Fully Bio-Based Adhesive from Tannin and Sucrose for Plywood Manufacturing with High Performances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Tannin–Sucrose Adhesives and Plywood and the Test of the Bonding Strength
2.3. Orthogonal Experiment
2.4. Insoluble Substances Rate in Cured Adhesives
2.5. Fourier Transform-Infrared (FT-IR) Spectrometry
2.6. Thermogravimetric (TG) Analysis
2.7. X-ray Diffraction (XRD) Analysis
3. Results and Discussion
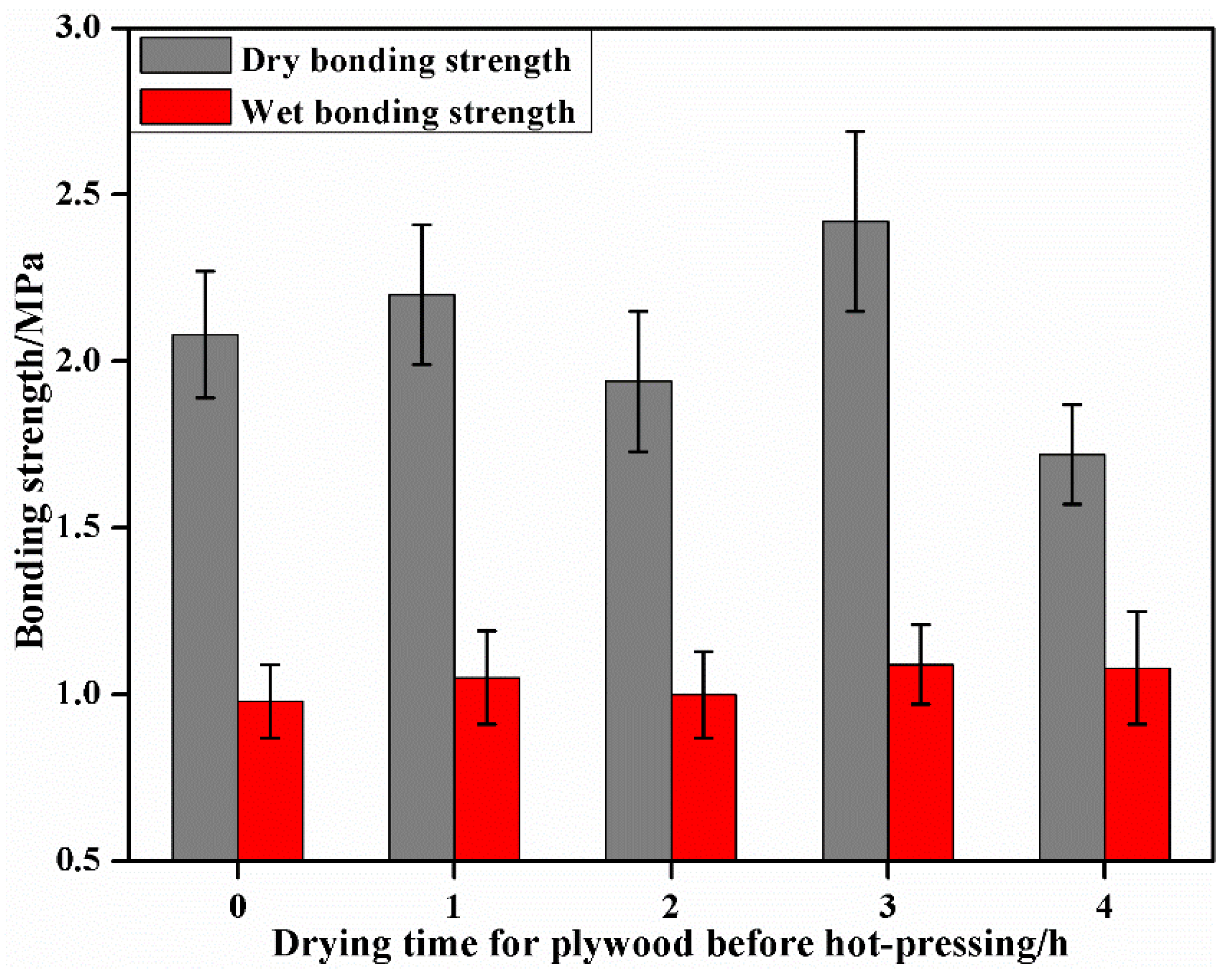
