Charge Distribution in Layered Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = Pr–Tb) Thermoelectric Materials
Abstract
1. Introduction
2. Experimental
3. Results and Discussion
3.1. X-ray Diffraction (XRD)
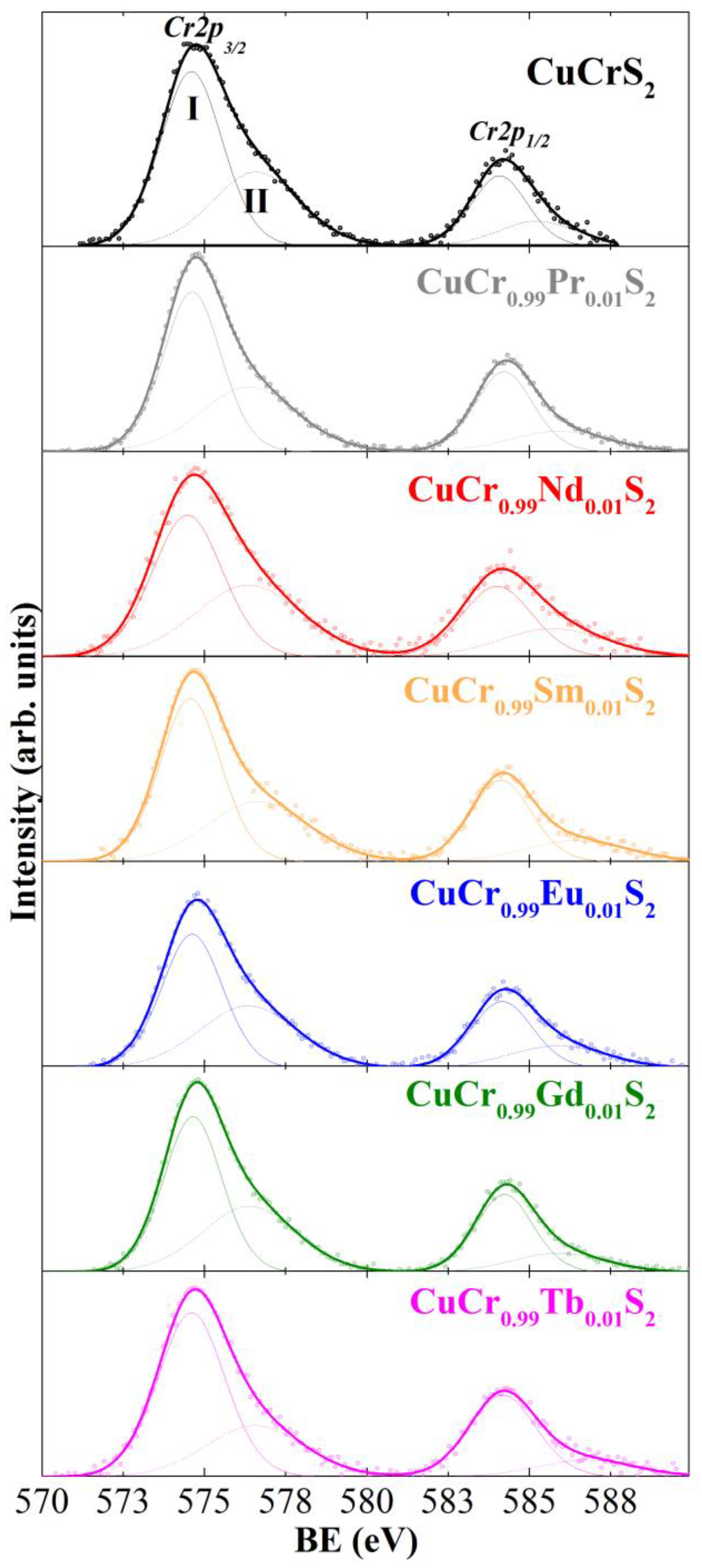
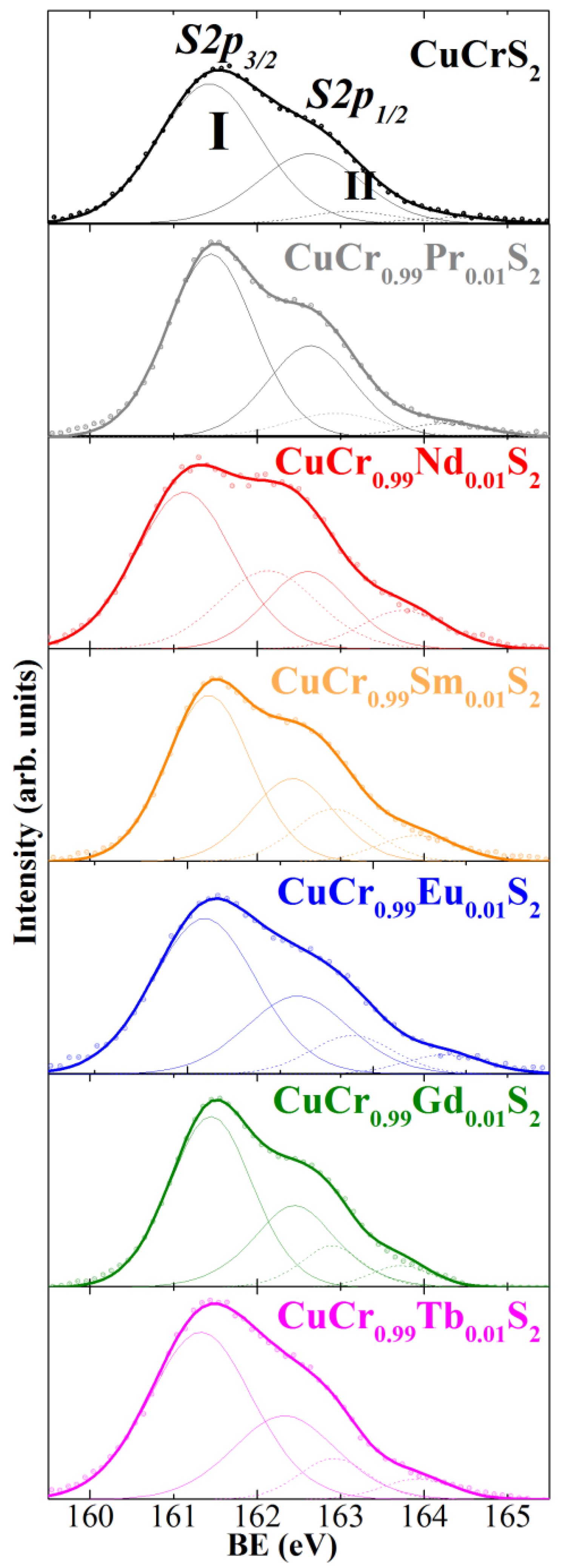
3.2. X-ray Photoelectron Spectroscopy (XPS)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mori, T.; Maignan, A. Thermoelectric Materials Developments: Past, Present, and Future. Sci. Technol. Adv. Mater. 2021, 22, 998–999. [Google Scholar] [CrossRef]
- Yakovleva, G.E.; Romanenko, A.I.; Ledneva, A.Y.; Belyavin, V.A.; Kuznetsov, V.A.; Berdinsky, A.S.; Burkov, A.T.; Konstantinov, P.P.; Novikov, S.V.; Han, M.K.; et al. Thermoelectric Properties of W1−xNbxSe2−ySy Polycrystalline Compounds. J. Am. Ceram. Soc. 2019, 102, 6060–6067. [Google Scholar] [CrossRef]
- Binwal, D.C.; Kaur, M.; Pramoda, K.; Rao, C.N.R. HER Activity of Nanosheets of 2D Solid Solutions of MoSe2 with MoS2 and MoTe2. Bull. Mater. Sci. 2020, 43, 313. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Pelmenev, K.G.; Zvereva, V.V.; Peregudova, N.N. Seebeck Coefficient of Cation-Substituted Disulfides CuCr1−xFexS2 and Cu1−xFexCrS2. J. Electron. Mater. 2018, 47, 3392–3397. [Google Scholar] [CrossRef]
- Srivastava, D.; Tewari, G.C.; Karppinen, M.; Nieminen, R.M. First-Principles Study of Layered Antiferromagnetic CuCrX2 (X = S, Se and Te). J. Phys. Condens. Matter 2013, 25, 105504. [Google Scholar] [CrossRef] [PubMed]
- Tsujii, N.; Kitazawa, H.; Kido, G. Insulator to Metal Transition Induced by Substitution in the Nearly Two-Dimensional Compound CuCr1–xVxS2. Phys. Status Solidi 2006, 3, 2775–2778. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Basu, R.; Bhatt, R.; Pitale, S.; Singh, A.; Aswal, D.K.; Gupta, S.K.; Navaneethan, M.; Hayakawa, Y. CuCrSe2: A High Performance Phonon Glass and Electron Crystal Thermoelectric Material. J. Mater. Chem. A 2013, 1, 11289–11294. [Google Scholar] [CrossRef]
