Study on Microstructure and Properties of Black Micro-Arc Oxidation Coating on AZ31 Magnesium Alloy by Orthogonal Experiment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Coating Preparation Procedures
2.2. Experiment Process
2.3. Characterization
3. Results
3.1. Orthogonal Experimental Results and Analysis
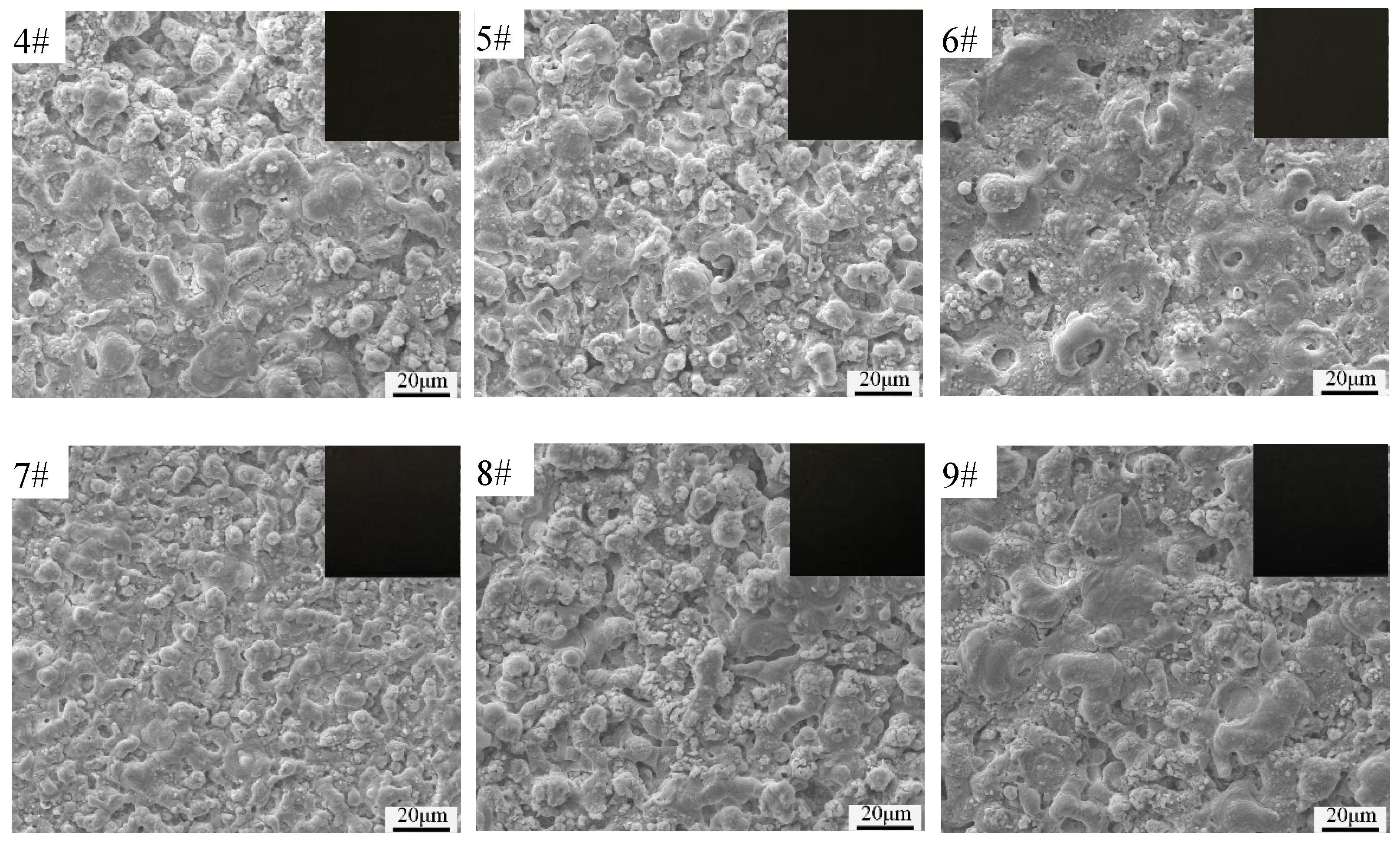
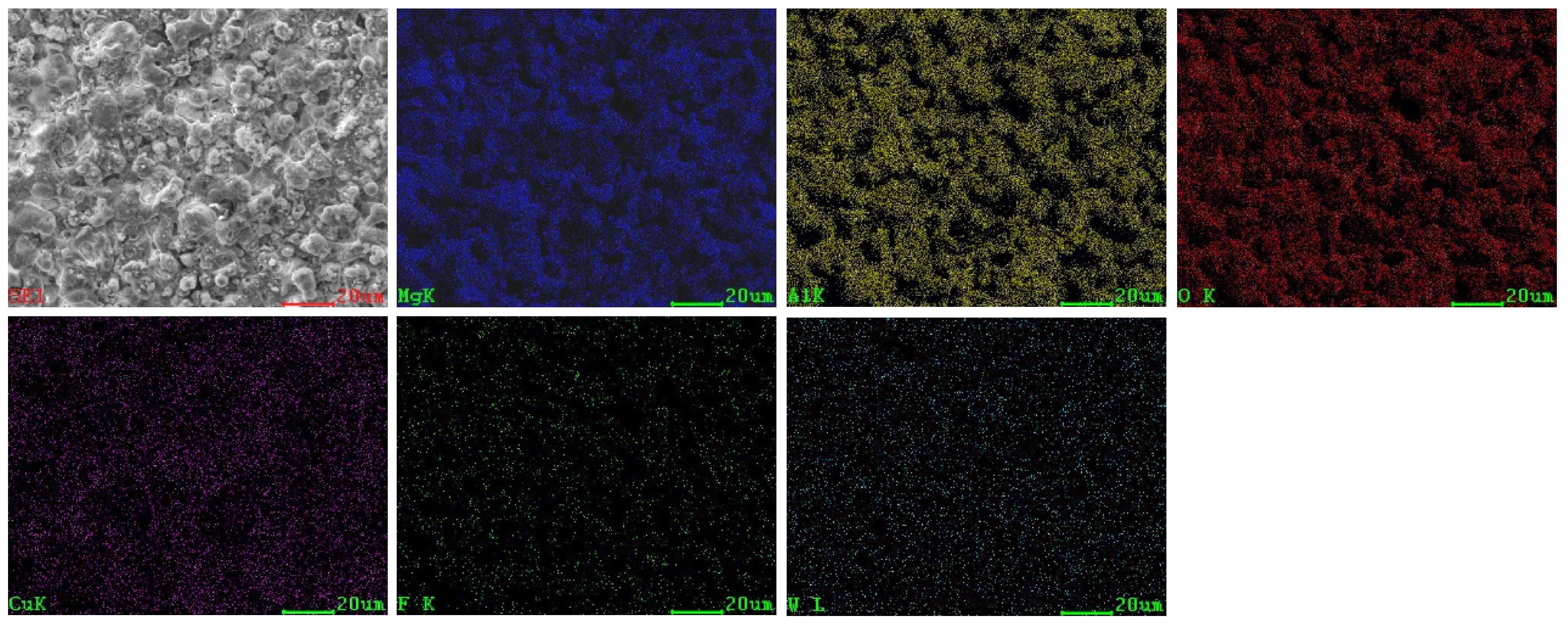
3.2. Surface Morphologies and Elemental Composition of Black MAO Coating
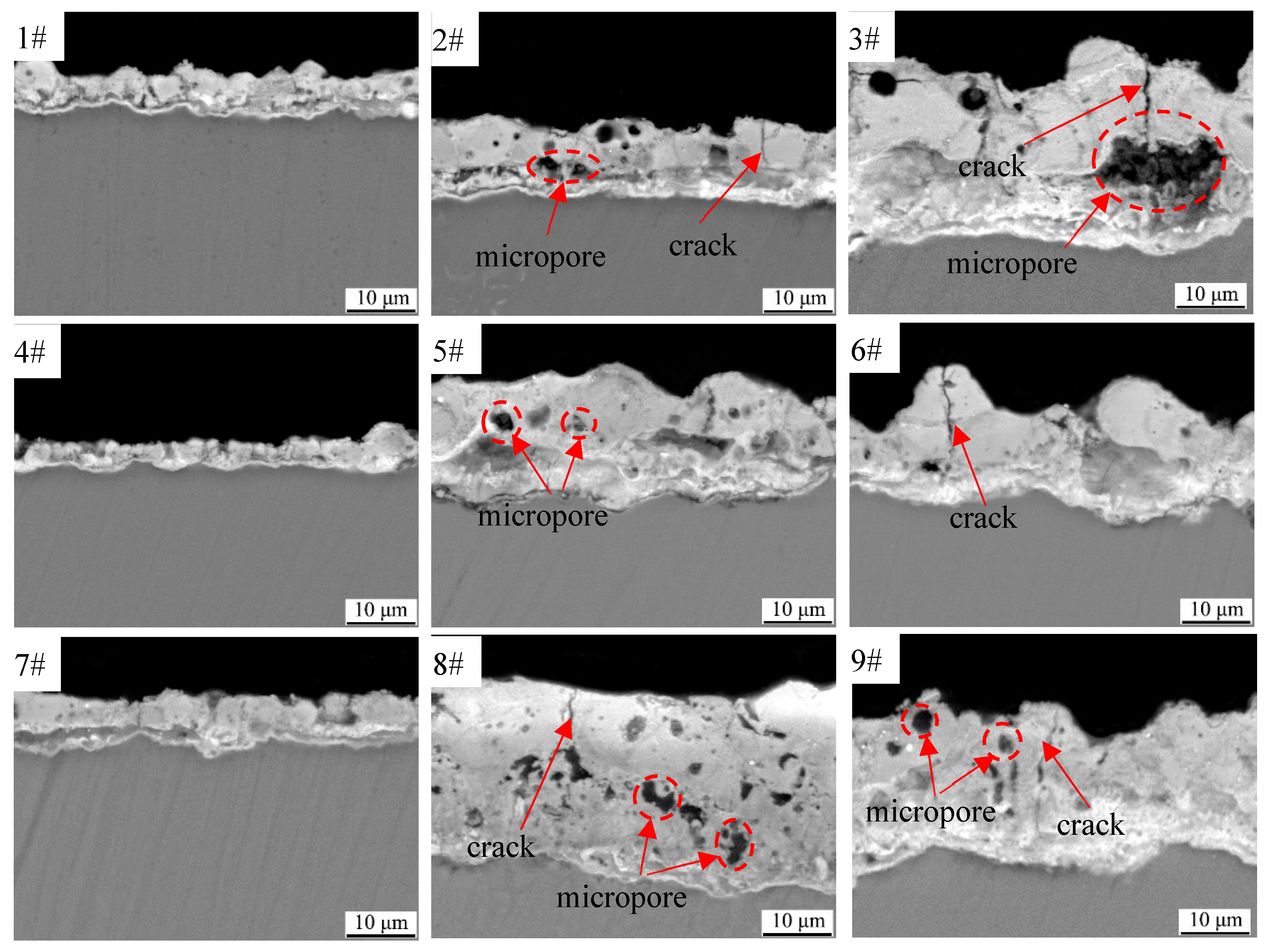
3.3. Cross-Sectional Morphologies and Elemental Composition of Black MAO Coating
3.4. Phase and Chemical Composition of Black MAO Coating
3.5. Electrochemical Corrosion Test of Black MAO Coating
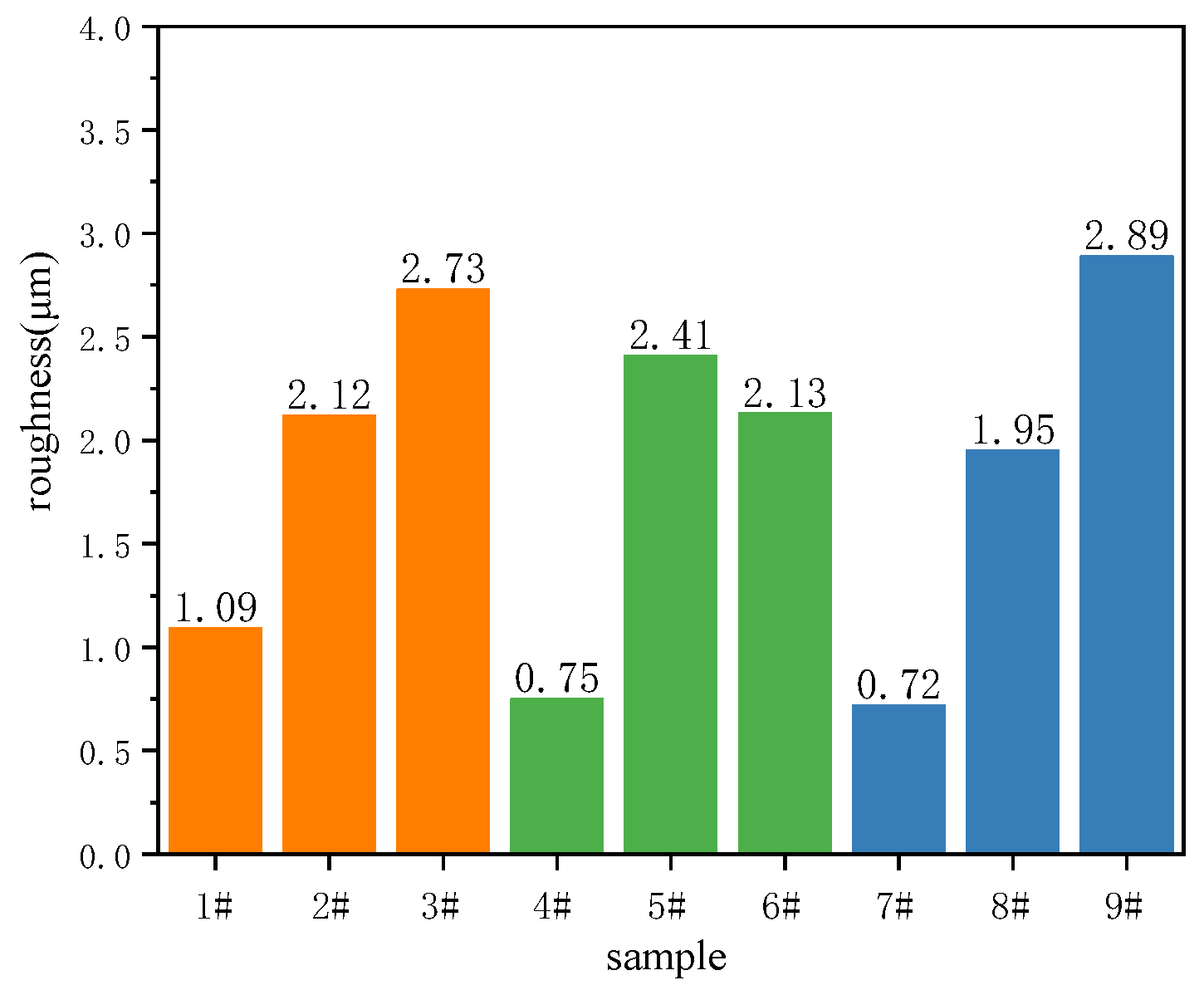
3.6. Hemisphere Emissivity of the Black MAO Coating
4. Discussion
5. Conclusions
- The results of the orthogonal experiment showed that the influences of each factor on corrosion current density and the hemisphere emissivity are as follows: voltage > treating time > CuSO4 concentration. The CuSO4 concentration, voltage and reaction time have no obvious effect on the blackness of the coating. The best process scheme for the preparation of black MAO coating is A2B3C2, which means the parameter combination is 1.5 g/L CuSO4, oxidation voltage is 500 V and treating time is 10 min;
- The black MAO coatings on the AZ31B magnesium alloy have a typical porous structure and black appearance. The coatings are mainly composed of WO3, MgAl2O4, CuAl2O4, MgO, CuO and MgF2. The black color of the coating is mainly caused by CuO and CuAl2O4 phases;
- Sample 6# has the best corrosion resistance. The corrosion current density of sample 6# is two magnitudes lower than the substrate, and the polarization resistance value is three orders of magnitude higher than the substrate;