3.1. The Effect of Pre-Drying before Hot-Pressing on the Bonding Performance of Plywood
3.2. The Effect of Solid Content of Tannin–Sucrose Adhesives on the Bonding Performance of Plywood
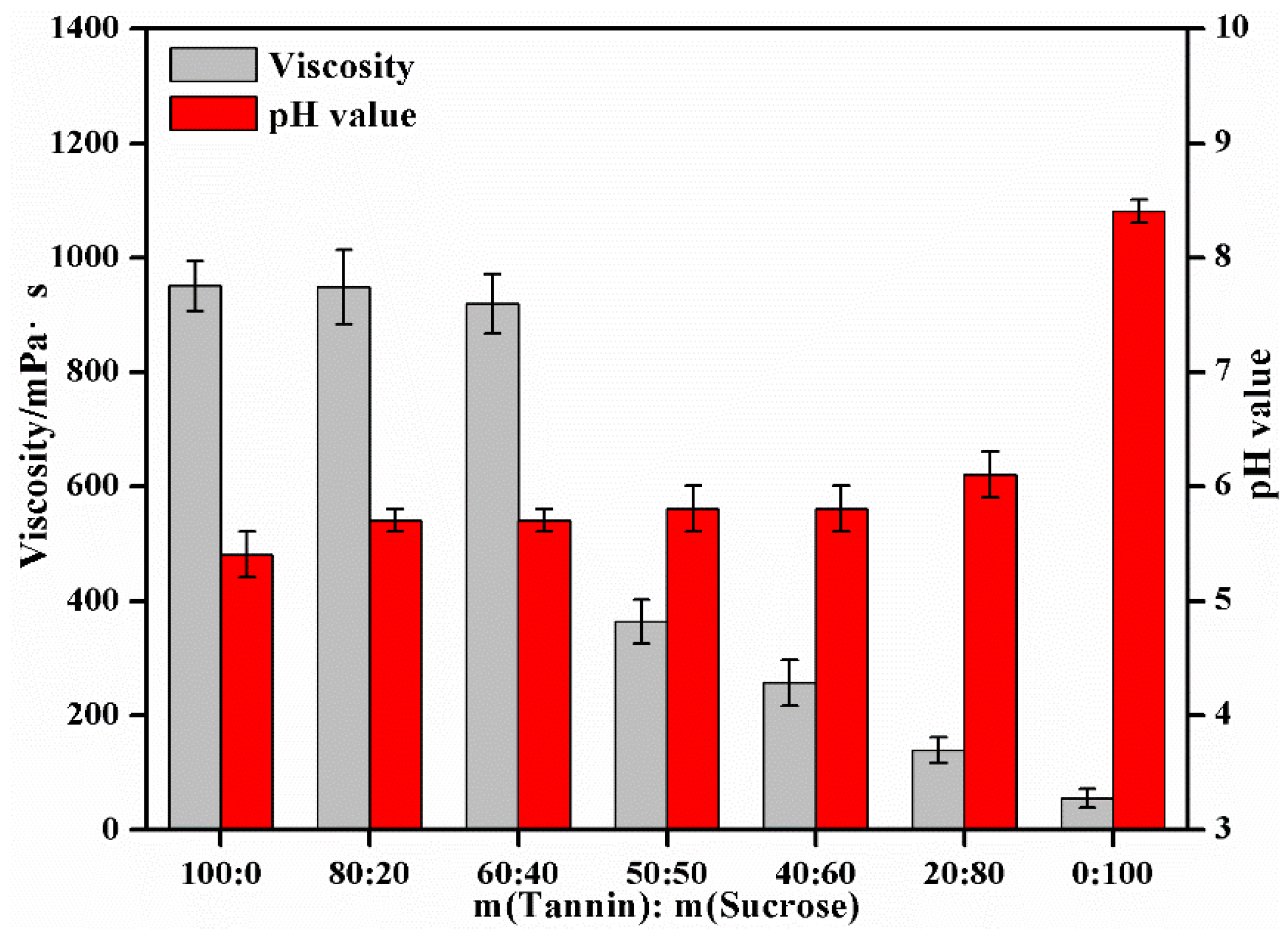
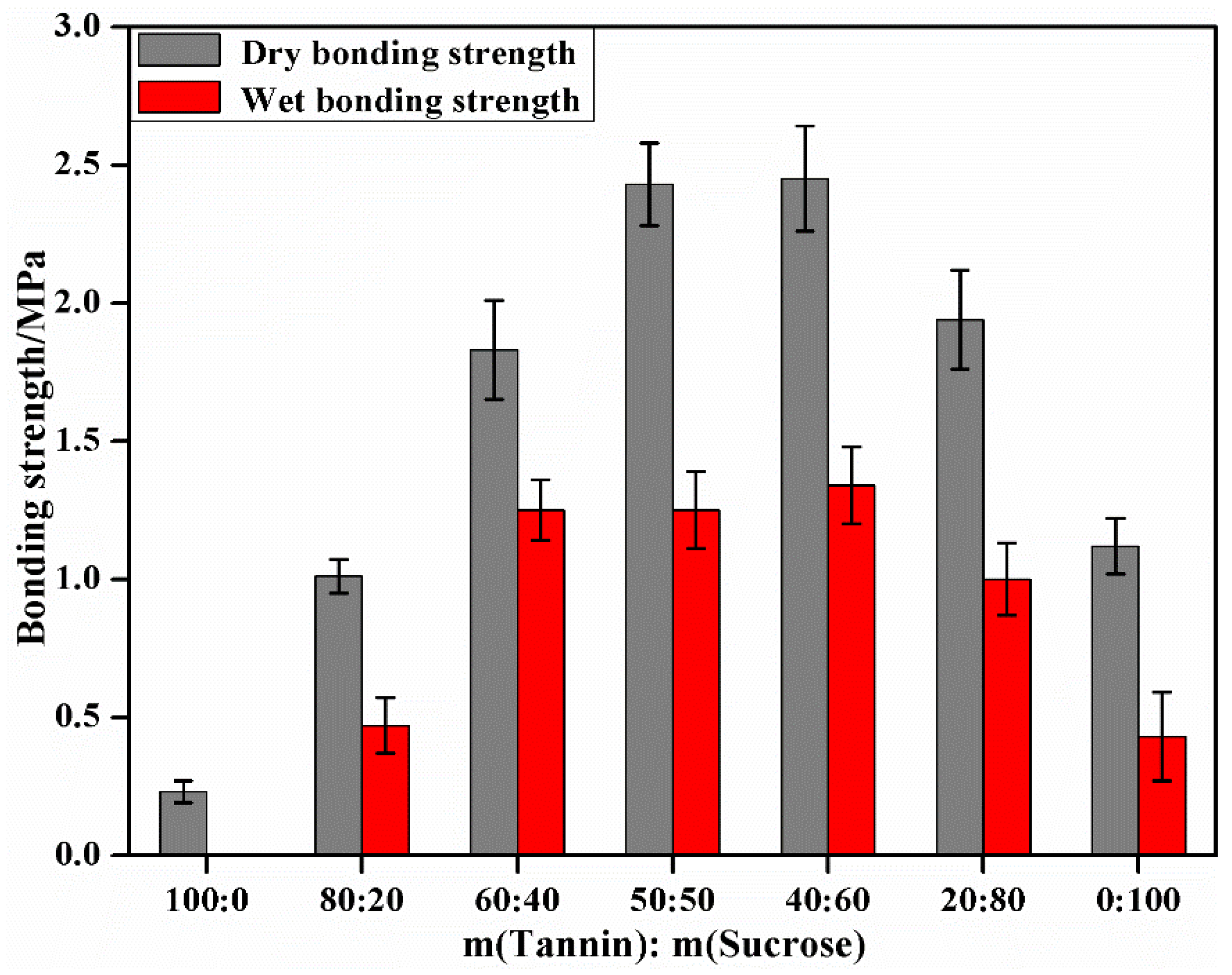
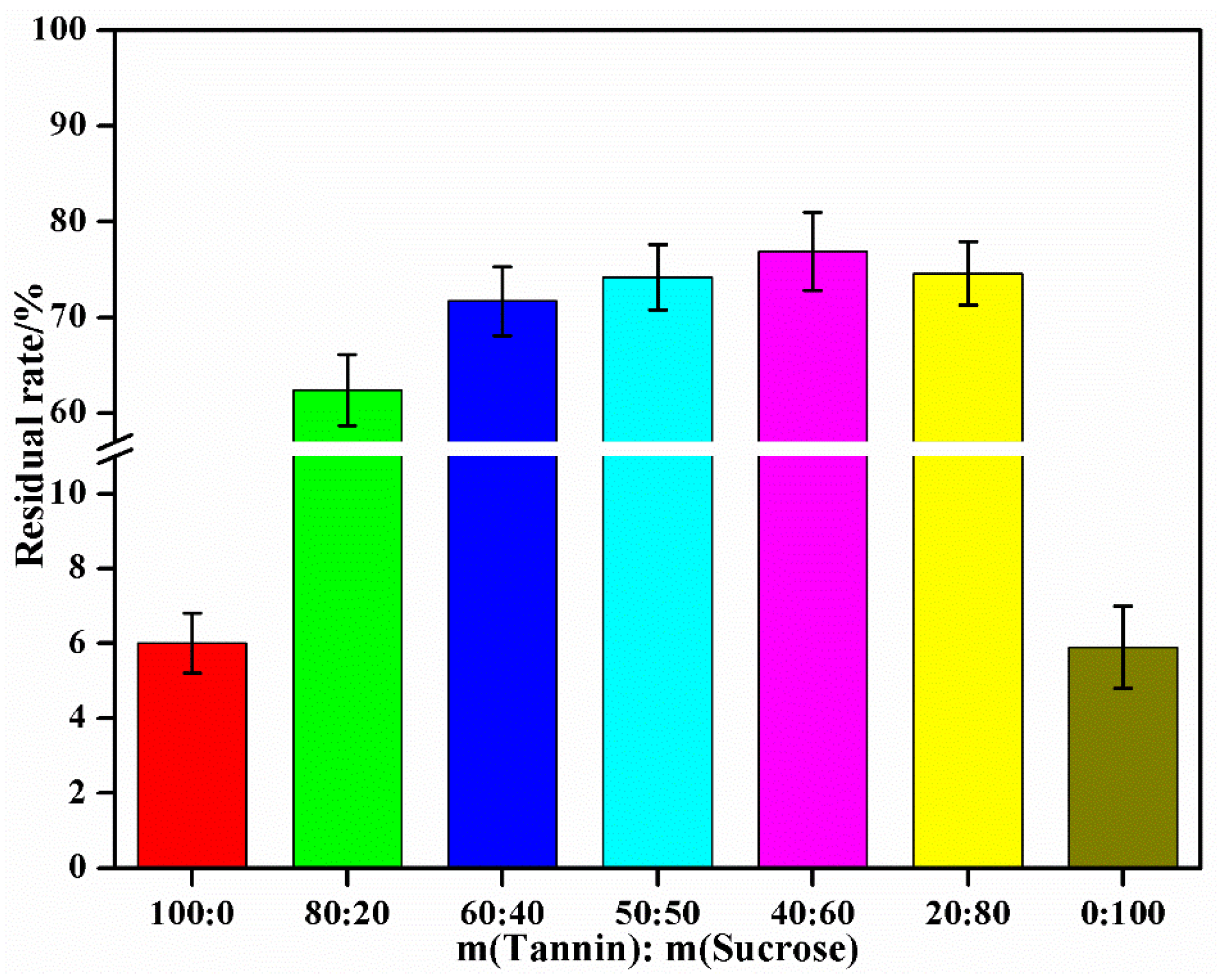
3.3. The Effect of Mass Ratio of the Tannin to Sucrose on the Performances of Tannin–Sucrose Adhesives
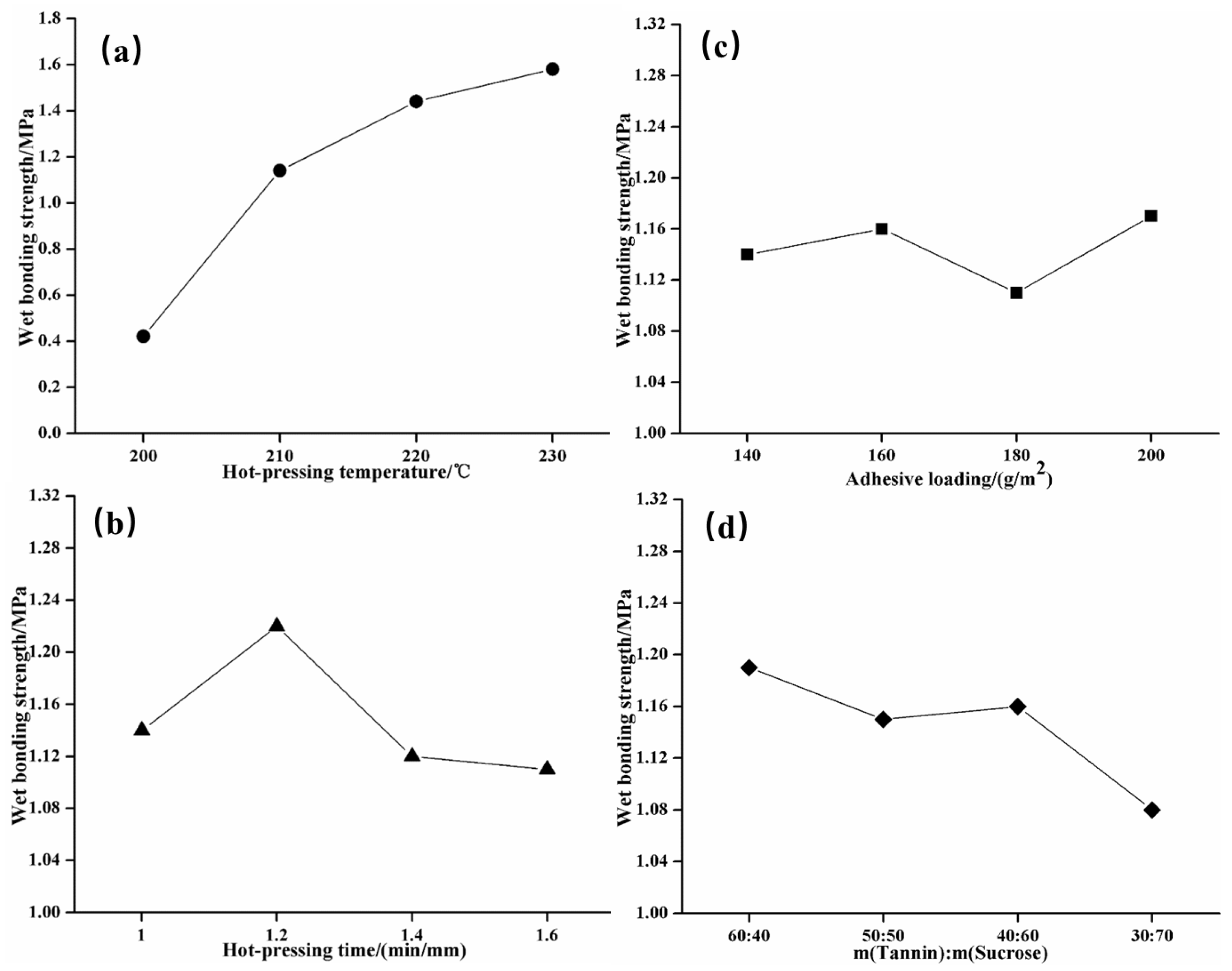
3.4. Results of Orthogonal Experiments and Analysis
