Use of Magnesium Silicate Contaminated with Organic Compounds in Ceramic Materials as a Pore Modifier
Abstract
:1. Introduction
2. Materials and Methods
3. Results
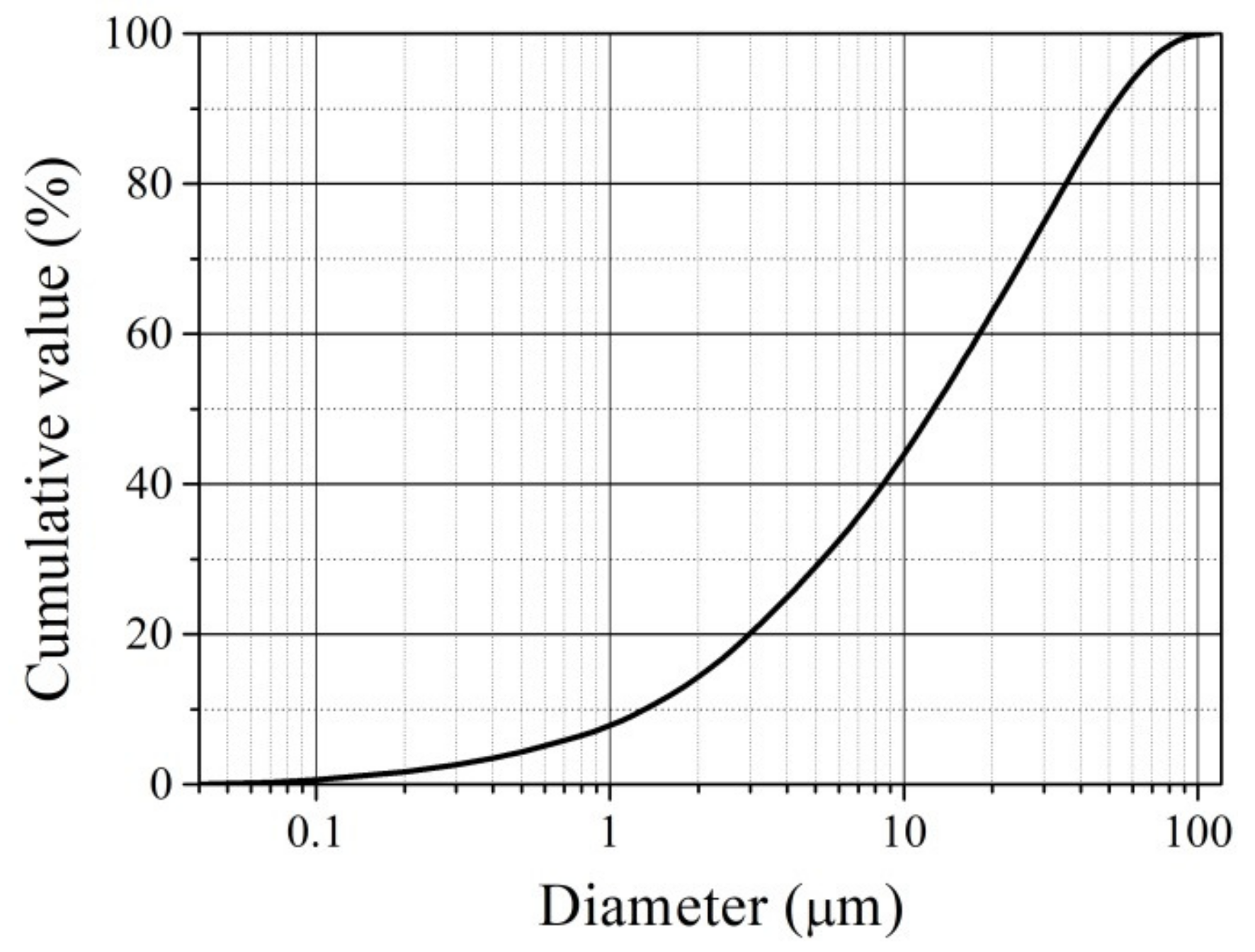
3.1. Characterisation of Used Materials
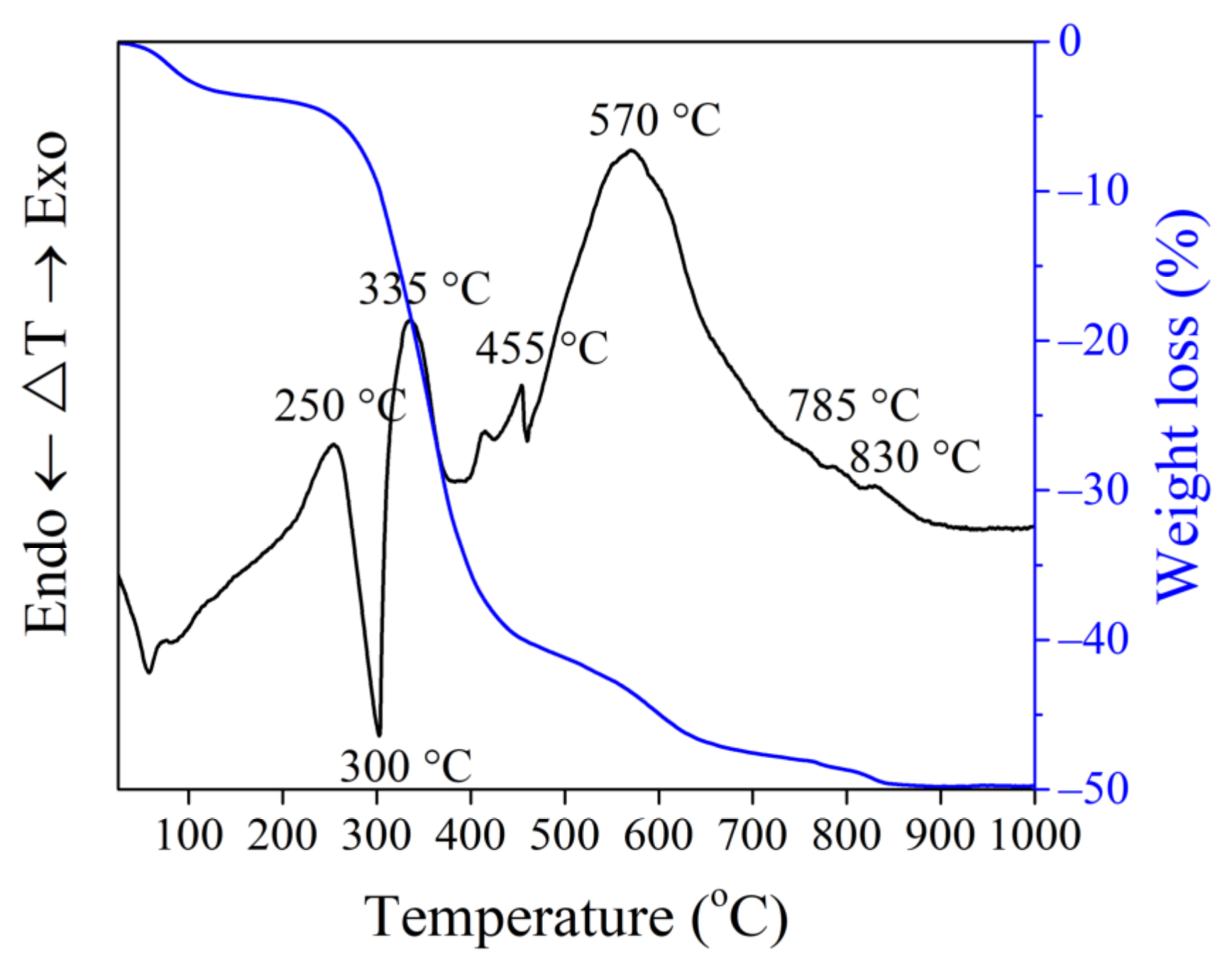
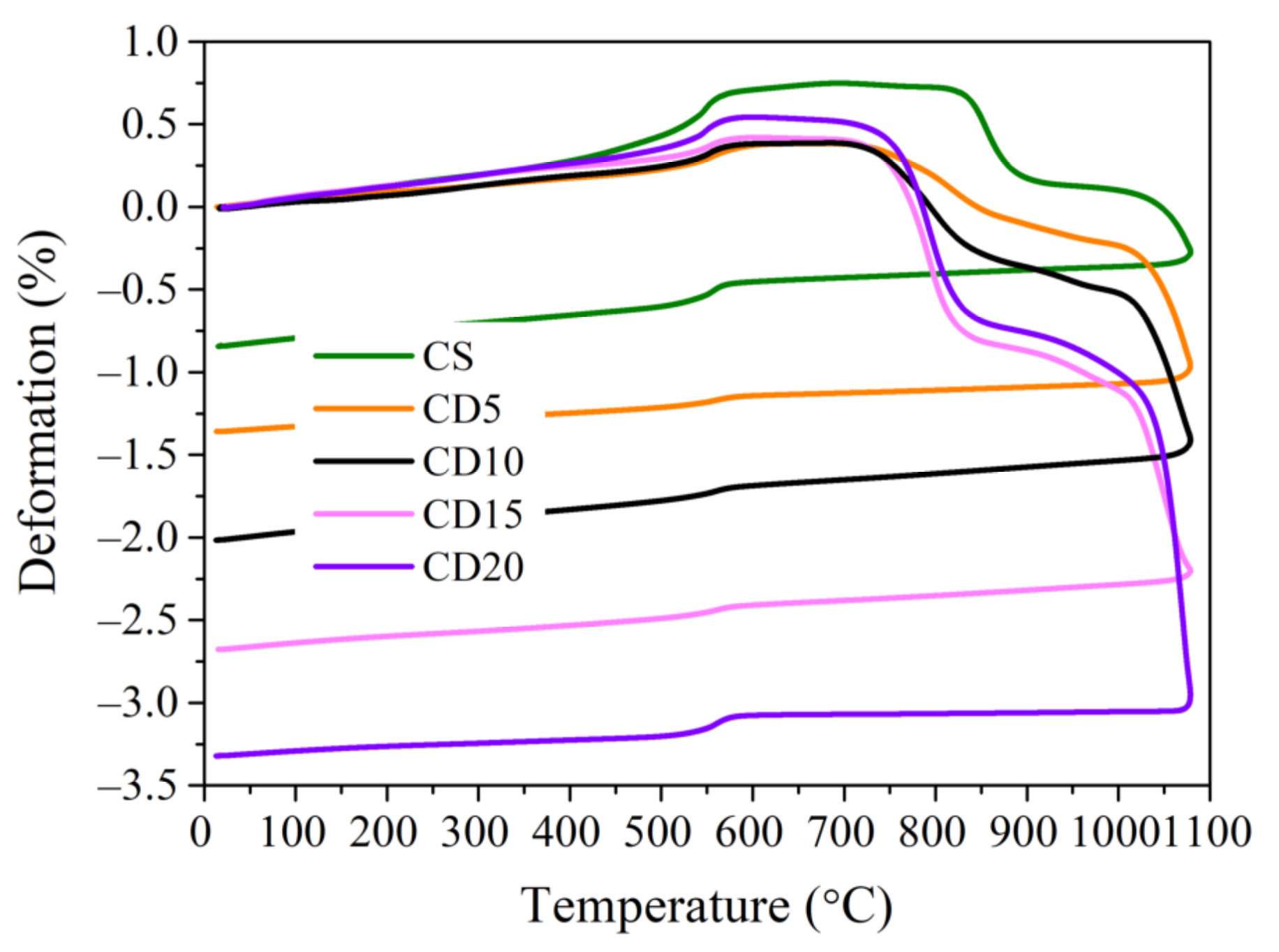
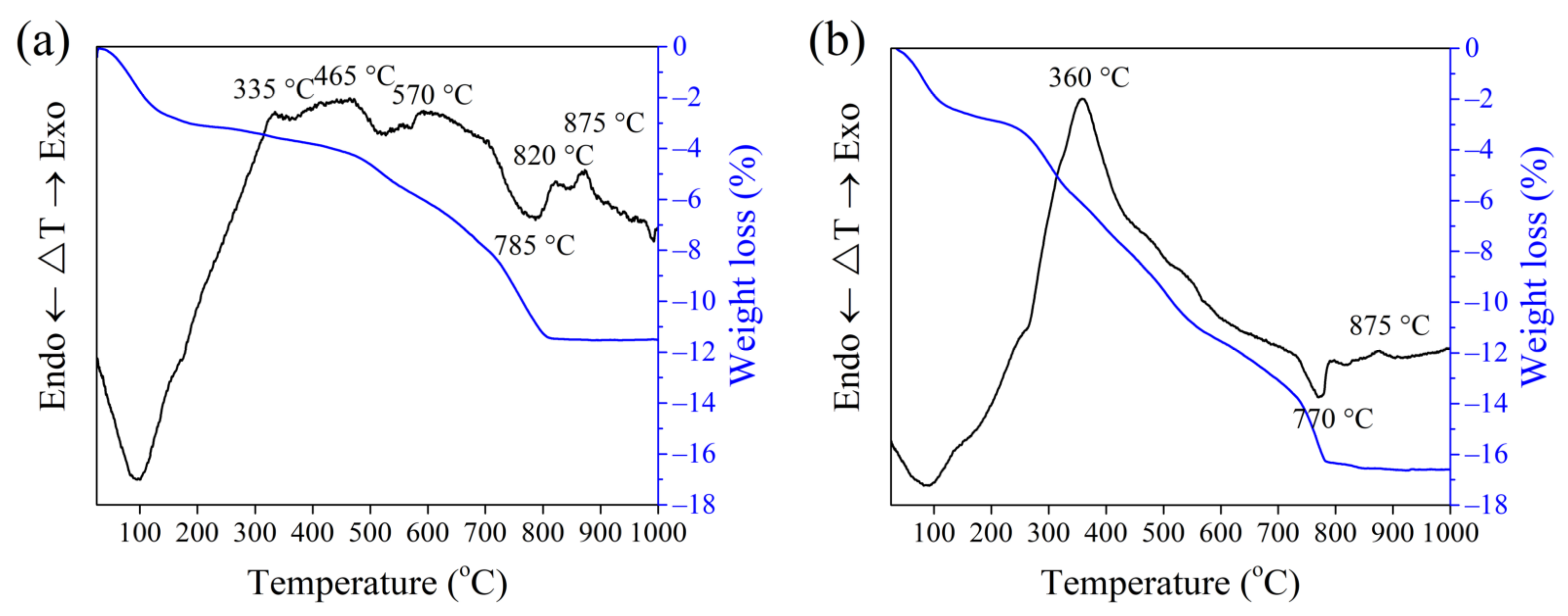
3.2. Thermal Analysis
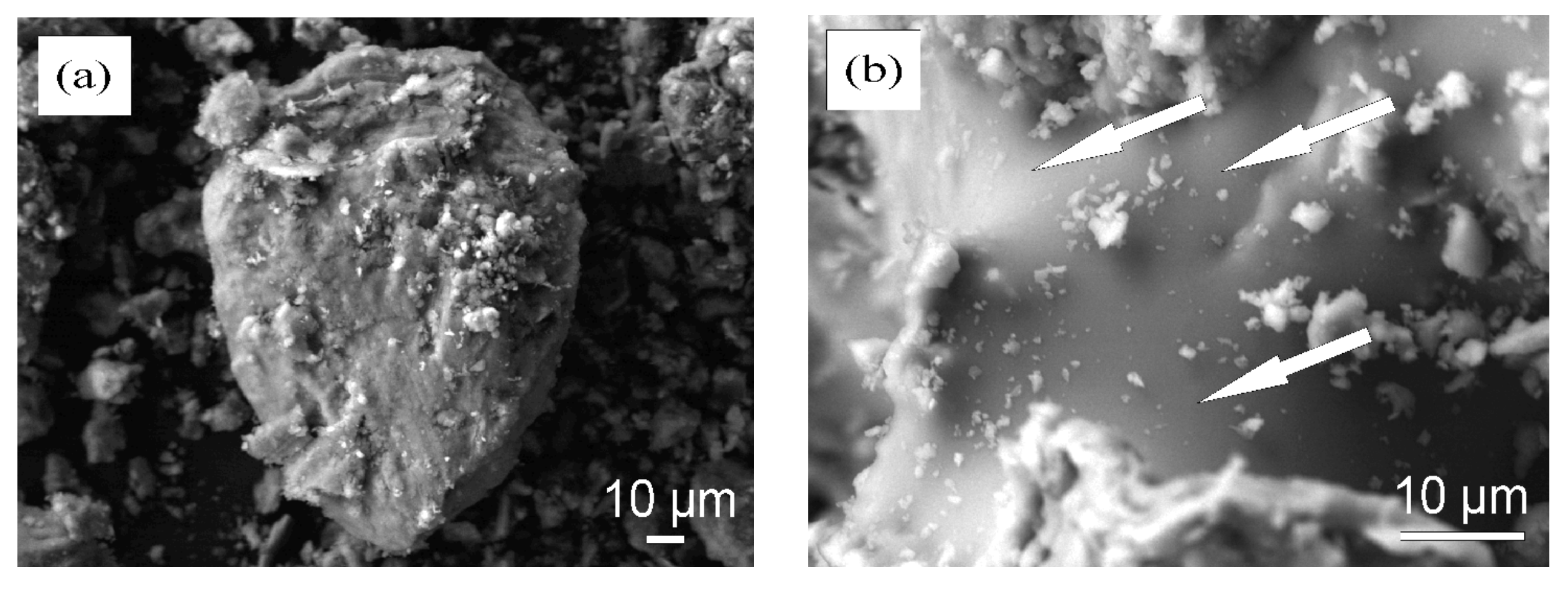
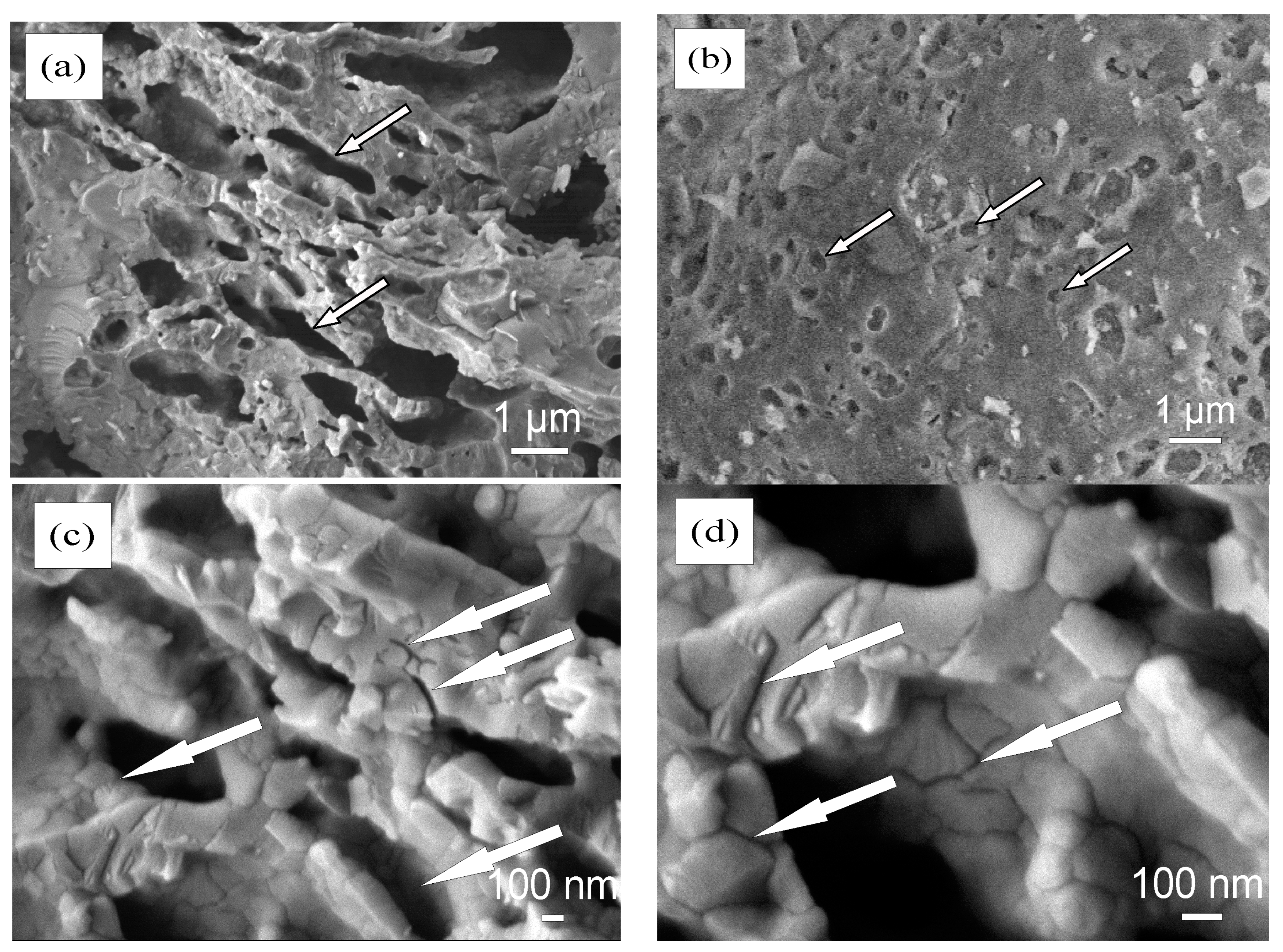
3.3. Microstructure
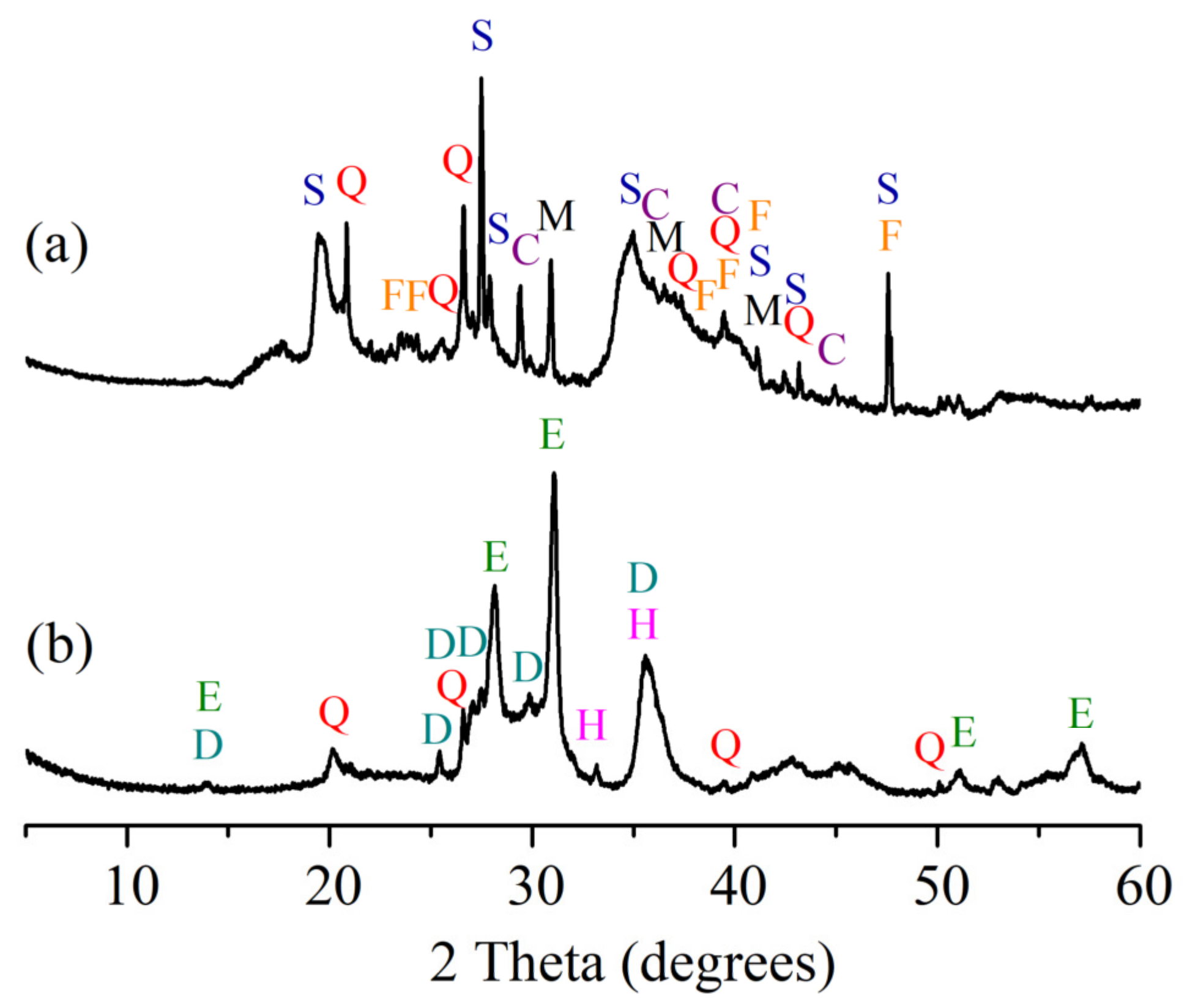
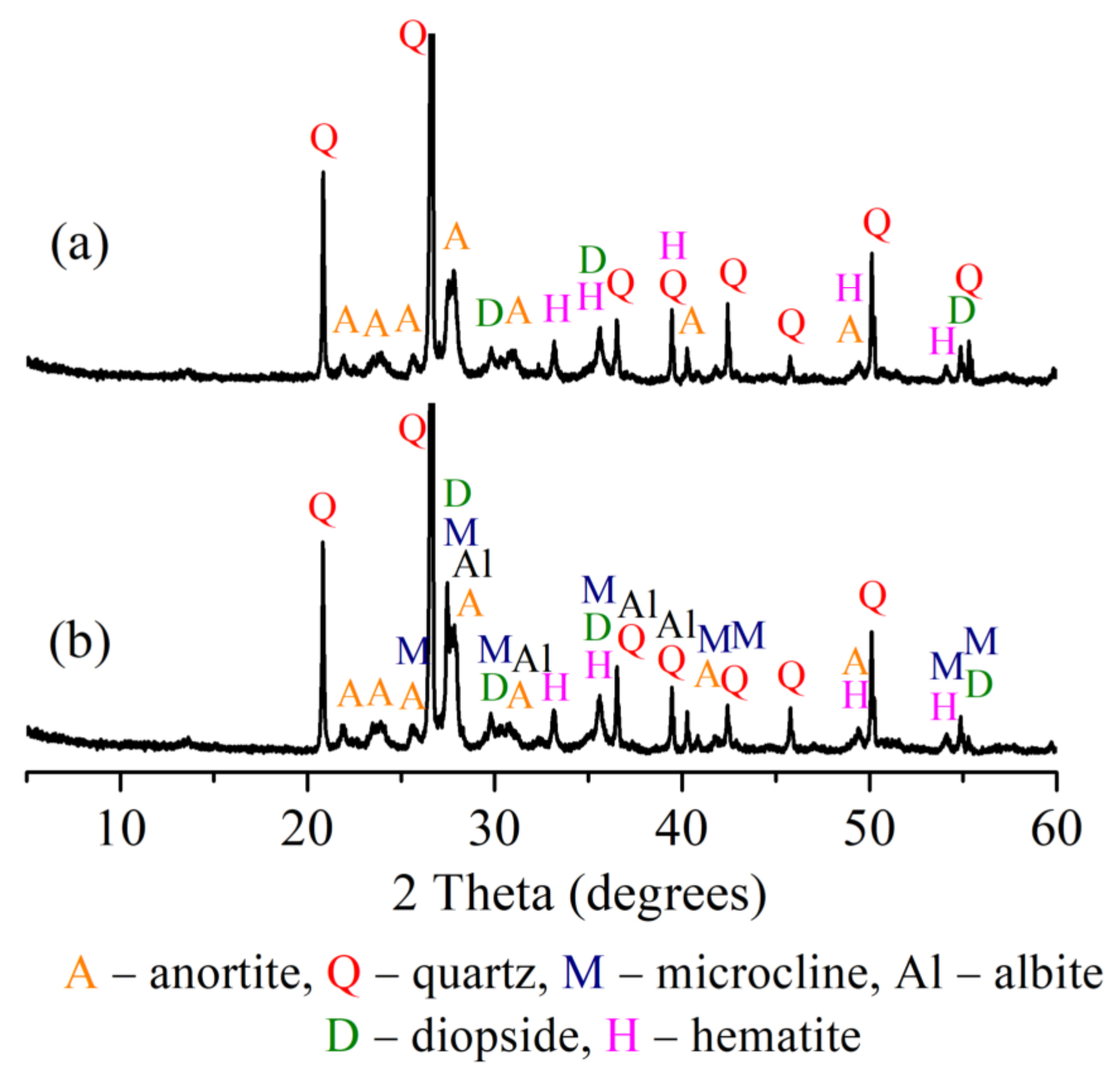
3.4. XRD Analysis
3.5. Water Absorption, Apparent Porosity, and Calculated Structural Parameters of Ceramic Samples
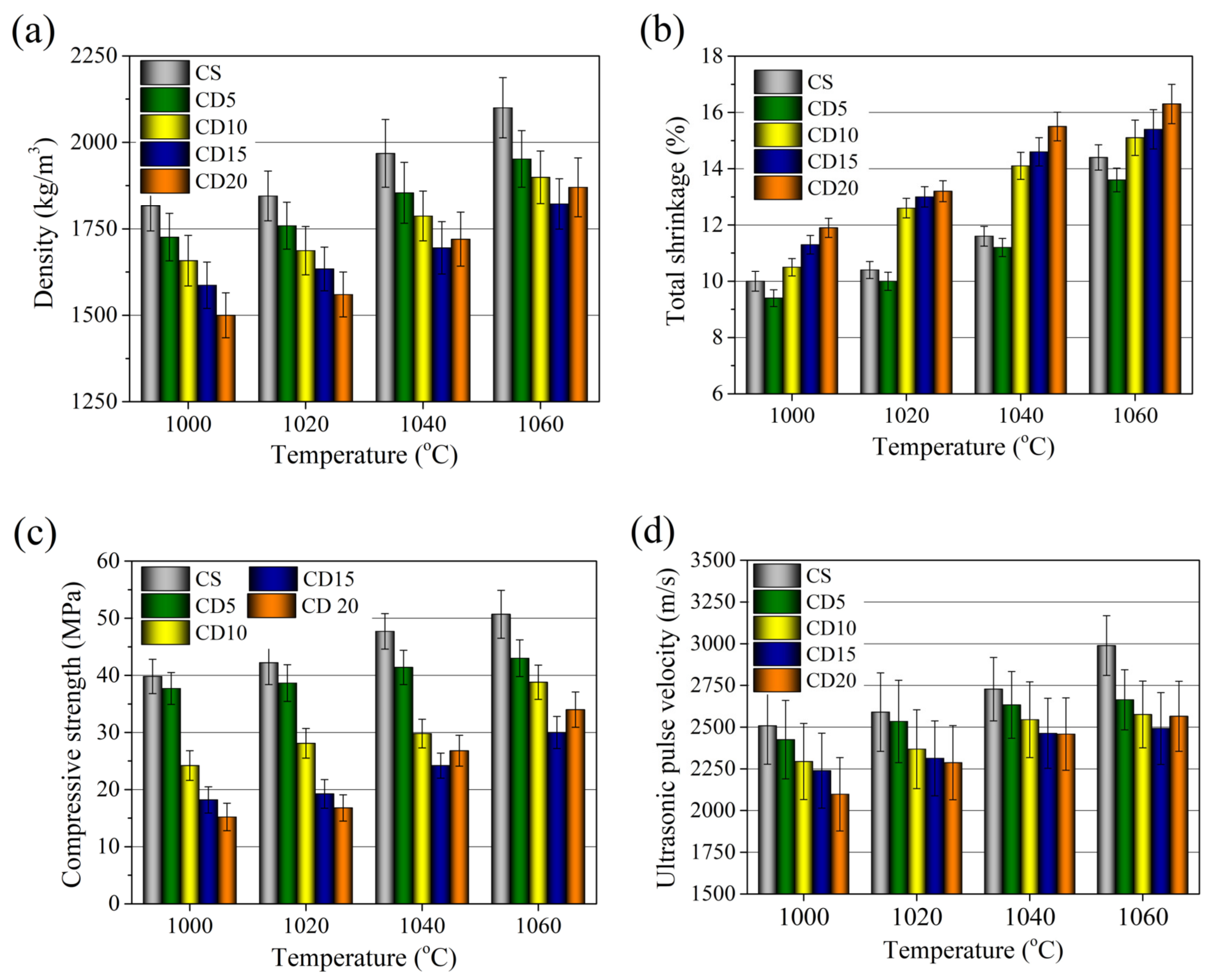
3.6. Physical and Mechanical Properties
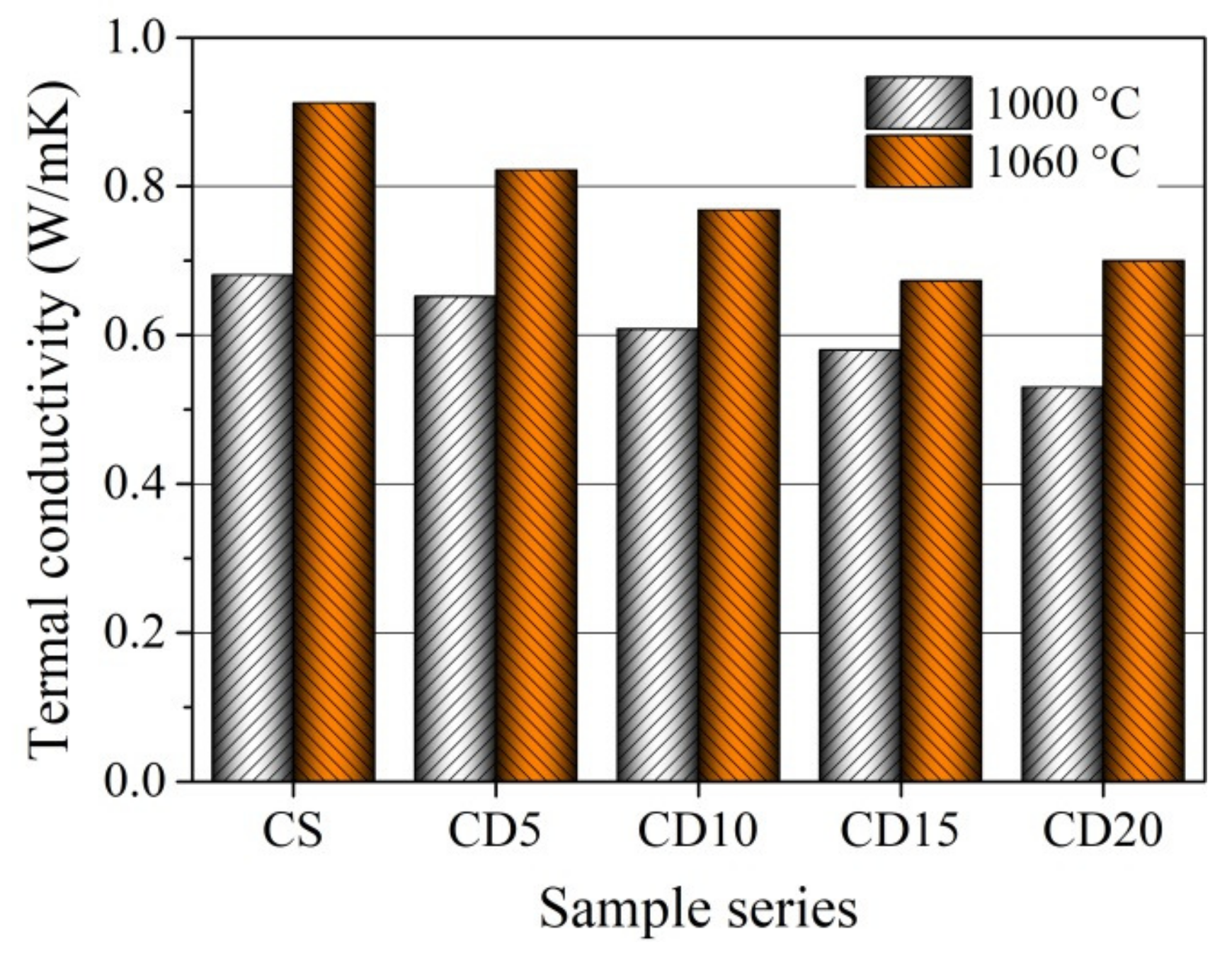
3.7. Thermal Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, Y.; Awan, U.; Ahmad, S.; Tan, Z. How do technological innovation and fiscal decentralization affect the environment? A story of the fourth industrial revolution and sustainable growth. Technol. Forecast. Soc. Change 2021, 162, 120398. [Google Scholar] [CrossRef]
- Song, Y.; Li, B.; Yang, E.H.; Liu, Y.; Ding, T. Feasibility study on utilization of municipal solid waste incineration bottom ash as aerating agent for the production of autoclaved aerated concrete. Cem. Concr. Compos. 2015, 56, 51–58. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Liu, H.; Han, C. Recycling of waste red mud for production of ceramic floor tile with high strength and lightweight. J. Alloys Compd. 2018, 748, 876–881. [Google Scholar] [CrossRef]
- Li, H.; Dong, L.; Jiang, Z.; Yang, X.; Yang, Z. Study on utilization of red brick waste powder in the production of cement-based red decorative plaster for walls. J. Clean. Prod. 2016, 133, 1017–1026. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Eliche-Quesada, D.; Martín-Pascual, J.; Martín-Morales, M.; Zamorano, M. Comparative study of the use of different biomass from olive grove in the manufacture of sustainable ceramic lightweight bricks. Constr. Build. Mater. 2020, 231, 117103. [Google Scholar] [CrossRef]
- Arbolino, R.; De Simone, L.; Carlucci, F.; Yigitcanlar, T.; Ioppolo, G. Towards a sustainable industrial ecology: Implementation of a novel approach in the performance evaluation of Italian regions. J. Clean. Prod. 2018, 178, 220–236. [Google Scholar] [CrossRef] [Green Version]
