Effective Method for the Determination of the Unit Cell Parameters of New MXenes
Abstract
1. Introduction
2. Materials and Methods
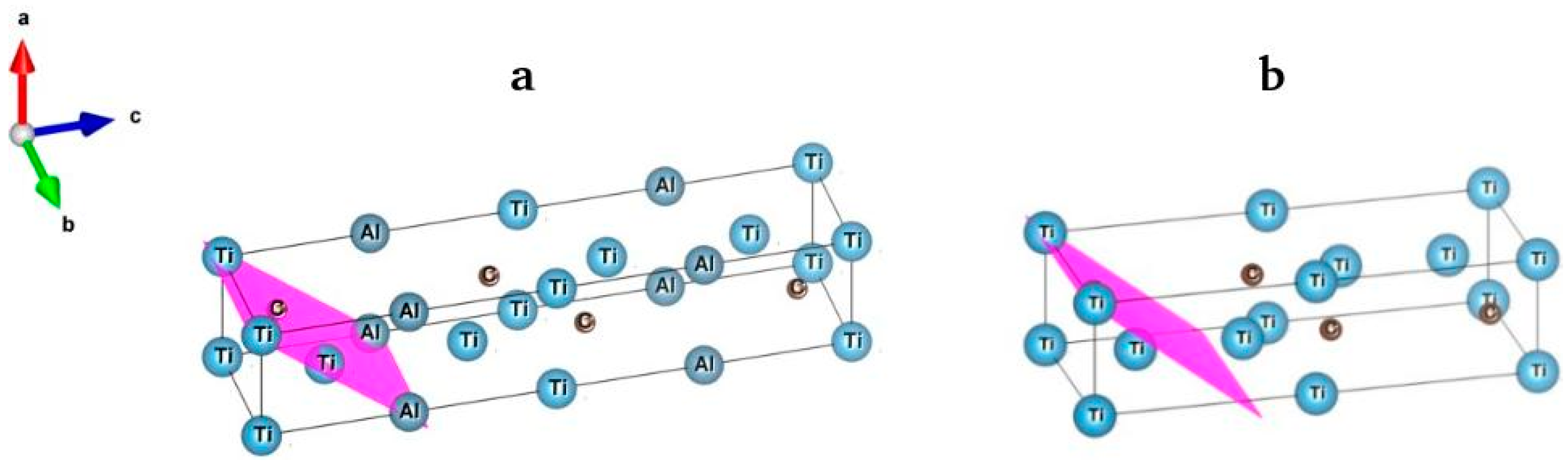
3. Results
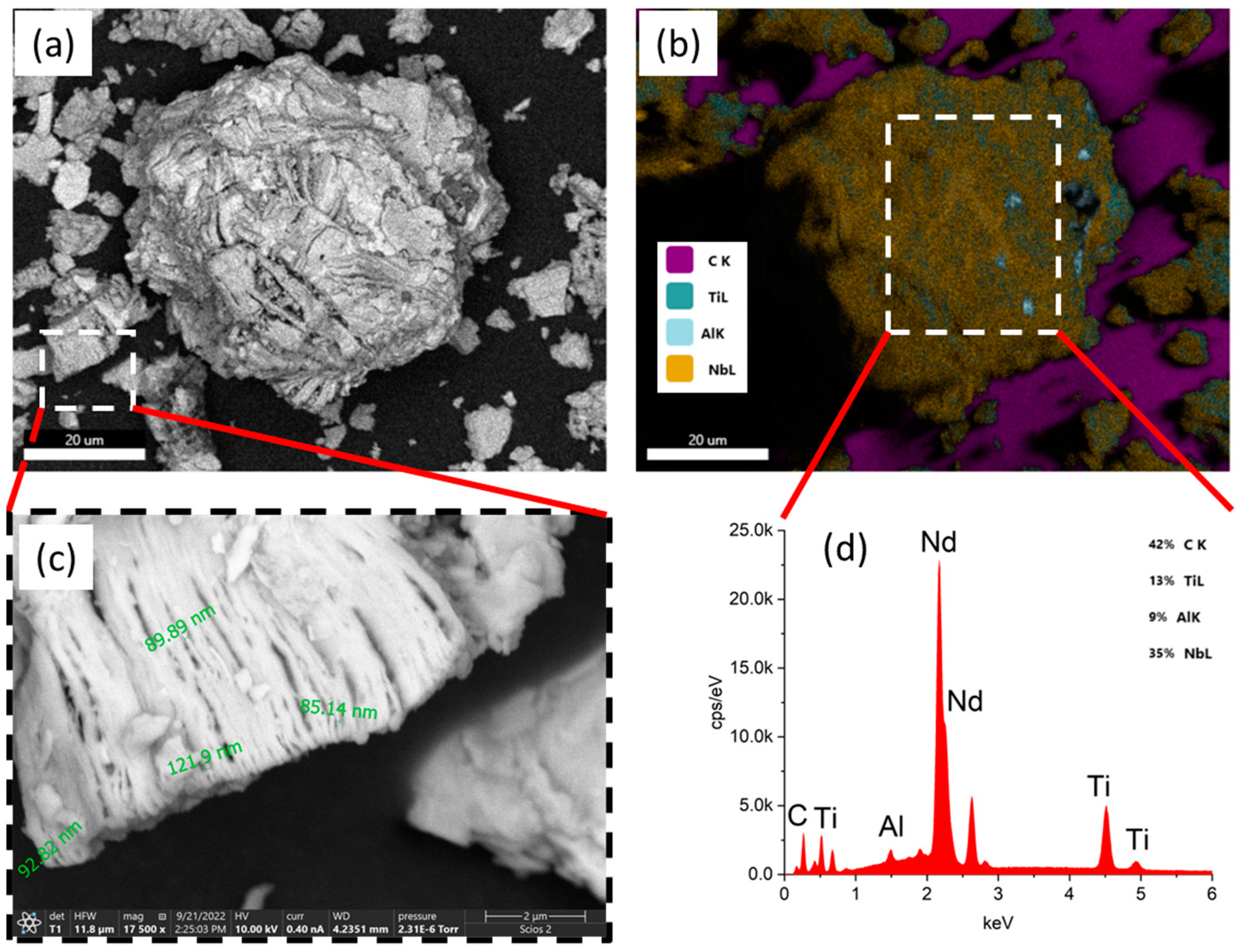
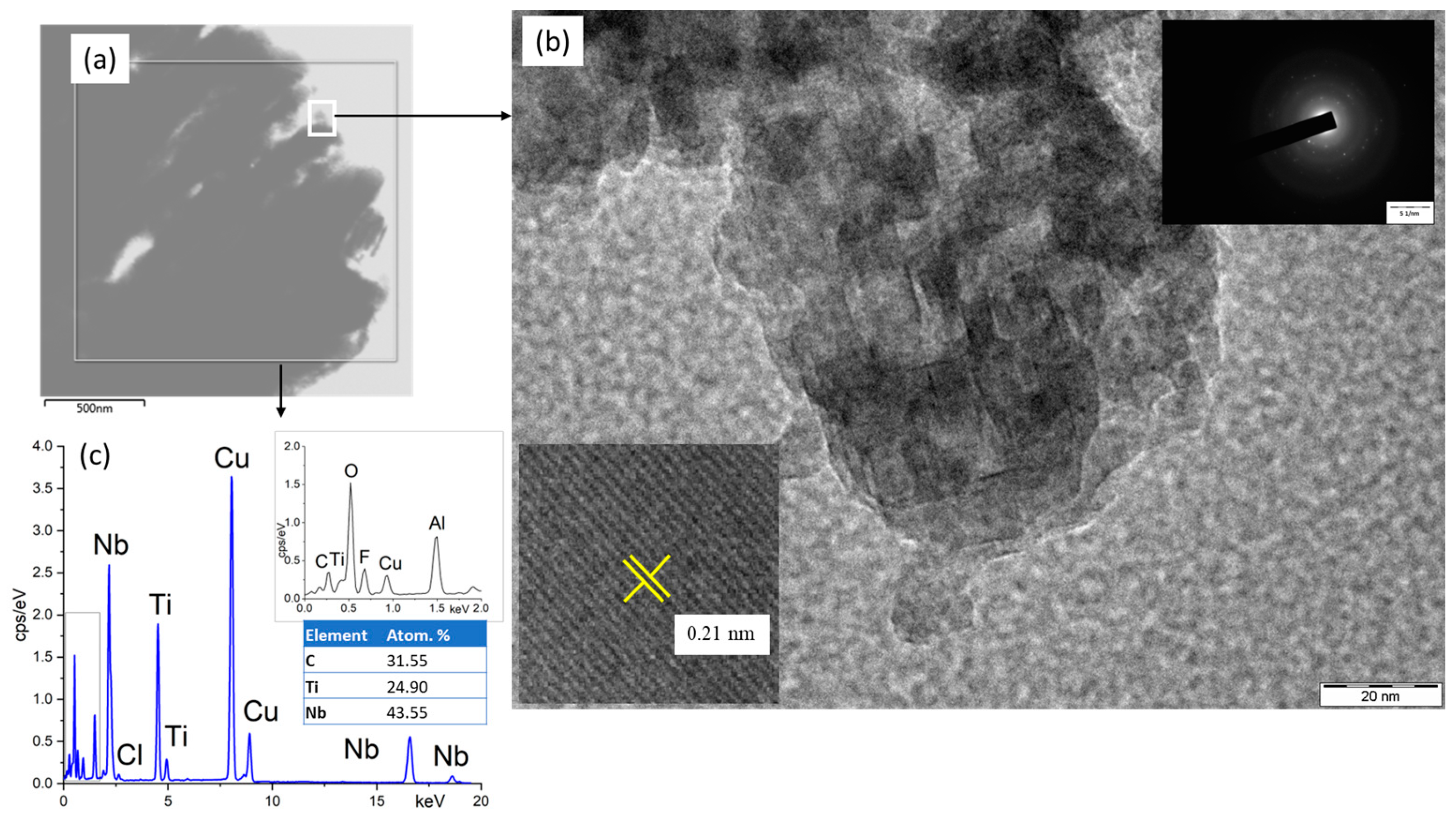
3.1. SEM and TEM Characterizations
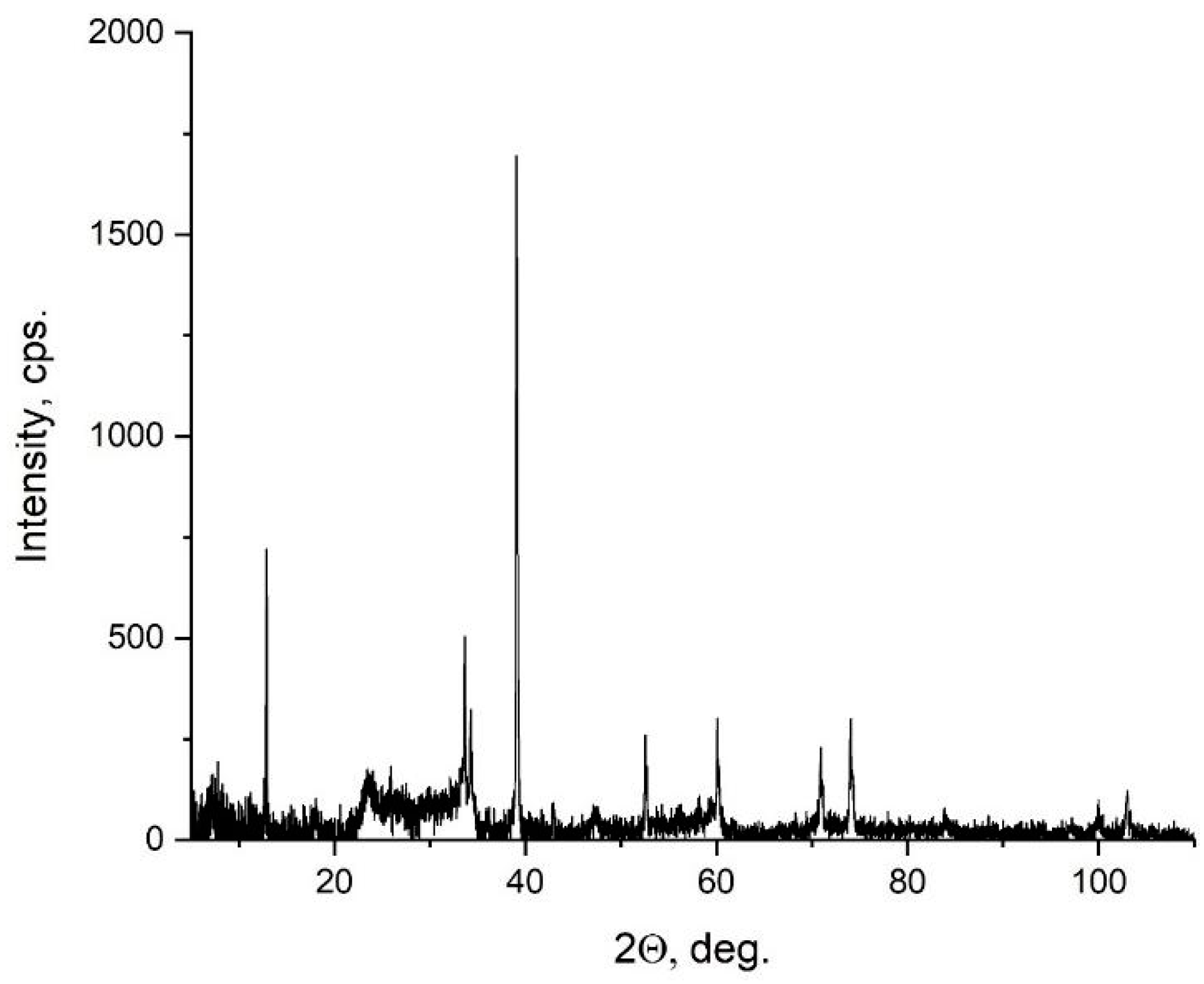
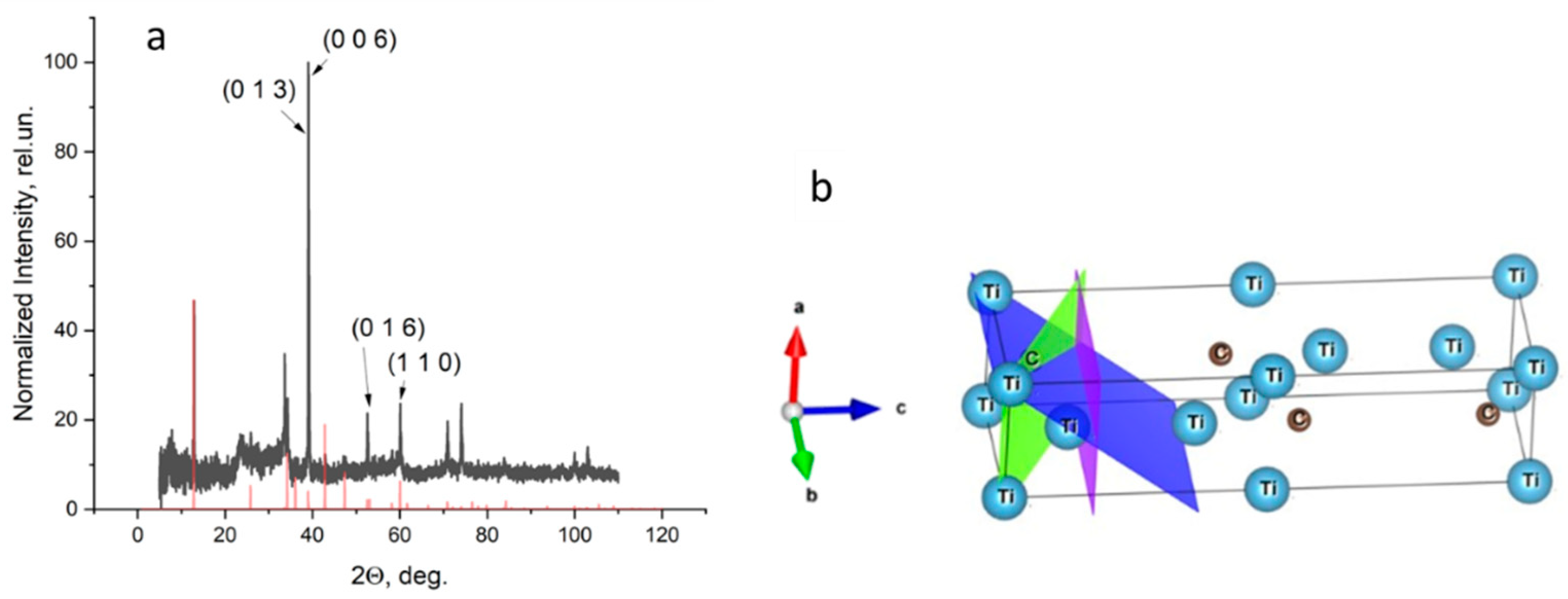
3.2. X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Anasori, B. The rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef] [PubMed]
- Vahid Mohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, eabf1581. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Huang, Q. MXenes: Two-dimensional building blocks for future materials and devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shah, D.; Boltasseva, A.; Gogotsi, Y. MXenes for photonics. ACS Photonics 2022, 9, 1108–1116. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Baek, D.S.; Joo, S.H.; Lee, K. MXene: An emerging two-dimensional material for future energy conversion and storage applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Esfarjani, K.; Bogdanovski, D.; Dronskowski, R.; Yunoki, S. Insights into exfoliation possibility of MAX phases to MXenes. Phys. Chem. Chem. Phys. 2018, 20, 8579–8592. [Google Scholar] [CrossRef]