- The variation trends of hemispherical emissivity and roughness of the black MAO coating are the same when the composition of the coatings is similar. The process conditions are 1.5 g/L CuSO4, 450 V of oxidation voltage and 20 min of treating time, and the obtained coating possessed the highest hemispherical emissivity of 0.84.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy. 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Kolawole, S.; Wang, J.; Su, X.; Tan, L.; Yang, K. Systems, Properties, Surface Modification and Applications of Biodegradable Magnesium Based Alloys: A Review. Materials 2022, 15, 5031. [Google Scholar] [CrossRef]
- Ren, L.; Fan, L.; Zhou, M.; Guo, Y.; Zhang, Y.; Boehlert, C.; Quan, G. Magnesium application in railway rolling stocks: A new challenge and opportunity for lightweighting. Int. J. Lightweight Mater. Manuf. 2018, 1, 81–88. [Google Scholar] [CrossRef]
- Toulabifard, A.; Rahmati, M.; Raeissi, K.; Hakimizad, A.; Santamaria, M. The Effect of Electrolytic Solution Composition on the Structure, Corrosion, and Wear Resistance of PEO Coatings on AZ31 Magnesium Alloy. Coatings 2020, 10, 937. [Google Scholar] [CrossRef]
- Jia, X.; Song, J.; Zhao, L.; Jiang, B.; Yang, H.; Zhao, T.; Zhao, H.; Liu, Q.; Pan, F. The corrosion behaviour of MAO coated AZ80 magnesium alloy with surface indentation of different sizes. Eng. Fail. Anal. 2022, 136, 106185. [Google Scholar] [CrossRef]
- Chen, Y.; Lua, X.; Lamaka, S.; Ju, P.; Blawert, C.; Zhang, T.; Wang, F.; Zheludkevich, M. Active protection of Mg alloy by composite PEO coating loaded with corrosion inhibitors. Appl. Surf. Sci. 2020, 504, 144462. [Google Scholar] [CrossRef]
- Han, J.; Cheng, Y.; Tu, W.; Zhan, T.; Cheng, Y. The black and white coatings on Ti-6Al-4V alloy or pure titanium by plasma electrolytic oxidation in concentrated silicate electrolyte. Appl. Surf. Sci. 2018, 428, 684–697. [Google Scholar] [CrossRef]