- Taurino, R.; Ferretti, D.; Cattani, L.; Bozzoli, F.; Bondioli, F. Lightweight clay bricks manufactured by using locally available wine industry waste. J. Build. Eng. 2019, 26, 100892. [Google Scholar] [CrossRef]
- Mohamed Soltan, A.M.; Pöhler, K.; Fuchs, F.; EL-Raoof, F.A.; El-Kaliouby, B.A.H.; Koenig, A.; Pöllmann, H. Clay-bricks from recycled rock tailings. Ceram. Int. 2016, 42, 16685–16696. [Google Scholar] [CrossRef]
- Stonys, R.; Kuznetsov, D.; Krasnikovs, A.; Škamat, J.; Baltakys, K.; Antonovič, V.; Černašejus, O. Reuse of ultrafine mineral wool production waste in the manufacture of refractory concrete. J. Environ. Manag. 2016, 176, 149–156. [Google Scholar] [CrossRef]
- Kazmi, S.M.S.; Abbas, S.; Munir, M.J.; Khitab, A. Exploratory study on the effect of waste rice husk and sugarcane bagasse ashes in burnt clay bricks. J. Build. Eng. 2016, 7, 372–378. [Google Scholar] [CrossRef]
- Yang, J.; Xiao, B.; Boccaccini, A.R. Preparation of low melting temperature glass-ceramics from municipal waste incineration fly ash. Fuel 2009, 88, 1275–1280. [Google Scholar] [CrossRef]
- Bernardo, E.; Bonomo, E.; Dattoli, A. Optimisation of sintered glass-ceramics from an industrial waste glass. Ceram. Int. 2010, 36, 1675–1680. [Google Scholar] [CrossRef]
- Coletti, C.; Maritan, L.; Cultrone, G.; Mazzoli, C. Use of industrial ceramic sludge in brick production: Effect on aesthetic quality and physical properties. Constr. Build. Mater. 2016, 124, 219–227. [Google Scholar] [CrossRef]
- Ducman, V.; Kopar, T. The influence of different waste additions to clay-product mixtures. Mater. Tehnol. 2007, 41, 289–293. [Google Scholar]
- Han, L.; Li, F.; Deng, X.; Wang, J.; Zhang, H.; Zhang, S. Foam-gelcasting preparation, microstructure and thermal insulation performance of porous diatomite ceramics with hierarchical pore structures. J. Eur. Ceram. Soc. 2017, 37, 2717–2725. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.; Nie, Y.; Li, R. Recycling of municipal solid waste incineration fly ash for ordinary Portland cement production: A real-scale test. Resour. Conserv. Recycl. 2010, 54, 1428–1435. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Wu, J.; Liu, J.; Li, C.; Ning, T.; Luo, Z.; Zhou, X.; Yang, Q.; Lu, A. The utilization of electrical insulators waste and red mud for fabrication of partially vitrified ceramic materials with high porosity and high strength. J. Clean. Prod. 2019, 223, 790–800. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Alexandre, J.; Margem, J.I.; Sánchez, R.; Vieira, C.M.F. Incorporation of sludge waste from water treatment plant into red ceramic. Constr. Build. Mater. 2008, 22, 1281–1287. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Hakkou, R.; Mansori, M. Natural clay substitution by calamine processing wastes to manufacture fired bricks. J. Clean. Prod. 2016, 135, 847–858. [Google Scholar] [CrossRef]