- Murali, G.; Modigunta, J.K.R.; Park, Y.H.; Lee, J.-H.; Rawal, J.; Lee, S.-Y.; In, I.; Park, S.-J. A review on MXene synthesis, stability, and photocatalytic applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Leong, Z.; Jin, H.; Wong, Z.M.; Nemani, K.; Anasori, B.; Tan, T.L. Elucidating the chemical order and disorder in high-entropy MXenes: A high-throughput survey of the atomic configurations in TiVNbMoC3 and TiVCrMoC3. Chem. Mater. 2022, 34, 9062–9071. [Google Scholar] [CrossRef]
- Zhang, T.; Matthews, K.; Vahid Mohammadi, A.; Han, M.; Gogotsi, Y. Pseudocapacitance of vanadium carbide MXenes in basic and acidic aqueous electrolytes. ACS Energy Lett. 2022, 7, 3864–3870. [Google Scholar] [CrossRef]
- Sokol, M.; Natu, V.; Kota, S.; Barsoum, M.W. Chemical diversity of the MAX phases. Trends Chem. 2019, 1, 210–223. [Google Scholar] [CrossRef]
- Gutiérrez-Ojeda, S.J.; Ponce-Pérez, R.; Maldonado-Lopez, D.; Hoat, D.M.; Guerrero-Sánchez, J.; Moreno-Armenta, M.G. Strain effects on the two-dimensional Cr2N MXene: An ab initio study. ACS Omega 2022, 7, 33884–33894. [Google Scholar] [CrossRef]