- Wu, D.; Huang, S.; Feng, D.; Li, B.; Chen, Y.; Zhang, J.; He, J. Revisiting AgCrSe2 as a Promising Thermoelectric Material. Phys. Chem. Chem. Phys. 2016, 18, 23872–23878. [Google Scholar] [CrossRef]
- Dmitriev, A.V.; Zvyagin, I.P. Current Trends in the Physics of Thermoelectric Materials. Physics-Uspekhi 2010, 53, 789–803. [Google Scholar] [CrossRef]
- Jabar, B.; Li, F.; Zheng, Z.; Mansoor, A.; Zhu, Y.; Liang, C.; Ao, D.; Chen, Y.; Liang, G.; Fan, P.; et al. Homo-Composition and Hetero-Structure Nanocomposite Pnma Bi2SeS2—Pnnm Bi2SeS2 with High Thermoelectric Performance. Nat. Commun. 2021, 12, 7192. [Google Scholar] [CrossRef]
- Tewari, G.C.; Tripathi, T.S.; Kumar, P.; Rastogi, A.K.; Pasha, S.K.; Gupta, G. Increase in the Thermoelectric Efficiency of the Disordered Phase of Layered Antiferromagnetic CuCrS2. J. Electron. Mater. 2011, 40, 2368–2373. [Google Scholar] [CrossRef]
- Hansen, A.-L.; Dankwort, T.; Groß, H.; Etter, M.; König, J.; Duppel, V.; Kienle, L.; Bensch, W. Structural Properties of the Thermoelectric Material CuCrS2 and of Deintercalated CuxCrS2 on Different Length Scales: X-Ray Diffraction, Pair Distribution Function and Transmission Electron Microscopy Studies. J. Mater. Chem. C 2017, 5, 9331–9338. [Google Scholar] [CrossRef]
- Kaltzoglou, A.; Vaqueiro, P.; Barbier, T.; Guilmeau, E.; Powell, A.V. Ordered-Defect Sulfides as Thermoelectric Materials. J. Electron. Mater. 2013, 43, 2029–2034. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Zhang, B.-P.; Ge, Z.-H.; Shang, P.-P. Preparation and Thermoelectric Properties of Ternary Superionic Conductor CuCrS2. J. Solid State Chem. 2012, 186, 109–115. [Google Scholar] [CrossRef]
- Vasilyeva, I.G. Chemical Aspect of the Structural Disorder in CuCrS2 and CuCr1–xVxS2 Solid Solutions. J. Struct. Chem. 2017, 58, 1009–1017. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Peregudova, N.N.; Mazalov, L.N.; Sokolov, V.V.; Kalinkin, A.V.; Kryuchkova, N.A.; Dikov, Y.P.; Buleev, M.I.; Filatova, I.Y.; Pichugin, A.Y. Photoelectron Spectra of Powder and Single Crystalline Chromium-Copper Disulfides. J. Struct. Chem. 2013, 54, 255–258. [Google Scholar] [CrossRef]