- Mymrin, V.; Klitzke, W.; Alekseev, K.; Catai, R.E.; Nagalli, A.; dos Santos Izzo, R.L.; Romano, C.A. Red clay application in the utilization of paper production sludge and scrap glass to fabricate ceramic materials. Appl. Clay Sci. 2015, 107, 28–35. [Google Scholar] [CrossRef]
- Awan, U.; Kraslawski, A.; Huiskonen, J. Governing Interfirm Relationships for Social Sustainability: The Relationship between Governance Mechanisms, Sustainable Collaboration, and Cultural Intelligence. Sustainability 2018, 10, 4473. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Felipe-Sesé, M.A.; López-Pérez, J.A.; Infantes-Molina, A. Characterization and evaluation of rice husk ash and wood ash in sustainable clay matrix bricks. Ceram. Int. 2017, 43, 463–475. [Google Scholar] [CrossRef]
- Muñoz, V.P.; Morales, O.M.P.; Letelier, G.V.; Mendívil, G.M.A. Fired clay bricks made by adding wastes: Assessment of the impact on physical, mechanical and thermal properties. Constr. Build. Mater. 2016, 125, 241–252. [Google Scholar] [CrossRef]
- Sutcu, M.; Ozturk, S.; Yalamac, E.; Gencel, O. Effect of olive mill waste addition on the properties of porous fired clay bricks using Taguchi method. J. Environ. Manag. 2016, 181, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Sutcu, M.; Akkurt, S. Utilization of recycled paper processing residues and clay of different sources for the production of porous anorthite ceramics. J. Eur. Ceram. Soc. 2010, 30, 1785–1793. [Google Scholar] [CrossRef] [Green Version]
- Mekki, H.; Anderson, M.; Benzina, M.; Ammar, E. Valorization of olive mill wastewater by its incorporation in building bricks. J. Hazard. Mater. 2008, 158, 308–315. [Google Scholar] [CrossRef]
- Veiseh, S.; Yousefi, A.A. The use of polystyrene in lightweight brick production. Iran. Polym. J. 2003, 12, 323–329. [Google Scholar]
- Sokolář, R.; Vodová, L.; Grygarová, S.; Štubňa, I.; Šín, P. Mechanical properties of ceramic bodies based on calcite waste. Ceram. Int. 2012, 38, 6607–6612. [Google Scholar] [CrossRef]