3.5. FT-IR Analysis
3.6. XRD Analysis
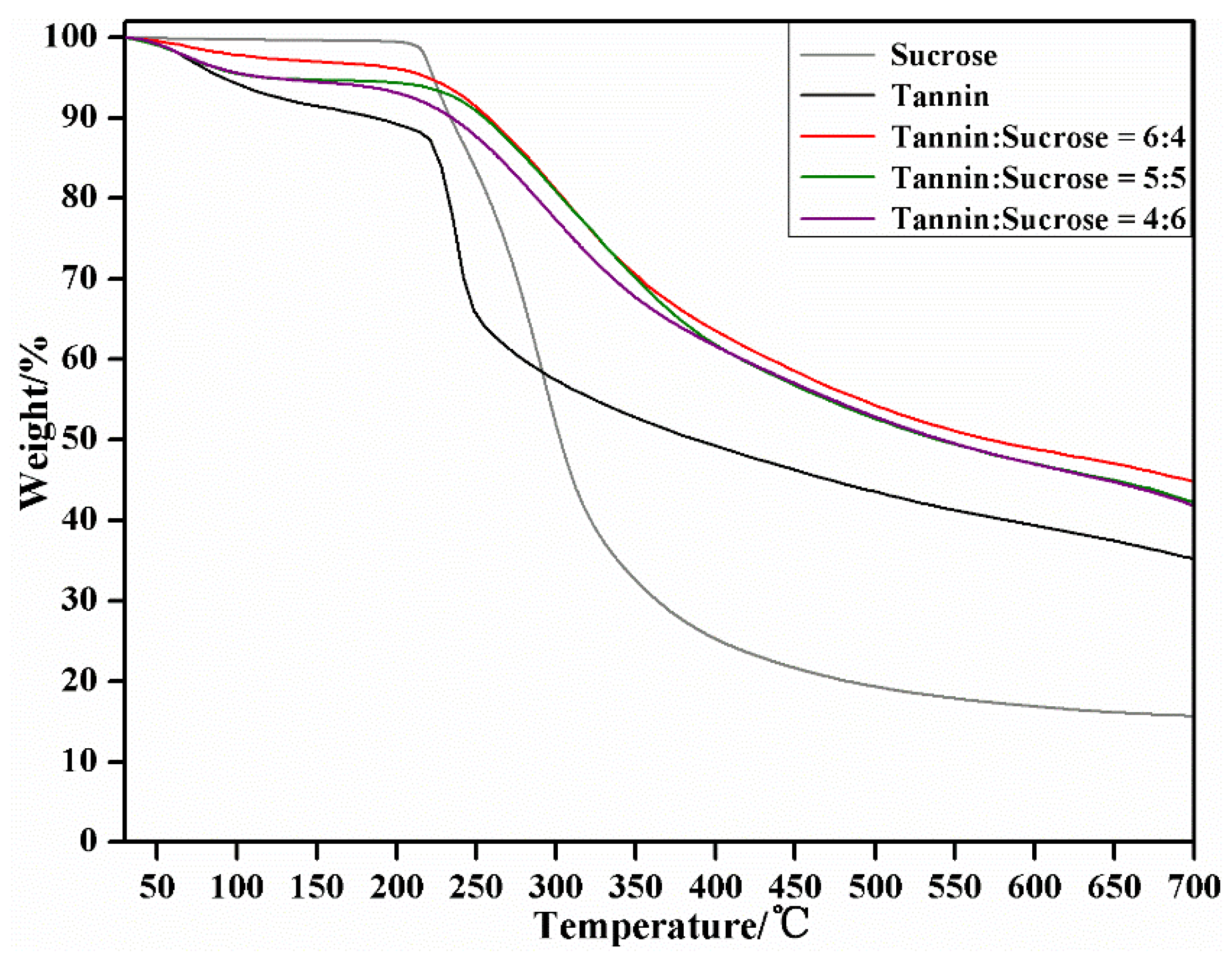
3.7. TG-DTG Analysis
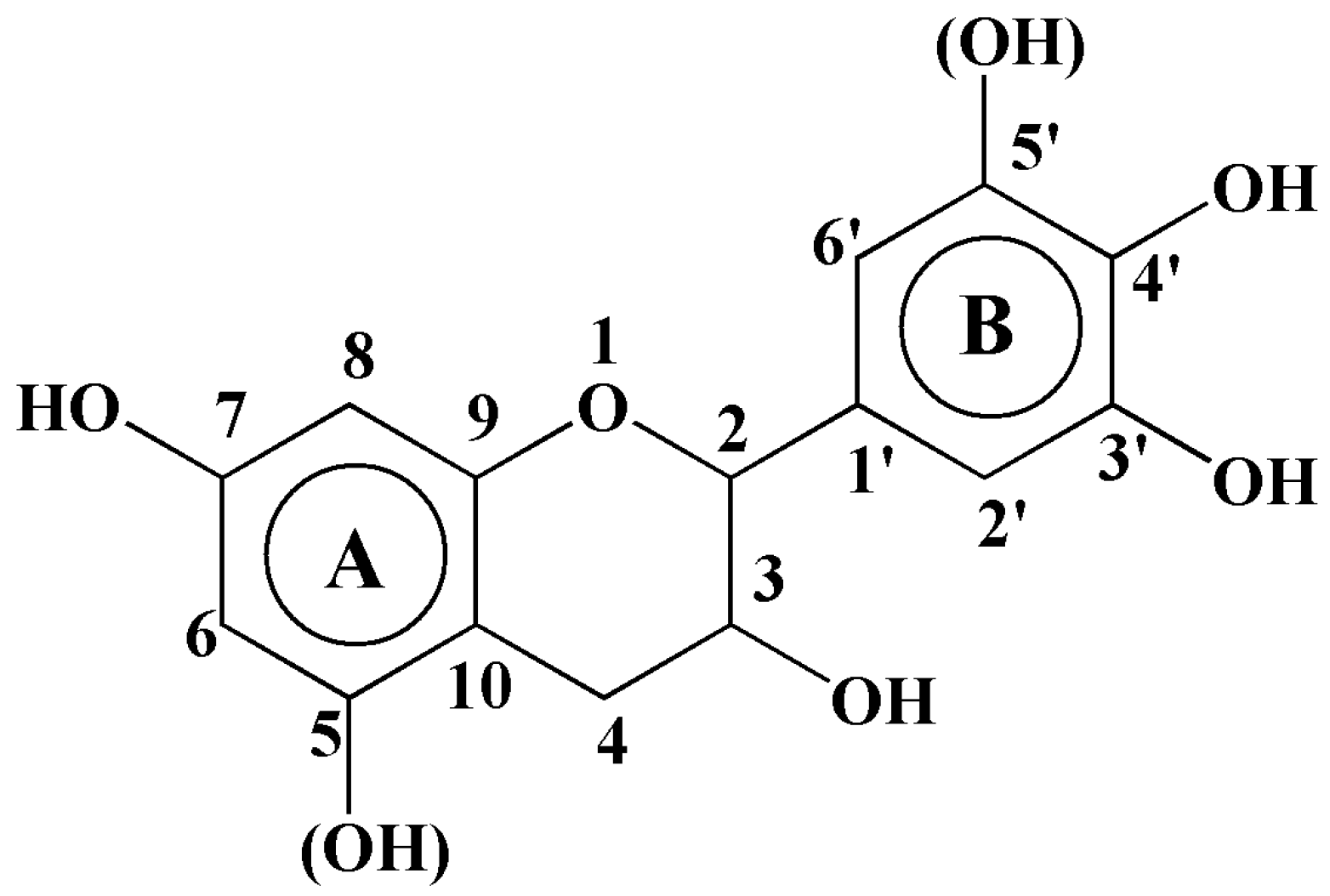
3.8. Bonding Mechanism Analysis of the Tannin–Sucrose Adhesive
4. Conclusions
- (1)
- With a solid content of up to 70% and a low viscosity, tannin–sucrose adhesives have the potential to prepare particleboard and fiberboard.
- (2)
- An appropriate tannin–sucrose mass ratio was key to acquiring adhesives with superior performance. Sucrose is converted into 5-HMF, which reacts with the tannins to exert an indirect crosslinking action, and the hydrophilia of sucrose had a viscosity reduction effect on the adhesive system. The macromolecular structure of tannins provided enough cohesive strength for the adhesive system, and acidic tannins could more easily promote the crosslinking reaction between tannins and sucrose.
- (3)
- The hot-pressing temperature played a decisive role in the performance of the tannin–sucrose adhesives. The good performance of plywood could be guaranteed only when the temperature was 210 °C or above. The optimal process of plywood preparation based on tannin–sucrose adhesives is presented as follows: hot-pressing temperature of 210 °C, time of 1.2 min/mm, m(tannin):m(sucrose) of 60:40, and adhesive loading of 160 g/m2. The wet bonding strength of the plywood prepared under such conditions was 0.89 MPa, meeting the strength requirements for Type II plywood in GB/T 17657-2013.