- Romanenko, A.I.; Chebanova, G.E.; Katamanin, I.N.; Drozhzhin, M.V.; Artemkina, S.B.; Han, M.-K.; Kim, S.-J.; Wang, H. Enhanced Thermoelectric Properties of Polycrystalline CuCrS2−xSex (x = 0, 0.5, 1.0, 1.5, 2) Samples by Replacing Chalcogens and Sintering. J. Phys. D. Appl. Phys. 2021, 55, 135302. [Google Scholar] [CrossRef]
- Mulla, R.; Dunnill, C.W. Core–Shell Nanostructures for Better Thermoelectrics. Mater. Adv. 2022, 3, 125–141. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Trubina, S.V.; Nikolenko, A.D.; Ivlyushkin, D.V.; Zavertkin, P.S.; Sotnikov, A.V.; Kriventsov, V.V. XANES Investigation of Novel Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = La, Ce) Solid Solutions. Appl. Phys. A 2020, 126, 537. [Google Scholar] [CrossRef]
- Shevelkov, A.V. Chemical Aspects of the Design of Thermoelectric Materials. Russ. Chem. Rev. 2008, 77, 1–19. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, C.; Xia, K.; Yu, J.; Zhao, X.; Pan, H.; Felser, C.; Zhu, T.; Liu, Y.T.; Xia, K.Y.; et al. Lanthanide Contraction as a Design Factor for High-Performance Half-Heusler Thermoelectric Materials. Adv. Mater. 2018, 30, 1800881. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; He, R.; Gong, S.; Xie, T.; Gorai, P.; Nielsch, K.; Grossman, J.C. Charting Lattice Thermal Conductivity for Inorganic Crystals and Discovering Rare Earth Chalcogenides for Thermoelectrics. Energy Environ. Sci. 2021, 14, 3559–3566. [Google Scholar] [CrossRef]
- Sotnikov, A.V.; Syrokvashin, M.M.; Bakovets, V.V.; Filatova, I.Y.; Korotaev, E.V.; Agazhanov, A.S.; Samoshkin, D.A. Figure of Merit Enhancement in Thermoelectric Materials Based on γ-Ln 0.8 Yb 0.2 S 1.5-y (Ln = Gd, Dy) Solid Solutions. J. Am. Ceram. Soc. 2022, 105, 2813–2822. