Anti-Corros. Methods Mater. 2018, 65, 506–514. [Google Scholar] [CrossRef]
- Boudalia, M.; Fernández-Domene, R.M.; Tabyaoui, M.; Bellaouchou, A.; Guenbour, A.; Garcia-Anton, J. Green Approach to Corrosion Inhibition of Stainless Steel in Phosphoric Acid of Artemesia Herba Albamedium Using Plant Extract. J. Mater. Res. Technol. 2019, 8, 5763–5773. [Google Scholar] [CrossRef]
- Lin, B.; Shao, J.; Xu, Y.; Lai, Y.; Zhao, Z. Adsorption and Corrosion of Renewable Inhibitor of Pomelo Peel Extract for Mild Steel in Phosphoric Acid Solution. Arab. J. Chem. 2021, 14, 103114. [Google Scholar] [CrossRef]



| Inhibitor | Chemical Structure | Content (%) |
|---|---|---|
| Fraxinus excelsior L. |  | 11.42 |
 | 6.15 | |
| Zingiber zerumbet L. | 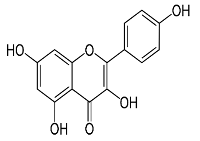 | 33.60 |
 | 17.40 | |
| Isatis tinctoria L. |  | 40.00 |
 | 20.89 |
| Cinh (g L−1) | CRWL × 105 (g cm−2 min−1) | IEWL% | ||||
|---|---|---|---|---|---|---|
| FEAE | ZZAE | ITAE | FEAE | ZZAE | ITAE | |
| 0.0 | 5.848 ± 0.014 | - | - | - | ||
| 0.005 | 3.017 ± 0.013 | 3.881 ± 0.023 | 5.219 ± 0.003 | 48.4 | 33.6 | 10.8 |
| 0.01 | 2.387 ± 0.009 | 3.232 ± 0.034 | 5.041 ± 0.007 | 59.2 | 44.7 | 13.8 |
| 0.025 | 0.931 ± 0.017 | 2.478 ± 0.005 | 3.573 ± 0.012 | 84.1 | 57.6 | 38.9 |
| 0.05 | 0.662 ± 0.005 | 2.289 ± 0.002 | 3.293 ± 0.023 | 88.7 | 60.9 | 43.7 |
| 0.1 | 0.577 ± 0.003 | 2.035 ± 0.012 | 2.543 ± 0.002 | 90.1 | 65.2 | 56.5 |
| 0.25 | 0.516 ± 0.012 | 1.924 ± 0.006 | 2.022 ± 0.005 | 91.2 | 67.1 | 65.4 |
| 0.5 | 0.427 ± 0.022 | 1.145 ± 0.017 | 1.396 ± 0.013 | 92.7 | 80.4 | 76.1 |
| 1.0 | 0.342 ± 0.006 | 0.802 ± 0.008 | 0.836 ± 0.003 | 94.2 | 86.3 | 85.7 |
| 1.5 | 0.312 ± 0.005 | 0.631 ± 0.021 | 0.713 ± 0.009 | 94.7 | 89.2 | 87.8 |
| 2.0 | 0.306 ± 0.016 | 0.614 ± 0.013 | 0.678 ± 0.011 | 94.8 | 89.5 | 88.4 |
| 2.5 | 0.286 ± 0.013 | 0.486 ± 0.015 | 0.622 ± 0.021 | 95.1 | 91.7 | 89.4 |
| 3.0 | 0.281 ± 0.003 | 0.420 ± 0.004 | 0.488 ± 0.004 | 95.2 | 92.8 | 91.7 |
| Cinh (g L−1) | Rs (ohm cm2) | Rp (ohm cm2) | Cdl (µF cm−2) | CPE “n” | Goodness of Fit (χ2) × 10−3 | IER% |
|---|---|---|---|---|---|---|
| FEAE | ||||||
| 0.0 | 2.50 ± 0.05 | 6.94 ± 0.5 | 365.01 | 0.99 ± 0.005 | 1.22 | - |
| 0.025 | 2.81 ± 0.07 | 18.17 ± 0.8 | 165.22 | 0.80 ± 0.006 | 1.29 | 61.8 |
| 0.05 | 2.85 ± 0.12 | 54.13 ± 2.2 | 88.85 | 0.81 ± 0.003 | 3.81 | 87.2 |
| 0.1 | 2.62 ± 0.06 | 84.04 ± 2.5 | 65.30 | 0.84 ± 0.007 | 2.44 | 91.8 |