- Sutcu, M.; Del Coz Díaz, J.J.; Álvarez Rabanal, F.P.; Gencel, O.; Akkurt, S. Thermal performance optimization of hollow clay bricks made up of paper waste. Energy Build. 2014, 75, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Boltakova, N.V.; Faseeva, G.R.; Kabirov, R.R.; Nafikov, R.M.; Zakharov, Y.A. Utilization of inorganic industrial wastes in producing construction ceramics. Review of Russian experience for the years 2000–2015. Waste Manag. 2017, 60, 230–246. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Lv, Y.; Jian, S.; Liu, G.; Jiang, W.; Jiang, D. Utilization of municipal sewage sludge and waste glass powder in production of lightweight aggregates. Constr. Build. Mater. 2020, 256, 119413. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Vieira, C.M.F. On the production of fired clay bricks from waste materials: A critical update. Constr. Build. Mater. 2014, 68, 599–610. [Google Scholar] [CrossRef]
- Galán-Arboledas, R.J.; Cotes-Palomino, M.T.; Bueno, S.; Martínez-García, C. Evaluation of spent diatomite incorporation in clay based materials for lightweight bricks processing. Constr. Build. Mater. 2017, 144, 327–337. [Google Scholar] [CrossRef]
- Bories, C.; Borredon, M.E.; Vedrenne, E.; Vilarem, G. Development of eco-friendly porous fired clay bricks using pore-forming agents: A review. J. Environ. Manag. 2014, 143, 186–196. [Google Scholar] [CrossRef]
- Pérez-Villarejo, L.; Corpas-Iglesias, F.A.; Martínez-Martínez, S.; Artiaga, R.; Pascual-Cosp, J. Manufacturing new ceramic materials from clay and red mud derived from the aluminium industry. Constr. Build. Mater. 2012, 35, 656–665. [Google Scholar] [CrossRef]
- Kizinievič, O.; Balkevičius, V.; Pranckevičiene, J.; Kizinievič, V. Investigation of the usage of centrifuging waste of mineral wool melt (CMWW), contaminated with phenol and formaldehyde, in manufacturing of ceramic products. Waste Manag. 2014, 34, 1488–1494. [Google Scholar] [CrossRef]
- El-Shimy, Y.N.; Amin, S.K.; El-Sherbiny, S.A.; Abadir, M.F. The use of cullet in the manufacture of vitrified clay pipes. Constr. Build. Mater. 2014, 73, 452–457. [Google Scholar] [CrossRef]
- Saparuddin, D.I.; Hisham, N.A.N.; Aziz, S.A.; Matori, K.A.; Honda, S.; Iwamoto, Y.; Zaid, M.H.M. Effect of sintering temperature on the crystal growth, microstructure and mechanical strength of foam glass-ceramic from waste materials. J. Mater. Res. Technol. 2020, 9, 5640–5647. [Google Scholar] [CrossRef]
- Zhang, L. Production of bricks from waste materials—A review. Constr. Build. Mater. 2013, 47, 643–655. [Google Scholar] [CrossRef]