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Trubina, S.V.; Nikolenko, A.D.; Ivlyushkin, D.V.; Zavertkin, P.S.; Kriventsov, V.V. The Conduction Band of the Lanthanide Doped Chromium Disulfides CuCr0.99Ln0.01S2 (Ln=La, Ce, Gd): XANES Investigations. AIP Conf. Proc. 2020, 2299, 080004. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Kalinkin, A.V.; Sotnikov, A.V. Valence Band Structure and Charge Distribution in the Layered Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = La, Ce) Solid Solutions. Sci. Rep. 2021, 11, 18934. [Google Scholar] [CrossRef]
- Abramova, G.M.; Petrakovskii, G.A. Metal-Insulator Transition, Magnetoresistance, and Magnetic Properties of 3d-Sulfides. Low Temp. Phys. 2006, 32, 725–734. [Google Scholar] [CrossRef]
- Sotnikov, A.V.; Bakovets, V.V.; Sokolov, V.V.; Filatova, I.Y. Lanthanum Oxide Sulfurization in Ammonium Rhodanide Vapor. Inorg. Mater. 2014, 50, 1024–1029. [Google Scholar] [CrossRef]
- Jaziri, N.; Boughamoura, A.; Müller, J.; Mezghani, B.; Tounsi, F.; Ismail, M. A Comprehensive Review of Thermoelectric Generators: Technologies and Common Applications. Energy Rep. 2020, 6, 264–287. [Google Scholar] [CrossRef]
- Terasaki, I. Introduction to Thermoelectricity. Mater. Energy Convers. Devices A Vol. Woodhead Publ. Ser. Electron. Opt. Mater. 2005, 339–357. [Google Scholar] [CrossRef]
- Shalimova, K.V. Semiconductors Physics; Lan: St. Petersburg, Russia, 2021; ISBN 978-5-8114-0922-8. [Google Scholar]
- Moeller, T. The Chemistry of the Lanthanides; Elsevier: Amsterdam, The Netherlands, 1973; ISBN 9780080188782. [Google Scholar]
- Niemantsverdriet, J.W. Spectroscopy in Catalysis; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2000; ISBN 9783527614127. [Google Scholar]
- Kraus, W.; Nolze, G. POWDER CELL—A Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-Ray Powder Patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- CasaXPS Software. Available online: http://www.casaxps.com/ (accessed on 14 November 2022).