- Shekhirev, M.; Busa, J.; Shuck, C.E.; Torres, A.; Bagheri, S.; Sinitskii, A.; Gogotsi, Y. Ultralarge flakes of Ti3C2Tx MXene via soft delamination. ACS Nano 2022, 16, 13695–13703. [Google Scholar] [CrossRef] [PubMed]
- Asad, S.; Zahoor, A.; Butt, F.A.; Hashmi, S.; Raza, F.; Ahad, I.U.; Hakami, J.; Ullah, S.; Al-Ahmed, A.; Khan, F.; et al. Recent advances in titanium carbide MXene (Ti3C2Tx) cathode material for lithium–air battery. ACS Appl. Energy Mater. 2022, 5, 11933–11946. [Google Scholar] [CrossRef]
- Meng, F.L.; Zhou, Y.C.; Wang, J.Y. Strengthening of Ti2AlC by substituting Ti with V. Scr. Mater. 2005, 53, 1369–1372. [Google Scholar] [CrossRef]
- Tian, W.; Sun, Z.; Hashimoto, H.; Du, Y. Synthesis, microstructure and properties of (Cr1−xVx)2AlC solid solutions. J. Alloys Compd. 2009, 484, 130–133. [Google Scholar] [CrossRef]
- Finkel, P.; Seaman, B.; Harrell, K.; Palma, J.; Hettinger, J.D.; Lofland, S.E.; Ganguly, A.; Barsoum, M.W.; Sun, Z.; Li, S.; et al. Electronic, thermal, and elastic properties of Ti3Si1−xGexC2 solid solutions. Phys. Rev. B 2004, 70, 085104. [Google Scholar] [CrossRef]
- Barsoum, M.W.; Salama, I.; El-Raghy, T.; Golczewski, J.; Seifert, H.J.; Aldinger, F.; Porter, W.D.; Wang, H. Thermal and electrical properties of Nb2AlC, (Ti, Nb)2AlC and Ti2AlC. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2002, 33, 2775–2779. [Google Scholar] [CrossRef]