- Yao, W.; Wu, L.; Wang, J.; Jiang, B.; Zhang, D.; Serdechnova, M.; Shulha, T.; Blawert, C.; Zheludkevich, M.; Pan, F. Micro-arc oxidation of magnesium alloys: A review. J. Mater. Sci. Technol. 2022, 118, 158–180. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, L.; Zeng, M.; Zeng, R.; Kannan, M.; Lin, C.; Zheng, Y. Biodegradation behavior of micro-arc oxidation coating on magnesium alloy-from a protein perspective. Bioact. Mater. 2020, 5, 398–409. [Google Scholar] [CrossRef]
- Bai, L.; Kou, G.; Zhao, K.; Chen, G.; Yan, F. Effect of in-situ micro-arc oxidation coating on the galvanic corrosion of AZ31Mg coupled to aluminum alloys. J. Alloys Compd. 2019, 775, 1077–1085. [Google Scholar] [CrossRef]
- Lou, B.; Yen, C.; Chen, Y.; Lee, J. Effects of processing parameters on the adhesion and corrosion resistance of oxide coatings grown by plasma electrolytic oxidation on AZ31 magnesium alloys. J. Mater. Res. Technol. 2021, 10, 1355–1371. [Google Scholar] [CrossRef]
- Xia, Q.; Li, X.; Yao, Z.; Jiang, Z. Investigations on the thermal control properties and corrosion resistance of MAO coatings prepared on Mg-5Y-7Gd-1Nd-0.5Zr alloy. Surf. Coat. Technol. 2021, 409, 126874. [Google Scholar] [CrossRef]
- Xia, Q.; Zhang, D.; Li, D.; Jiang, Z.; Yao, Z. Preparation of the plasma electrolytic oxidation coating on Mg-Li alloy and its thermal control performance. Surf. Coat. Technol. 2019, 369, 252–256. [Google Scholar] [CrossRef]
- Yao, R.; Li, Y.; Yao, Z.; Zhang, P.; Lu, S.; Wu, X. Black PEO coating with enhanced thermal stability on titanium alloy and its thermal control properties. Surf. Coat. Technol. 2022, 429, 127934. [Google Scholar] [CrossRef]