- (4)
- How to achieve good bonding performances for tannin–sucrose adhesives at a low curing temperature will be studied in subsequent research work.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pizzi, A. Handbook of Adhesive Technology; Marcel Dekker: New York, NY, USA, 2003. [Google Scholar]
- Zhang, B.; Wu, Z.; Liang, J.; Yu, L.; Xi, X.; Lei, H.; Du, G. Effects of polyethylene glycol on the flexibility of cold-setting melamine-urea-formaldehyde resin. Eur. J. Wood Prod. 2022, 80, 975–984. [Google Scholar] [CrossRef]
- Wang, J.; Du, G.; Yang, H.; Ren, J.; Ran, X.; Li, Z.; Yang, L. Synthesis and adhesive properties of highly branched polyurea with polyethyleneimine and urea. J. For. Eng. 2022, 7, 97–102. [Google Scholar]
- Jiang, S.; Hu, M.; Zhou, X.; Duan, Z.; Du, G.; Li, T. Effects of highly branched polyurea on the water resistance and formaldehyde emission of urea-formaldehyde adhesive. J. For. Eng. 2022, 7, 107–114. [Google Scholar]
- Pizzi, A. Wood Adhesives: Chemistry and Technology; Deeker: New York, NY, USA, 1989. [Google Scholar]
- Liang, J.; Li, Q.; Wu, Z.; Du, G.; Li, T.; Lei, H.; Li, L. Competitive polycondensation of model compound melamine-urea-formaldehyde (MUF) resin system by 13C NMR. J. Bioresour. Bioprod. 2020, 5, 60–66. [Google Scholar] [CrossRef]
- Song, J.; Tian, H.; Lei, H.; Xu, G.; Wang, J.; Pu, L.; Chen, Q. Modification of urea-formaldehyde resin by nano MnO2. J. For. Eng. 2022, 7, 91–96. [Google Scholar]
- Zhang, J.; Xi, X.; Liang, J.; Pizzi, A.; Du, G.; Deng, S. Tannin-based adhesive cross-linked by furfuryl alcohol-glyoxal and epoxy resins. Int. J. Adhes. Adhes. 2019, 94, 47–52. [Google Scholar] [CrossRef]
- Huo, Z. Crosslinking and Reinforcement of Tannin Adhesive by Cellulose Nanofibrils Grafted with Hyperbranched Polyamides. Master’s Thesis, Nanjing Forestry University, Nanjing, China, 2017. [Google Scholar]
- Yuan, C.; Chen, M.; Luo, J.; Li, X.; Gao, Q.; Li, J. A novel water-based process produces eco-friendly bio-adhesive made from green cross-linked soybean soluble polysaccharide and soy protein. Carbohyd. Polym. 2017, 169, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Chen, M.; Gao, Q.; Li, J. A high-performance and low-cost soy flour adhesive with a hydroxymethyl melamine prepolymer. Polymers 2018, 10, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Xu, Y.; Chen, M.; Zhang, Y.; Li, J.; Gao, Q.; Shi, S.Q. Soy protein adhesive with bio-based epoxidized daidzein for high strength and mildew resistance. Chem. Eng. J. 2020, 390, 124622. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by PTGE and PAM. Ind. Crop. Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Gao, Q.; Zhang, S.; Li, J. Properties of soybean-flour-based adhesives enhanced by attapulgite and glycerol polyglycidyl ether. Ind. Crop. Prod. 2014, 59, 35–40. [Google Scholar] [CrossRef]
- Xia, Z.; Li, J.; Zhang, J.; Zhang, X.; Zheng, X.; Zhang, J. Processing and valorization of cellulose, lignin and lignocellulose using ionic liquids. J. Bioresour. Bioprod. 2020, 5, 79–95. [Google Scholar] [CrossRef]
- Zhang, N.; Li, Z.; Xiao, Y.; Pan, Z.; Jia, P.; Feng, G.; Bao, C.; Zhou, Y.; Hua, L. Lignin-based phenolic resin modified with whisker silicon and its application. J. Bioresour. Bioprod. 2020, 5, 67–77. [Google Scholar] [CrossRef]
- Li, R.; Gutierrez, J.; Chung, Y.; Frank, C.; Billington, S.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Nacas, A.M.; Ito, N.M.; De Sousa, R.R., Jr.; Spinacé, M.A.; Dos Santos, D.J. Effects of NCO:OH ratio on the mechanical properties and chemical structure of Kraft lignin–based polyurethane adhesive. J. Adhesion 2016, 93, 18–29. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef] [Green Version]
- Pizzi, A.; Papadopoulos, A.N.; Policardi, F. Wood Composites and Their Polymer Binders. Polymers 2020, 12, 1115. [Google Scholar] [CrossRef]
- Siahkamari, M.; Emmanuel, S.; Hodge, D.B.; Nejad, M. Lignin-Glyoxal: A Fully Biobased Formaldehyde-Free Wood Adhesive for Interior Engineered Wood Products. ACS Sustain. Chem. Eng. 2022, 10, 3430–3441. [Google Scholar] [CrossRef]
- Pizzi, A.; Meikleham, N.; Stephanou, A. Induced accelerated autocondensation of polyflavonoid tannins for phenolic polycondensates. II: Cellulose effect and application. J. Appl. Polym. Sci. 1995, 55, 929–933. [Google Scholar] [CrossRef]