| 0.5 | 2.50 ± 0.11 | 120.53 ± 2.4 | 45.64 | 0.85 ± 0.006 | 1.31 | 94.3 |
| 1.0 | 2.86 ± 0.08 | 136.43 ± 1.7 | 33.22 | 0.88 ± 0.002 | 2.76 | 94.9 |
| 2.0 | 2.49 ± 0.07 | 155.08 ± 2.6 | 24.12 | 0.88 ± 0.008 | 2.14 | 95.5 |
| 2.5 | 2.38 ± 0.11 | 181.58 ± 1.9 | 19.61 | 0.88 ± 0.004 | 1.54 | 96.2 |
| ZZAE | ||||||
| 0.0 | 2.50 ± 0.05 | 6.94 ± 0.5 | 365.01 | 0.99 ± 0.005 | 1.22 | - |
| 0.025 | 2.37 ± 0.12 | 10.05 ± 0.8 | 280.90 | 1.00 ± 0.004 | 1.14 | 30.9 |
| 0.05 | 3.56 ± 0.08 | 12.77 ± 0.6 | 215.23 | 0.99 ± 0.005 | 3.21 | 45.7 |
| 0.1 | 3.54 ± 0.06 | 16.73 ± 1.1 | 174.37 | 0.89 ± 0.002 | 2.53 | 58.5 |
| 0.5 | 2.88 ± 0.11 | 62.52 ± 2.2 | 94.50 | 0.83 ± 0.001 | 1.65 | 88.9 |
| 1.0 | 2.74 ± 0.13 | 88.80 ± 2.5 | 69.43 | 0.87 ± 0.006 | 2.39 | 92.2 |
| 2.0 | 2.69 ± 0.09 | 138.44 ± 1.8 | 41.69 | 0.88 ± 0.008 | 3.22 | 94.9 |
| 2.5 | 2.51 ± 0.13 | 180.93 ± 2.7 | 26.41 | 0.88 ± 0.003 | 2.37 | 96.2 |
| ITAE | ||||||
| 0.0 | 2.50 ± 0.05 | 6.94 ± 0.5 | 365.01 | 0.99 ± 0.005 | 1.22 | - |
| 0.025 | 2.21 ± 0.07 | 9.85 ± 0.7 | 291.28 | 0.99 ± 0.003 | 3.35 | 29.6 |
| 0.05 | 3.07 ± 0.12 | 12.1 ± 0.4 | 226.37 | 0.87 ± 0.006 | 0.81 | 42.7 |
| 0.1 | 2.69 ± 0.11 | 15.03 ± 0.9 | 188.52 | 0.81 ± 0.002 | 0.67 | 53.9 |
| 0.5 | 2.92 ± 0.09 | 38.11 ± 1.1 | 122.37 | 0.82 ± 0.004 | 1.55 | 81.8 |
| 1.0 | 2.76 ± 0.06 | 58.05 ± 1.3 | 98.63 | 0.85 ± 0.001 | 2.08 | 88.1 |
| 2.0 | 2.84 ± 0.14 | 68.14 ± 1.1 | 74.25 | 0.86 ± 0.002 | 2.55 | 89.8 |
| 2.5 | 3.26 ± 0.07 | 79.04 ± 1.6 | 52.79 | 0.86 ± 0.004 | 2.56 | 91.2 |
| Cinh (g L−1) | −Ecorr (mV) | icorr (mA cm−2) | Rp (ohm cm2) | IEi% | ||
|---|---|---|---|---|---|---|
| FEAE | ||||||
| 0.0 | 473 ± 0.5 | 3.43 ± 0.05 | 71 ± 3.5 | 113 ± 2.4 | 5.54 | - |
| 0.025 | 453 ± 1.2 | 1.90 ± 0.03 | 53 ± 2.3 | 127 ± 3.5 | 8.53 | 44.6 |
| 0.05 | 446 ± 0.7 | 1.28 ± 0.06 | 48 ± 2.6 | 125 ± 3.7 | 11.72 | 62.7 |
| 0.1 | 445 ± 1.5 | 0.81 ± 0.02 | 50 ± 1.7 | 126 ± 2.8 | 19.24 | 76.2 |
| 0.5 | 438 ± 0.8 | 0.51 ± 0.03 | 48 ± 1.2 | 126 ± 1.3 | 29.42 | 85.1 |
| 1.0 | 445 ± 0.3 | 0.39 ± 0.01 | 49 ± 2.2 | 130 ± 2.5 | 39.79 | 88.5 |
| 2.0 | 442 ± 1.1 | 0.32 ± 0.02 | 49 ± 3.1 | 131 ± 1.7 | 48.66 | 90.6 |
| 2.5 | 431 ± 0.6 | 0.23 ± 0.01 | 44 ± 1.4 | 129 ± 2.2 | 61.44 | 93.2 |
| ZZAE | ||||||
| 0.0 | 473 ± 0.5 | 3.43 ± 0.05 | 71 ± 3.5 | 113 ± 2.4 | 5.54 | - |
| 0.025 | 467 ± 0.3 | 2.66 ± 0.02 | 59 ± 1.6 | 105 ± 1.3 | 6.14 | 22.3 |
| 0.05 | 461 ± 1.2 | 2.17 ± 0.01 | 61 ± 1.4 | 113 ± 2.1 | 7.91 | 36.7 |