- Ramakrishnan, E.; Premchand, C.; Manojkumar, P.; Rameshbabu, N. Development of thermal control coatings on AA7075 by plasma electrolytic oxidation (PEO) process. Mater. Today Proc. 2021, 46, 1085–1090. [Google Scholar] [CrossRef]
- He, R.; Wang, B.; Xiang, J.; Pan, T. Effect of copper additive on microstructure and anti-corrosion performance of black MAO films grown on AZ91 alloy and coloration mechanism. J. Alloys Compd. 2021, 889, 161501. [Google Scholar] [CrossRef]
- Lu, S.; Qin, W.; Wu, X.; Wang, X.; Zhao, G. Effect of Fe3+ ions on the thermal and optical properties of the ceramic coating grown in-situ on AZ31 Mg Alloy. Mater. Chem. Phys. 2012, 135, 58–62. [Google Scholar] [CrossRef]
- Lee, S.; Do, L. Effects of copper additive on micro-arc oxidation coating of LZ91 magnesium-lithium alloy. Surf. Coat. Technol. 2016, 307, 781–789. [Google Scholar] [CrossRef]
- Baia, L.; Dong, B.; Chen, G.; Xin, T.; Wu, J.; Sun, X. Effect of positive pulse voltage on color value and corrosion property of magnesium alloy black micro-arc oxidation ceramic coating. Surf. Coat. Technol. 2019, 374, 402–408. [Google Scholar] [CrossRef]
- Tu, W.; Zhu, Z.; Zhuang, X.; Cheng, Y.; Skeldon, P. Effect of frequency on black coating formation on AZ31 magnesium alloy by plasma electrolytic oxidation in aluminate-tungstate electrolyte. Surf. Coat. Technol. 2019, 372, 34–44. [Google Scholar] [CrossRef]
- Wang, X.; Ju, P.; Lu, X.; Yan Chen, Y.; Wang, F. Influence of Cr2O3 particles on corrosion, mechanical and thermal control properties of green PEO coatings on Mg alloy. Ceram. Int. 2022, 48, 3615–3627. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Ju, P.; Chen, Y.; Zhang, T.; Wang, F. Influence of ZnO on thermal control property and corrosion resistance of plasma electrolytic oxidation coatings on Mg alloy. Surf. Coat. Technol. 2021, 409, 126905. [Google Scholar] [CrossRef]