- Eliche-Quesada, D.; Corpas-Iglesias, F.A. Utilisation of spent filtration earth or spent bleaching earth from the oil refinery industry in clay products. Ceram. Int. 2014, 40, 16677–16687. [Google Scholar] [CrossRef]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; Sánchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Martinovic, S.; Vlahovic, M.; Boljanac, T.; Pavlovic, L. Preparation of filter aids based on diatomites. Int. J. Miner. Process. 2006, 80, 255–260. [Google Scholar] [CrossRef]
- Ivanov, S.É.; Belyakov, A.V. Diatomite and its applications. Glas. Ceram. 2008, 65, 48–51. [Google Scholar] [CrossRef]
- Reka, A.; Anovski, T.; Bogoevski, S.; Pavlovski, B.; Boškovski, B. Physical-chemical and mineralogical-petrographic examinations of diatomite from deposit near village of Rožden, Republic of Macedonia. Geol. Maced. 2014, 28, 121–126. [Google Scholar]
- Reka, A.A.; Pavlovski, B.; Makreski, P. New optimized method for low-temperature hydrothermal production of porous ceramics using diatomaceous earth. Ceram. Int. 2017, 43, 12572–12578. [Google Scholar] [CrossRef]
- Arik, H. Synthesis of Si3N4 by the carbo-thermal reduction and nitridation of diatomite. J. Eur. Ceram. Soc. 2003, 23, 2005–2014. [Google Scholar] [CrossRef]
- Wei, H. An overview of wax production, requirement and supply in the world market. Eur. Chem. Bull. 2012, 1, 266–268. [Google Scholar]
- Hadjar, H.; Hamdi, B.; Jaber, M.; Brendlé, J.; Kessaïssia, Z.; Balard, H.; Donnet, J.B. Elaboration and characterisation of new mesoporous materials from diatomite and charcoal. Microporous Mesoporous Mater. 2008, 107, 219–226. [Google Scholar] [CrossRef]
- Khraisheh, M.A.M.; Al-degs, Y.S.; Mcminn, W.A.M. Remediation of wastewater containing heavy metals using raw and modified diatomite. Chem. Eng. J. 2004, 99, 177–184. [Google Scholar] [CrossRef]
- Pimraksa, K.; Chindaprasirt, P. Lightweight bricks made of diatomaceous earth, lime and gypsum. Ceram. Int. 2009, 35, 471–478. [Google Scholar] [CrossRef]
- Escalera, E.; Garcia, G.; Terán, R.; Tegman, R.; Antti, M.L.; Odén, M. The production of porous brick material from diatomaceous earth and Brazil nut shell ash. Constr. Build. Mater. 2015, 98, 257–264. [Google Scholar] [CrossRef]
- Netinger Grubeša, I.; Vračević, M.; Ranogajec, J.; Vučetić, S. Influence of Pore-Size Distribution on the Resistance of Clay Brick to Freeze–Thaw Cycles. Materials 2020, 13, 2364. [Google Scholar] [CrossRef]