| 0.1 | 462 ± 1.5 | 1.69 ± 0.03 | 57 ± 0.8 | 103 ± 2.2 | 9.40 | 50.8 |
| 0.5 | 449 ± 0.4 | 0.73 ± 0.01 | 54 ± 2.1 | 122 ± 3.5 | 22.27 | 78.7 |
| 1.0 | 450 ± 0.2 | 0.56 ± 0.04 | 53 ± 1.5 | 123 ± 1.4 | 28.63 | 83.7 |
| 2.0 | 447 ± 1.1 | 0.39 ± 0.02 | 48 ± 1.7 | 122 ± 1.8 | 38.41 | 88.6 |
| 2.5 | 446 ± 0.4 | 0.32 ± 0.01 | 54 ± 1.1 | 124 ± 2.6 | 50.91 | 90.7 |
| ITAE | ||||||
| 0.0 | 473 ± 0.5 | 3.43 ± 0.05 | 71 ± 3.5 | 113 ± 2.4 | 5.54 | - |
| 0.025 | 462 ± 1.1 | 2.76 ± 0.01 | 55 ± 0.7 | 124 ± 2.4 | 6.01 | 19.5 |
| 0.05 | 461 ± 0.6 | 2.34 ± 0.03 | 60 ± 1.1 | 122 ± 2.5 | 7.50 | 31.8 |
| 0.1 | 459 ± 0.4 | 1.81 ± 0.02 | 53 ± 2.1 | 126 ± 3.3 | 8.96 | 47.1 |
| 0.5 | 451 ± 1.3 | 1.02 ± 0.04 | 55 ± 1.2 | 125 ± 2.6 | 16.27 | 70.2 |
| 1.0 | 451 ± 0.7 | 0.70 ± 0.01 | 52 ± 0.6 | 128 ± 1.2 | 22.81 | 79.6 |
| 2.0 | 448 ± 1.1 | 0.45 ± 0.03 | 53 ± 1.3 | 125 ± 1.1 | 35.72 | 86.9 |
| 2.5 | 453 ± 0.3 | 0.38 ± 0.02 | 53 ± 1.5 | 121 ± 1.4 | 42.35 | 88.9 |
| Inhibitor | Frequency (cm−1) | Band Assignment | |
|---|---|---|---|
| Plant Extract-Powder | Film Adsorbed on Mild Steel | ||
| FEAE | 3395.74 | 3437.03 | O-H stretching |
| 2926.79 | 2924.48 | C-H stretching | |
| 2855.92 | 2852.60 | ||
| 1662.83 | 1636.81 | C=O (ester), C=C stretching | |
| 1120.22 | 1113.20 | C-O stretching | |
| 1078.14 | 1059.60 | ||
| ZZAE | 3421.43 | 3435.93 | O-H stretching |
| 2925.48 | 2927.05 | C-H stretching | |
| 2854.16 | 2855.53 | ||
| 1639.10 | 1634.43 | C=O (ketone), C=C stretching | |
| 1078.57 | 1059.73 | C-O stretching | |
| ITAE | 3401.95 | 3444.71 | N-H stretching |
| 1603.18 | 1633.75 | C=O (amide), C=C stretching | |
| Technique | Langmuir Parameters | ||||
|---|---|---|---|---|---|
| Inhibitor | Slope | Kads (Lg−1) | −ΔGads (kJ mol−1) | r2 | |
| FEAE | |||||
| WL | 1.05 | 136.42 | 29.77 | 0.999 | |
| EIS | 1.04 | 98.68 | 28.96 | 0.999 | |
| PDP | 1.07 | 27.07 | 25.70 | 0.999 | |
| ZZAE | |||||
| WL | 1.09 | 21.90 | 25.17 | 0.999 | |
| EIS | 1.02 | 16.53 | 24.46 | 0.999 | |
| PDP | 1.07 | 10.69 | 23.36 | 0.999 | |
| ITAE | |||||
| WL | 1.10 | 15.00 | 24.22 | 0.999 | |
| EIS | 1.07 | 14.69 | 24.16 | 0.999 | |
| PDP | 1.09 | 8.05 | 22.65 | 0.999 | |
| Inhibitor | A (g cm−2 min−1) | ||||
|---|---|---|---|---|---|
| Free acid | 42.65 | 103 × 1.35 | 40.01 | −193.88 | 2.64 |
| FEAE | 52.56 | 103 × 3.35 | 49.92 | −186.29 | 2.64 |
| ZZAE | 52.34 | 103 × 4.90 | 49.70 | −183.15 | 2.64 |
| ITAE | 57.37 | 104 × 4.77 | 54.73 | −164.22 | 2.64 |