- Hadzima, B.; Kajánek, D.; Jambor, M.; Drábiková, J.; Brezina, M.; Buhagiar, J.; Pastorková, J.; Jacková, M. PEO of AZ31 Mg Alloy: Effect of Electrolyte Phosphate Content and Current Density. Metals 2020, 10, 1521. [Google Scholar] [CrossRef]
- Wu, G.; Zhao, D.; Lin, X.; Liu, J.; Ji, X. Investigation of an environmentally friendly coloring coating for magnesium-lithium alloy micro-arc oxidation. Surf. Interfaces 2020, 20, 100513. [Google Scholar] [CrossRef]
- Hariprasad, S.; Gowtham, S.; Arun, S.; Ashok, M.; Rameshbabu, N. Fabrication of duplex coatings on biodegradable AZ31 magnesium alloy by integrating cerium conversion (CC) and plasma electrolytic oxidation (PEO) processes. J. Alloys Compd. 2017, 722, 698–715. [Google Scholar] [CrossRef]
- Weng, W.; Wu, W.; Yu, X.; Sun, M.; Lin, Z.; Ibrahim, M.; Yang, H. Effect of Gel MA Hydrogel Coatings on Corrosion Resistance and Biocompatibility of MAO-Coated Mg Alloys. Materials 2020, 13, 3834. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Li, H.; Li, Z.; Chen, Y.; Wang, Y.; Geng, J. Effect of (NH4)2ZrF6, Voltage and Treating Time on Corrosion Resistance of Micro-Arc Oxidation Coatings Applied on ZK61M Magnesium Alloys. Materials 2021, 14, 7410. [Google Scholar] [CrossRef]
- An, L.; Ma, Y.; Liu, Y.; Sun, L.; Wang, S.; Wang, Z. Effects of additives, voltage and their interactions on PEO coatings formed on magnesium alloys. Surf. Coat. Technol. 2018, 354, 226–235. [Google Scholar] [CrossRef]
- Park, J.; Seo, E.; Lee, M.; Kim, D. Fabrication of a CuO composite PEO and effect of post-treatment on improving its thermal properties and corrosion resistance of magnesium alloy AZ31. Surf. Coat. Technol. 2022, 447, 128828. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wang, J.; Xia, X.; Lv, Y.; Cai, G.; Liu, H.; Xiao, J.; Liu, B.; Dong, Z. Microstructure, formation mechanism and antifouling property of multilayered Cu-incorporated Al2O3 coating fabricated through plasma electrolytic oxidation. Ceram. Int. 2020, 46, 2901–2909. [Google Scholar] [CrossRef]