- Zhang, Z.; Duan, X.; Jia, D.; Zhou, Y.; van der Zwaag, S. On the formation mechanisms and properties of MAX phases: A review. J. Eur. Ceram. Soc. 2021, 41, 3851–3878. [Google Scholar] [CrossRef]
- Li, Y.-M.; Chen, W.-G.; Guo, Y.-L.; Jiao, Z.-Y. Theoretical investigations of TiNbC MXenes as anode materials for Li-ion batteries. J. Alloys Compd. 2019, 778, 53–60. [Google Scholar] [CrossRef]
- Zhang, L.; Su, W.; Huang, Y.; Li, H.; Fu, L.; Song, K.; Huang, X.; Yu, J.; Lin, C.-T. In situ high-pressure X-ray diffraction and Raman spectroscopy study of Ti3C2Tx MXene. Nanoscale Res. Lett. 2018, 13, 343. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [PubMed]
- Gonze, X.; Amadon, B.; Anglade, P.M.; Beuken, J.-M.; Bottin, F.; Boulanger, P.; Bruneval, F.; Caliste, D.; Caracas, R.; Cote, M.; et al. ABINIT: First-principles approach to material and nanosystem properties. Comput. Phys. Commun. 2009, 180, 2582–2615. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Williams, T.; Kelley, C.; Merritt, E.A.; Bersch, C.; Bröker, H.-B.; Campbell, J.; Cunningham, R.; Denholm, D.; Elber, G.; Fearick, R.; et al. Gnuplot 5.2: An Interactive Plotting Program. 2018. Available online: http://www.gnuplot.info (accessed on 6 December 2022).