| Natural Plant | Metal/Medium | Performance of Optimum Inhibitor Concentration (%) | Reference |
|---|---|---|---|
| Rosemary oil | Steel/2 M H3PO4 | 73.0 | [15] |
| Artemisia oil | Steel/2 M H3PO4 | 79.4 | [16] |
| Apricot juice | Mild steel/1 M H3PO4 | 75.0 | [17] |
| Red cabbage dye | Mild steel/1 N H3PO4 | 76.5 | [73] |
| Black tea | High carbon steel/1 M H3PO4 | 93.7 | [18] |
| Psidium guajava (guava) leaf | Mild steel/1 M H3PO4 | 89.0 | [19] |
| Red onion seeds and peels | Steel/0.75 M H3PO4 | 90.0 (s) 74.7 (p) | [74] |
| Guar gum | Carbon steel/2 M H3PO4 | 95.9 | [20] |
| pomegranate peel | Mild steel/2 M H3PO4 | 91.6 | [75] |
| Artemisia herba-alba oil | Stainless steel/1 M H3PO4 | 88.0 | [76] |
| Pomelo peel | Mild steel/1 M H3PO4 | 95.0 | [77] |
| Fraxinus excelsior L. seeds | Mild steel/2 M H3PO4 | 96.2 | Present work |
| Zingiber zerumbet L. roots | 96.2 | ||
| Isatis tinctoria L. leaves | 91.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Moubaraki, A.H.; Chaouiki, A.; Alahmari, J.M.; Al-hammadi, W.A.; Noor, E.A.; Al-Ghamdi, A.A.; Ko, Y.G. Development of Natural Plant Extracts as Sustainable Inhibitors for Efficient Protection of Mild Steel: Experimental and First-Principles Multi-Level Computational Methods. Materials 2022, 15, 8688. https://doi.org/10.3390/ma15238688
Al-Moubaraki AH, Chaouiki A, Alahmari JM, Al-hammadi WA, Noor EA, Al-Ghamdi AA, Ko YG. Development of Natural Plant Extracts as Sustainable Inhibitors for Efficient Protection of Mild Steel: Experimental and First-Principles Multi-Level Computational Methods. Materials. 2022; 15(23):8688. https://doi.org/10.3390/ma15238688
Chicago/Turabian StyleAl-Moubaraki, Aisha H., Abdelkarim Chaouiki, Jamilah M. Alahmari, Wesam A. Al-hammadi, Ehteram A. Noor, Azza A. Al-Ghamdi, and Young Gun Ko. 2022. "Development of Natural Plant Extracts as Sustainable Inhibitors for Efficient Protection of Mild Steel: Experimental and First-Principles Multi-Level Computational Methods" Materials 15, no. 23: 8688. https://doi.org/10.3390/ma15238688
APA StyleAl-Moubaraki, A. H., Chaouiki, A., Alahmari, J. M., Al-hammadi, W. A., Noor, E. A., Al-Ghamdi, A. A., & Ko, Y. G. (2022). Development of Natural Plant Extracts as Sustainable Inhibitors for Efficient Protection of Mild Steel: Experimental and First-Principles Multi-Level Computational Methods. Materials, 15(23), 8688. https://doi.org/10.3390/ma15238688