- Osman, Z.; Pizzi, A. Comparison of gelling reaction effectiveness of procyanidin tannins for wood adhesives. Eur. J. Wood Prod. 2002, 60, 328. [Google Scholar] [CrossRef]
- Meikleham, N.E.; Pizzi, A. Acid-catalyzed and alkali-catalyzed tannin-based rigid foams. J. Appl. Polym. Sci. 1994, 53, 1547–1556. [Google Scholar] [CrossRef]
- Rossouw, D. Reaction Kinetics of Phenols and Tannin with Aldehydes; University of South Africa: Gauteng, South Africa, 1979. [Google Scholar]
- Sun, G.; Sun, H.; Liu, Y.; Zhao, B.; Zhu, N.; Hu, K. Comparative study on the curing kinetics and mechanism of a lignin-based-epoxy/anhydride resin system. Polymer 2007, 48, 330–337. [Google Scholar] [CrossRef]
- Li, K.; Geng, X.; Simonsen, J.; Karchesy, J. Novel wood adhesives from condensed tannins and polyethylenimine. Int. J. Adhes. Adhes. 2004, 24, 327–333. [Google Scholar] [CrossRef]
- Ghahri, S.; Pizzi, A. Improving soy-based adhesives for wood particleboard by tannins addition. Wood Sci. Technol. 2018, 52, 261–279. [Google Scholar] [CrossRef]
- Xi, X.; Pizzi, A.; Frihart, C.R.; Lorenz, L.; Gerardin, C. Tannin plywood bioadhesives with non-volatile aldehydes generation by specific oxidation of mono- and disaccharides. Int. J. Adhes. Adhes. 2020, 98, 102499. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. 2. Effect of pressing temperature and time on board properties, and characterization of adhesive. Bioresources 2015, 10, 2444–2460. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Umemura, K.; Kanayama, K. Effects of the addition of citric acid on tannin-sucrose adhesive and physical properties of the particleboard. Bioresources 2015, 11, 1319–1333. [Google Scholar] [CrossRef]
- Merlin, A.; Pizzi, A. An ESR study of the silica-induced autocondensation of polyflavonoid tannins. J. Appl. Polym. Sci. 1996, 59, 945–952. [Google Scholar] [CrossRef]
- Masson, E.; Pizzi, A.; Merlin, A. Comparative kinetics of the induced radical autocondensation of polyflavonoid tannins. II: Flavonoid units effects. J. Appl. Polym. Sci. 1997, 64, 243–265. [Google Scholar] [CrossRef]
- Masson, E.; Pizzi, A.; Merlin, A. Comparative kinetics of the induced radical autocondensation of polyflavonoid tannins. III: Micellar reactions vs. cellulose surface catalysis. J. Appl. Polym. Sci. 1996, 60, 1655–1664. [Google Scholar] [CrossRef]
- Deng, X.; Wu, Z.; Zhang, B.; Lei, H.; Liang, J.; Li, L.; Tu, Y.; Li, D.; Xiao, G. A new wood adhesive based on recycling Camellia oleifera cake-protein: Preparation and properties. Materials 2022, 15, 1659. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Pizzi, A.; Gerardin, C.; Chen, X.; Amirou, S. Soy protein isolate-based polyamides as wood adhesives. Wood Sci. Technol. 2019, 54, 89–102. [Google Scholar] [CrossRef]
- Luo, J. Cross-Linking Architecture Regulation and Reinforcement Mechanism of Soy Protein-Based Adhesive; Beijing Forestry University: Beijing, China, 2018. [Google Scholar]
- Xu, G.; Tian, H.; Xi, X.; Song, J.; Lei, H.; Du, G. Preparation and characterization of urea–formaldehyde adhesives modified with glyoxalated tannin. Eur. J. Wood. Prod. 2022, 80, 1215–1223. [Google Scholar] [CrossRef]
- Qiu, J.; Liu, M.; Shi, J.; Guo, X. Catalytic dehydration of sucrose into 5-hydroxymethylfurfural over H-Beta zeolite in [EMIM] Br. Acta Pet. Sin. 2012, 28, 407–412. [Google Scholar]
- Hu, F.; Li, L.; Wu, Z.; Yu, L.; Liu, B.; Cao, Y.; Xu, H. Surface characteristics of thermally modified bamboo fibers and its utilization potential for bamboo plastic composites. Materials 2022, 15, 4481. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, B.; Wu, Z.; Yu, L.; Li, L.; Xi, X. Effects of steam heat-treatment on properties of Pinus massoniana wood and its bonding performance. J. Renew. Mater. 2021, 9, 789–801. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, M.; Umemura, K.; Zhao, Z. Investigation and Characterization of Synthesis Conditions on Sucrose-ammonium Dihydrogen Phosphate (SADP) Adhesive: Bond Performance and Chemical Transformation. Materials 2019, 12, 4078. [Google Scholar] [CrossRef] [Green Version]