- Ponmudi, S.; Sivakumar, R.; Sanjeeviraja, C.; Gopalakrishnan, C.; Jeyadheepan, K. Facile fabrication of spinel structured n-type CuAl2O4 thin film with nanograss like morphology by sputtering technique. Appl. Surf. Sci. 2019, 483, 601–615. [Google Scholar] [CrossRef]
- Das, S.; Rahman, M.; Mondal, P.; Mahapatra, P.; Saha, D. Screen-printed MgAl2O4 semi-thick film based highly sensitive and stable capacitive humidity sensor. Ceram. Int. 2021, 47, 33515–33524. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Chen, X.; Tong, G.; Zhang, L. Microstructure and corrosion behavior of PEO-LDHs-SDS superhydrophobic composite film on magnesium alloy. Corros. Sci. 2022, 208, 110699. [Google Scholar] [CrossRef]
- Dohcevic-Mitrovic, Z.; Stojadinovic, S.; Lozzi, L.; Aškrabic, S.; Rosic, M.; Tomic, N.; Paunovic, N.; Lazovic, S.; Nikolic, M.; Santucci, S. WO3/TiO2 composite coatings: Structural, optical and photocatalytic properties. Mater. Res. Bull. 2016, 83, 217–224. [Google Scholar] [CrossRef]
- Zehra, T.; Patil, S.; Shrestha, N.; Fattah-alhosseini, A.; Kaseema, M. Anionic assisted incorporation of WO3 nanoparticles for enhanced electrochemical properties of AZ31 Mg alloy coated via plasma electrolytic oxidation. J. Alloys Compd. 2022, 916, 165445. [Google Scholar] [CrossRef]
- Li, H.; Lu, S.; Qin, W.; Wu, X. In-situ grown MgO-ZnO ceramic coating with high thermal emittance on Mg alloy by plasma electrolytic oxidation. Acta Astronaut. 2017, 136, 230–235. [Google Scholar] [CrossRef]
- Qin, Y.; Wu, G.; Atrens, A.; Zhang, X.; Zhang, L.; Ding, W. Effect of NaOH concentration on microstructure and corrosion resistance of MAO coating on cast Al−Li alloy. Nonferrous Met. Soc. China 2021, 31, 913–924. [Google Scholar] [CrossRef]