- Maage, M. Frost resistance and pore size distribution in bricks. Matériaux Constr. 1984, 17, 345–350. [Google Scholar] [CrossRef]
- Mačiulaitis, R.; Malaiškiene, J. Possibilities to control ceramics properties by changing firing cycles. Constr. Build. Mater. 2009, 23, 226–232. [Google Scholar] [CrossRef]
- Mačiulaitis, R. Fasadinės Keramikos Atsparumas Šalčiui ir Ilgaamžiškumas [Frost Resistance and Durability of Ceramic Facade Products]; Technika: Vilnius, Lithuania, 1996. [Google Scholar]
- Христoфoрoв, А.И.; Христoфoрoва, И.А. Расчет Физикo-Химических Свoйств Стекoл: Учебнoе Пoсoбие [Calculation of Physico-Chemical Properties of Glasses: Handbook]; Vladimir State University: Vladimir, Russia, 2004. [Google Scholar]
- Tatarintseva, O.S.; Khodakova, N.N.; Uglova, T.K. Dependence of the viscosity of basalt melts on the chemical composition of the initial mineral material. Glas. Ceram. 2012, 68, 323–326. [Google Scholar] [CrossRef]
- Pisciotta, A.; Perevozchikov, B.V.; Osovetsky, B.M.; Menshikova, E.A.; Kazymov, K.P. Quality Assessment of Melanocratic Basalt for Mineral Fiber Product, Southern Urals, Russia. Nat. Resour. Res. 2015, 24, 329–337. [Google Scholar] [CrossRef]
- Farouk, M.; Soltan, A.M.; Schlüter, S.; Hamzawy, E.; Farrag, A.; El-Kammar, A.; Yahia, A.; Pollmann, H. Optimization of microstructure of basalt-based fibers intended for improved thermal and acoustic insulations. J. Build. Eng. 2021, 34, 101904. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Jiang, Z.; Li, C.; Cang, D.; Liu, X.; Xuan, Y.; Ding, Y. Diatomite-based porous ceramics with high apparent porosity: Pore structure modification using calcium carbonate. Ceram. Int. 2019, 45, 6085–6092. [Google Scholar] [CrossRef]
- Kizinievič, O.; Žurauskienė, R.; Kizinievič, V.; Yakovlev, G.; Bur’yanov, A. Use of Sludge from Drinking Water Purification in the Production of Effective Ceramic Articles. Glas. Ceram. 2016, 73, 58–61. [Google Scholar] [CrossRef]
- Salem, A.; Jazayeri, S.H.; Rastelli, E.; Timellini, G. Dilatometeric study of shrinkage during sintering process for porcelain stoneware body in presence of nepheline syenite. J. Mater. Process. Technol. 2009, 209, 1240–1246. [Google Scholar] [CrossRef]
- Sánchez Muñoz, L.; Carda Castelló, J.B. Transformaciones con la temperatura. In Materias Primas y Aditivos Cerámicos; Enciclopedia Cerámica; Faenza Editrice Ibérica: Madrid, Spain, 2003; Volume 2, pp. 88–120. [Google Scholar]