- Qiu, G.; Wang, X.; Huang, C.; Zhang, P.; Li, Y.; Chen, B. Synthesis of 5-Hydroxymethylfurfural from Sucrose Catalyzed by Phosphotungstate. Chin. J. Org. Chem. 2018, 38, 940. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Zhou, X.; Li, L.; Yu, L.; Liao, J.; Du, G. Influence of Single/Collective Use of Curing Agents on the Curing Behavior and Bond Strength of Soy Protein-Melamine-Urea-Formaldehyde (SMUF) Resin for Plywood Assembly. Polymers 2019, 11, 1995. [Google Scholar] [CrossRef] [Green Version]
- Kim, S. Environment-friendly adhesives for surface bonding of wood-based flooring using natural tannin to reduce formaldehyde and TVOC emission. Bioresour. Technol. 2009, 100, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Lara-Serrano, M.; Morales-Delarosa, M.; Campos-Martin, J.M.; Abdelkader-Fernández, V.K.; Cunha-Silva, L.; Balula, S. One-Pot Conversion of Glucose into 5-Hydroxymethylfurfural using MOFs and Bronsted-Acid Tandem Catalysts. Adv. Sustain. Syst. 2022, 5, 6. [Google Scholar] [CrossRef]
- Kong, S.; Liu, S.; Li, L.; Yu, S. Preparation of 5-Hydroxymethylfurfural by Dehydration of Sucrose. Chem. Ind. For. Prod. 2014, 34, 73–76. [Google Scholar]
- Desir, P.; Vlachos, D. Intensified reactive extraction for the acid-catalyzed conversion of fructose to 5-hydroxymethyl furfural. Chem. Eng. J. 2022, 428, 132556. [Google Scholar] [CrossRef]





| Samples | Tannin/g | Sucrose/g | Water/g |
|---|---|---|---|
| 100:0 | 50 | 0 | 33.3 |
| 80:20 | 40 | 10 | 33.3 |
| 60:40 | 30 | 20 | 33.3 |
| 50:50 | 25 | 25 | 33.3 |
| 40:60 | 20 | 30 | 33.3 |
| 20:80 | 10 | 40 | 33.3 |
| 0:100 | 0 | 50 | 33.3 |
| Levels | Factors | |||
|---|---|---|---|---|
| Hot-Pressing Temperature/°C | Hot-Pressing Time/(min/mm) | Adhesive Loading /(g/m2) | m(Tannin):m(Sucrose) | |
| 1 | 200 | 1.0 | 140 | 60:40 |
| 2 | 210 | 1.2 | 160 | 50:50 |
| 3 | 220 | 1.4 | 180 | 40:60 |
| 4 | 230 | 1.6 | 200 | 30:70 |
| NO. | Hot-Pressing Temperature/°C | Hot-Pressing Time/(min/mm) | Adhesive Loading/(g/m2) | m(Tannin):m(Sucrose) | Wet Bonding Strength/MPa |
|---|---|---|---|---|---|
| 1 | 200 | 1.0 | 140 | 60:40 | 0.38 ± 0.07 |
| 2 | 200 | 1.2 | 160 | 50:50 | 0.53 ± 0.02 |
| 3 | 200 | 1.4 | 180 | 40:60 | 0.33 ± 0.06 |
| 4 | 200 | 1.6 | 200 | 30:70 | 0.43 ± 0.03 |
| 5 | 210 | 1.0 | 160 | 40:60 | 1.25 ± 0.18 |
| 6 | 210 | 1.2 | 140 | 30:70 | 1.11 ± 0.15 |
| 7 | 210 | 1.4 | 200 | 60:40 | 1.21 ± 0.14 |
| 8 | 210 | 1.6 | 180 | 50:50 | 0.99 ± 0.10 |
| 9 | 220 | 1.0 | 180 | 30:70 | 1.36 ± 0.11 |
| 10 | 220 | 1.2 | 200 | 40:60 | 1.48 ± 0.11 |
| 11 | 220 | 1.4 | 140 | 50:50 | 1.49 ± 0.16 |
| 12 | 220 | 1.6 | 160 | 60:40 | 1.44 ± 0.10 |
| 13 | 230 | 1.0 | 200 | 50:50 | 1.57 ± 0.12 |
| 14 | 230 | 1.2 | 180 | 60:40 | 1.74 ± 0.15 |
| 15 | 230 | 1.4 | 160 | 30:70 | 1.43 ± 0.06 |
| 16 | 230 | 1.6 | 140 | 40:60 | 1.59 ± 0.15 |
| K1 | 0.42 | 1.14 | 1.14 | 1.19 | — |
| K2 | 1.14 | 1.22 | 1.16 | 1.15 | — |
| K3 | 1.44 | 1.12 | 1.11 | 1.16 | — |
| K4 | 1.58 | 1.11 | 1.17 | 1.08 | — |
| R | 1.17 | 0.10 | 0.07 | 0.11 | — |
| Factors | Sum of Squares of Deviations (DEVSQ) | Degree of Freedom (DOF) | Mean Square Error (MSER) | Significance |
|---|---|---|---|---|
| Hot-pressing temperature | 3.237 | 3 | 294.273 | * |
| Hot-pressing time | 0.028 | 3 | 2.545 | |
| Adhesive loading | 0.011 | 3 | 1.000 | |
| m(tannin):m(sucrose) | 0.026 | 3 | 2.364 | |
| Error | 0.012 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, G.; Liang, J.; Li, D.; Tu, Y.; Zhang, B.; Gong, F.; Gu, W.; Tang, M.; Ding, X.; Wu, Z.; et al. Fully Bio-Based Adhesive from Tannin and Sucrose for Plywood Manufacturing with High Performances. Materials 2022, 15, 8725. https://doi.org/10.3390/ma15248725
Xiao G, Liang J, Li D, Tu Y, Zhang B, Gong F, Gu W, Tang M, Ding X, Wu Z, et al. Fully Bio-Based Adhesive from Tannin and Sucrose for Plywood Manufacturing with High Performances. Materials. 2022; 15(24):8725. https://doi.org/10.3390/ma15248725
Chicago/Turabian StyleXiao, Guoming, Jiankun Liang, De Li, Yuan Tu, Bengang Zhang, Feiyan Gong, Wen Gu, Min Tang, Xinyue Ding, Zhigang Wu, and et al. 2022. "Fully Bio-Based Adhesive from Tannin and Sucrose for Plywood Manufacturing with High Performances" Materials 15, no. 24: 8725. https://doi.org/10.3390/ma15248725
APA StyleXiao, G., Liang, J., Li, D., Tu, Y., Zhang, B., Gong, F., Gu, W., Tang, M., Ding, X., Wu, Z., & Lei, H. (2022). Fully Bio-Based Adhesive from Tannin and Sucrose for Plywood Manufacturing with High Performances. Materials, 15(24), 8725. https://doi.org/10.3390/ma15248725