- Inorganic Crystal Structure Database, version 2.1.0; Leibniz Institute for Information Infrastructure, FIZ Karlsruhe: Eggenstein-Leopoldshafen, Germany, 2014.
- Larsson, S. Satellites in ESCA Inner-Shell Spectra of 3d0 Transition Metal Complexes. J. Electron Spectros. Relat. Phenom. 1976, 8, 171–178. [Google Scholar] [CrossRef]
- NIST X-ray Photoelectron Spectroscopy (XPS) Database, Version 3.5. Available online: https://srdata.nist.gov/xps/ (accessed on 14 November 2022).
- XPS, AES, UPS and ESCA Database. Available online: http://www.lasurface.com/database/elementxps.php (accessed on 14 November 2022).
- Nefedov, V.A. X-ray Photoelectron Spectroscopy of Chemical Compounds; Khimiya: Moscow, Russia, 1984. [Google Scholar]
- Mikhlin, Y.L.; Tomashevich, Y.V.; Pashkov, G.L.; Okotrub, A.V.; Asanov, I.P.; Mazalov, L.N. Electronic Structure of the Non-Equilibrium Iron-Deficient Layer of Hexagonal Pyrrhotite. Appl. Surf. Sci. 1998, 125, 73–84. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Zvereva, V.V. Vanadium Doped Layered Copper-Chromium Sulfides: The Correlation between the Magnetic Properties and XES Data. Vacuum 2020, 179, 109390. [Google Scholar] [CrossRef]
- Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Zvereva, V.V. Magnetic Properties of Novel Layered Disulfides CuCr0.99Ln0.01S2 (Ln = La…Lu). Materials 2021, 14, 5101. [Google Scholar] [CrossRef] [PubMed]
- Nasluzov, V.; Shor, A.; Romanchenko, A.; Tomashevich, Y.; Mikhlin, Y. DFT + U and Low-Temperature XPS Studies of Fe-Depleted Chalcopyrite (CuFeS2) Surfaces: A Focus on Polysulfide Species. J. Phys. Chem. C 2019, 123, 21031–21041. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Vorobyev, S.; Romanchenko, A.; Likhatski, M.; Antsiferova, S.; Markosyan, S. Towards Understanding the Role of Surface Gas Nanostructures: Effect of Temperature Difference Pretreatment on Wetting and Flotation of Sulfide Minerals and Pb-Zn Ore. Nanomaterials 2020, 10, 1362. [Google Scholar] [CrossRef]
- Libralesso, L.; Lee, T.L.; Zegenhagen, J. Pr2O3 on Si(001): A Commensurate Interfacial Layer Overgrown by Silicate. Appl. Phys. Lett. 2007, 90, 222905. [Google Scholar] [CrossRef]
- Baltrus, J.P.; Keller, M.J. Rare Earth Oxides Eu2O3 and Nd2O3 Analyzed by XPS. Surf. Sci. Spectra 2019, 26, 014001. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Mrabet, D.; Do, T.O. Controlled Self-Assembly of Sm2O3 Nanoparticles into Nanorods: Simple and Large Scale Synthesis Using Bulk Sm2O3 Powders. J. Phys. Chem. C 2008, 112, 15226–15235. [Google Scholar] [CrossRef]
- Hong, M.; Lu, Z.H.; Kwo, J.; Kortan, A.R.; Mannaerts, J.P.; Krajewski, J.J.; Hsieh, K.C.; Chou, L.J.; Cheng, K.Y. Initial Growth of Ga2O3(Gd2O3) on GaAs: Key to the Attainment of a Low Interfacial Density of States. Appl. Phys. Lett. 2000, 76, 312–314. [Google Scholar] [CrossRef]