- Wu, T.; Blawert, C.; Serdechnova, M.; Karlova, P.; Dovzhenko, G.; Wieland, D.; Zheludkevich, M. PEO processing of AZ91Nd/Al2O3 MMC-the role of alumina fibers. J. Magnes. Alloy. 2022, 10, 423–439. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, B.; Liu, Y.; Cheng, Y. Plasma electrolytic oxidation of copper in an aluminate based electrolyte with the respective additives of Na3PO4, NaH2PO4 and NaH2PO2. Appl. Surf. Sci. 2021, 565, 150477. [Google Scholar] [CrossRef]







| Factors | A | B | C |
|---|---|---|---|
| Levels | CuSO4 Concentration (g/L) | Voltage (V) | Treating Time (min) |
| 1 | 1 | 400 | 10 |
| 2 | 1.5 | 450 | 15 |
| 3 | 2 | 500 | 20 |
| Experimental | A | B | C | Icorr (mA/cm2) | The Hemisphere Emissivity εH | Porosity (%) |
|---|---|---|---|---|---|---|
| 1# | 1 | 400 | 10 | 0.019 | 0.72 | 6.27 |
| 2# | 1 | 450 | 15 | 0.009 | 0.80 | 6.90 |
| 3# | 1 | 500 | 20 | 0.011 | 0.83 | 7.19 |
| 4# | 1.5 | 400 | 15 | 0.006 | 0.78 | 5.96 |
| 5# | 1.5 | 450 | 20 | 0.029 | 0.84 | 6.79 |
| 6# | 1.5 | 500 | 10 | 0.001 | 0.79 | 6.59 |
| 7# | 2 | 400 | 20 | 0.006 | 0.78 | 5.28 |
| 8# | 2 | 450 | 10 | 0.035 | 0.78 | 7.24 |
| 9# | 2 | 500 | 15 | 0.002 | 0.83 | 7.58 |
| k1 | 0.013 (0.783) (6.787) | 0.010 (0.760) (5.837) | 0.018 (0.763) (6.700) | |||
| k2 | 0.012 (0.803) (6.447) | 0.024 (0.807) (6.977) | 0.006 (0.803) (6.813) | |||
| k3 | 0.014 (0.797) (6.700) | 0.005 (0.817) (7.120) | 0.015 (0.817) (6.420) | |||
| R | 0.002 (0.020) (0.340) | 0.019 (0.057) (1.283) | 0.012 (0.054) (0.393) |
| Coating | Mg/at% | Al/at% | O/at% | Cu/at% | F/at% | W/at% |
|---|---|---|---|---|---|---|
| 1# | 14.00 | 30.43 | 46.99 | 4.16 | 2.84 | 1.58 |
| 2# | 16.98 | 28.51 | 45.17 | 4.43 | 2.78 | 2.11 |
| 3# | 21.65 | 24.55 | 44.72 | 4.04 | 2.44 | 2.60 |