- Serra, M.F.; Acebedo, M.F.; Conconi, M.S.; Suarez, G.; Aglietti, E.F.; Rendtorff, N.M. Thermal evolution of the mechanical properties of calcareous earthenware. Ceram. Int. 2014, 40, 1709–1716. [Google Scholar] [CrossRef]
- Šaponjić, A.; Stanković, M.; Majstorović, J.; Matović, B.; Ilić, S.; Egelja, A.; Kokunešoski, M. Porous ceramic monoliths based on diatomite. Ceram. Int. 2015, 41, 9745–9752. [Google Scholar] [CrossRef]
- García-Ten, J.; Orts, M.J.; Saburit, A.; Silva, G. Thermal conductivity of traditional ceramics: Part II: Influence of mineralogical composition. Ceram. Int. 2010, 36, 2017–2024. [Google Scholar] [CrossRef]
- Kashcheev, I.D.; Glyzina, A.É.; Finkel’shtein, A.B.; Sheffer, A.A.; Pavlova, I.A. Unmolded diatomite based heat insulating material for aluminum alloys. Refract. Ind. Ceram. 2019, 60, 362–364. [Google Scholar] [CrossRef]
- Bernardo, E.; Doyle, J.; Hampshire, S. Sintered feldspar glass-ceramics and glass-ceramic matrix composites. Ceram. Int. 2008, 34, 2037–2042. [Google Scholar] [CrossRef]
- Ptáček, P.; Lang, K.; Šoukal, F.; Opravil, T.; Bartoníčková, E.; Tvrdík, L. Preparation and properties of enstatite ceramic foam from talc. J. Eur. Ceram. Soc. 2014, 34, 515–522. [Google Scholar] [CrossRef]
- Mačiulaitis, R.; Malaiškiene, J. New quality regulation system for manufacture of ceramic products. Constr. Build. Mater. 2007, 21, 258–268. [Google Scholar] [CrossRef]
- Kizinievič, O.; Žurauskiene, R.; Kizinievič, V.; Žurauskas, R. Utilisation of sludge waste from water treatment for ceramic products. Constr. Build. Mater. 2013, 41, 464–473. [Google Scholar] [CrossRef]
- Malaiškiene, J.; Vaičiene, M.; Žurauskiene, R. Effectiveness of technogenic waste usage in products of building ceramics and expanded clay concrete. Constr. Build. Mater. 2011, 25, 3869–3877. [Google Scholar] [CrossRef]
- Stryszewska, T.; Kánka, S. Forms of Damage of Bricks Subjected to Cyclic Freezing and Thawing in Actual Conditions. Materials 2019, 12, 1165. [Google Scholar] [CrossRef] [Green Version]
- Bracka, A.; Rusin, Z. Comparison of pore characteristics and water absorption in ceramic materials with frost resistance factor, Fc. Struct. Environ. 2012, 4, 15–19. [Google Scholar]
- Il’ina, V.P.; Skamnitskaya, L.S.; Repnikova, E.A. Effect of feldspar concentrate composition on structure and properties of ceramic mixtures. Glas. Ceram. 1999, 56, 259–262. [Google Scholar] [CrossRef]
- Clauser, C.; Huenges, E. Rock Physics and Phase Relations: A Handbook of Physical Constants; American Geophysical Union: Washington, DC, USA, 1995. [Google Scholar] [CrossRef]