- Vaitkus, A.; Merkys, A.; Gražulis, S. Validation of the crystallography open database using the crystallographic information framework. J. Appl. Cryst. 2021, 54, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Quirós, M.; Gražulis, S.; Girdzijauskaitė, S.; Merkys, A.; Vaitkus, A. Using SMILES strings for the description of chemical connectivity in the Crystallography Open Database. J. Cheminform. 2018, 10, 23. [Google Scholar] [CrossRef]
- Merkys, A.; Vaitkus, A.; Butkus, J.; Okulič-Kazarinas, M.; Kairys, V.; Gražulis, S. COD::CIF::Parser: An error-correcting CIF parser for the Perl language. J. Appl. Cryst. 2016, 49, 292–301. [Google Scholar] [CrossRef]
- Gražulis, S.; Merkys, A.; Vaitkus, A.; Okulič-Kazarinas, M. Computing stoichiometric molecular composition from crystal structures. J. Appl. Cryst. 2015, 48, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An open-access collection of crystal structures. J. Appl. Cryst. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
| Energy, eV | Symmetry | Ti Atom Positions According to Figure S5 |
|---|---|---|
| 0.00 | P63/mmc (#194) | 1–2 |
| 2.56 | P3m1 (#156) | 1–5, 1–6, 2–3, 2–4 |