| 4# | 27.39 | 25.21 | 39.98 | 3.40 | 2.21 | 1.81 |
| 5# | 24.23 | 24.34 | 40.44 | 6.88 | 1.03 | 3.09 |
| 6# | 22.52 | 26.44 | 42.64 | 3.89 | 1.66 | 2.85 |
| 7# | 25.81 | 22.65 | 44.23 | 2.49 | 2.35 | 2.47 |
| 8# | 19.97 | 25.38 | 45.67 | 4.36 | 1.60 | 3.01 |
| 9# | 20.52 | 25.96 | 42.73 | 6.27 | 1.78 | 2.74 |
| Coating | Ecorr (V) | Icorr (A/cm2) | Βa (mV/dec) | −βc (mV/dec) | Rp (kΩ·cm2) |
|---|---|---|---|---|---|
| 1# | −1.377 | 1.910 × 10−5 | 99.08 | 230.75 | 1.528 × 103 |
| 2# | −1.377 | 8.973 × 10−6 | 73.07 | 276.68 | 2.798 × 103 |
| 3# | −1.362 | 1.052 × 10−5 | 99.53 | 314.82 | 3.118 × 103 |
| 4# | −1.387 | 6.109 × 10−6 | 53.93 | 158.72 | 2.861 × 103 |
| 5# | −1.487 | 2.894 × 10−5 | 57.46 | 208.19 | 6.754 × 102 |
| 6# | −1.371 | 1.536 × 10−6 | 101.43 | 203.87 | 1.915 × 104 |
| 7# | −1.419 | 6.394 × 10−6 | 44.63 | 219.91 | 2.519 × 103 |
| 8# | −1.422 | 3.492 × 10−5 | 43.36 | 218.22 | 4.497 × 103 |
| 9# | −1.424 | 2.595 × 10−6 | 88.08 | 144.4 | 9.151 × 103 |
| AZ31 | −1.522 | 2.582 × 10−4 | 72.22 | 187.41 | 8.767 × 101 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Wang, Y.; Geng, J.; Li, S.; Chen, Y. Study on Microstructure and Properties of Black Micro-Arc Oxidation Coating on AZ31 Magnesium Alloy by Orthogonal Experiment. Materials 2022, 15, 8755. https://doi.org/10.3390/ma15248755
Li H, Wang Y, Geng J, Li S, Chen Y. Study on Microstructure and Properties of Black Micro-Arc Oxidation Coating on AZ31 Magnesium Alloy by Orthogonal Experiment. Materials. 2022; 15(24):8755. https://doi.org/10.3390/ma15248755
Chicago/Turabian StyleLi, Hongzhan, Yifei Wang, Juanjuan Geng, Shaolong Li, and Yongnan Chen. 2022. "Study on Microstructure and Properties of Black Micro-Arc Oxidation Coating on AZ31 Magnesium Alloy by Orthogonal Experiment" Materials 15, no. 24: 8755. https://doi.org/10.3390/ma15248755