- Pan, T.M.; Chen, F.H.; Jung, J.S. Structural and Electrical Characteristics of High-k Tb2O3 and Tb2TiO5 Charge Trapping Layers for Nonvolatile Memory Applications. J. Appl. Phys. 2010, 108, 074501. [Google Scholar] [CrossRef]
- Ivliev, A.D. Electric Resistance of Rare-Earth Metals and Their Alloys at High Temperatures: The Role of Magnetic Scattering. Phys. Solid State 2020, 62, 1755–1761. [Google Scholar] [CrossRef]




| a, Å | c, Å | |
|---|---|---|
| CuCrS2 | 3.48 (3) | 18.68 (9) |
| CuCr0.99Pr0.01S2 | 3.48 (2) | 18.70 (1) |
| CuCr0.99Nd0.01S2 | 3.47 (9) | 18.68 (8) |
| CuCr0.99Sm0.01S2 | 3.47 (9) | 18.68 (6) |
| CuCr0.99Eu0.01S2 | 3.47 (9) | 18.68 (5) |
| CuCr0.99Gd0.01S2 | 3.48 (0) | 18.69 (6) |
| CuCr0.99Tb0.01S2 | 3.48 (0) | 18.69 (7) |
| BE ± 0.2 eV | Cu2p3/2 | Cr2p3/2 | S2p3/2 | Ln3d5/2 |
|---|---|---|---|---|
| CuCrS2 | 932.4 | 574.6 | 161.5 | – |
| 934.6 | 576.6 | 163.1 | ||
| CuCr0.99Pr0.01S2 | 932.2 | 574.6 | 161.5 | 117.9 (Pr4d) |
| 933.9 | 576.4 | 162.9 | ||
| CuCr0.99Nd0.01S2 | 932.2 | 574.5 | 161.1 | 988.6 |
| 933.2 | 576.4 | 162.6 | ||
| CuCr0.99Sm0.01S2 | 932.2 | 574.6 | 161.4 | 1083.6 |
| 934.4 | 576.7 | 162.9 | ||
| CuCr0.99Eu0.01S2 | 932.6 | 574.6 | 161.2 | 1135.5 |
| 934.7 | 576.4 | 162.8 | ||
| CuCr0.99Gd0.01S2 | 932.3 | 574.7 | 161.5 | 1187.1 |
| 933.7 | 576.4 | 162.9 | ||
| CuCr0.99Tb0.01S2 | 932.4 | 574.6 | 161.3 | 152.7 (Tb4d) |
| 935.2 | 576.6 | 162.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korotaev, E.V.; Syrokvashin, M.M.; Filatova, I.Y.; Sotnikov, A.V.; Kalinkin, A.V. Charge Distribution in Layered Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = Pr–Tb) Thermoelectric Materials. Materials 2022, 15, 8747. https://doi.org/10.3390/ma15248747
Korotaev EV, Syrokvashin MM, Filatova IY, Sotnikov AV, Kalinkin AV. Charge Distribution in Layered Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = Pr–Tb) Thermoelectric Materials. Materials. 2022; 15(24):8747. https://doi.org/10.3390/ma15248747
Chicago/Turabian StyleKorotaev, Evgeniy V., Mikhail M. Syrokvashin, Irina Yu. Filatova, Aleksandr V. Sotnikov, and Alexandr V. Kalinkin. 2022. "Charge Distribution in Layered Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = Pr–Tb) Thermoelectric Materials" Materials 15, no. 24: 8747. https://doi.org/10.3390/ma15248747
APA StyleKorotaev, E. V., Syrokvashin, M. M., Filatova, I. Y., Sotnikov, A. V., & Kalinkin, A. V. (2022). Charge Distribution in Layered Lanthanide-Doped CuCr0.99Ln0.01S2 (Ln = Pr–Tb) Thermoelectric Materials. Materials, 15(24), 8747. https://doi.org/10.3390/ma15248747