| 3.20 | P3m1 (#156) | 1–3, 1–4, 2–5, 2–6 |
| 5.12 | P-6m2 (#187) | 3–6, 4–5 |
| 5.13 | P63mc (#186) | 3–5, 4–6 |
| 5.22 | P-3m1 (#164) | 3–4, 5–6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syuy, A.; Shtarev, D.; Lembikov, A.; Gurin, M.; Kevorkyants, R.; Tselikov, G.; Arsenin, A.; Volkov, V. Effective Method for the Determination of the Unit Cell Parameters of New MXenes. Materials 2022, 15, 8798. https://doi.org/10.3390/ma15248798
Syuy A, Shtarev D, Lembikov A, Gurin M, Kevorkyants R, Tselikov G, Arsenin A, Volkov V. Effective Method for the Determination of the Unit Cell Parameters of New MXenes. Materials. 2022; 15(24):8798. https://doi.org/10.3390/ma15248798
Chicago/Turabian StyleSyuy, Alexander, Dmitry Shtarev, Alexey Lembikov, Mikhail Gurin, Ruslan Kevorkyants, Gleb Tselikov, Aleksey Arsenin, and Valentyn Volkov. 2022. "Effective Method for the Determination of the Unit Cell Parameters of New MXenes" Materials 15, no. 24: 8798. https://doi.org/10.3390/ma15248798
APA StyleSyuy, A., Shtarev, D., Lembikov, A., Gurin, M., Kevorkyants, R., Tselikov, G., Arsenin, A., & Volkov, V. (2022). Effective Method for the Determination of the Unit Cell Parameters of New MXenes. Materials, 15(24), 8798. https://doi.org/10.3390/ma15248798