- Propiedades Térmicas de Metales, Cerámicas y Polímeros 1 TEMA. 2008. Available online: http://ocw.uc3m.es/ciencia-e-oin/quimica-de-los-materiales/Material-de-clase (accessed on 5 January 2021).
- Nigay, P.M.; Cutard, T.; Nzihou, A. The impact of heat treatment on the microstructure of a clay ceramic and its thermal and mechanical properties. Ceram. Int. 2017, 43, 1747–1754. [Google Scholar] [CrossRef]




| Raw Materials | Composition of Formation Masses (% of the Mass) | ||||
|---|---|---|---|---|---|
| CD5 | CD10 | CD15 | CD20 | CS | |
| Clay | 75 | 70 | 65 | 60 | 80 |
| Sand | 20 | 20 | 20 | 20 | 20 |
| OCW | 5 | 10 | 15 | 20 | - |
| Chemical Composition (%) | OCW | Clay | Sand |
|---|---|---|---|
| SiO2 | 27.4 | 47.0 | 90.4 |
| Al2O3 + TiO2 | 2.50 | 16.0 | 4.00 |
| Fe2O3 | 1.10 | 5.31 | 0.65 |
| CaO | 3.50 | 10.3 | 2.05 |
| MgO | 14.0 | 4.37 | 0.49 |
| Na2O + K2O | 4.90 | 2.50 | 1.41 |
| Loss on ignition | 46.8 | 14.5 | 1.02 |
| Raw Materials | Chemical Composition (%) | |||||
|---|---|---|---|---|---|---|
| SiO2 | Al2O3 + TiO2 | Fe2O3 | CaO | MgO | Na2O + K2O | |
| CS | 63.13 | 15.42 | 4.96 | 9.81 | 4.07 | 2.59 |
| CD5 | 63.16 | 14.92 | 4.81 | 9.60 | 4.70 | 2.77 |
| CD10 | 63.19 | 14.41 | 4.65 | 9.38 | 5.36 | 2.97 |
| CD15 | 63.21 | 13.87 | 4.49 | 9.15 | 6.04 | 3.17 |
| CD20 | 63.23 | 13.31 | 4.32 | 8.91 | 6.74 | 3.37 |
| Forming Masses Mark | Temperature, °C (Tƞ) | Modulus of Acidity (Ma) | Melting Capability Modulus (Mk) |
|---|---|---|---|
| CS | 843 | 5.66 | 4.62 |
| CD5 | 833 | 5.46 | 4.02 |
| CD10 | 827 | 5.26 | 3.83 |
| CD15 | 818 | 5.07 | 3.65 |
| CD20 | 791 | 4.89 | 3.48 |
| Formation Mix | Ws (%) | Wr (%) | We (%) | Rp (%) | D |
|---|---|---|---|---|---|
| After Firing at 1000 °C | |||||
| CS | 13.42 | 30.70 | 24.74 | 19.41 | 2.26 |
| CD5 | 14.60 | 34.18 | 25.64 | 24.93 | 1.93 |
| CD10 | 15.72 | 37.01 | 26.59 | 28.10 | 1.70 |
| CD15 | 17.05 | 39.30 | 27.59 | 29.79 | 1.55 |
| CD20 | 18.25 | 41.40 | 28.48 | 31.19 | 1.44 |
| After firing at 1020 °C | |||||
| CS | 12.21 | 30.59 | 23.02 | 24.74 | 2.27 |
| CD5 | 12.17 | 33.35 | 22.00 | 34.05 | 2.00 |
| CD10 | 13.67 | 35.99 | 23.69 | 34.16 | 1.78 |
| CD15 | 14.36 | 38.29 | 24.03 | 37.26 | 1.61 |
| CD20 | 14.90 | 40.29 | 24.11 | 38.92 | 1.49 |
| After firing at 1040 °C | |||||
| CS | 6.66 | 21.94 | 13.37 | 39.03 | 3.55 |
| CD5 | 8.05 | 26.51 | 15.35 | 42.14 | 2.77 |
| CD10 | 8.64 | 29.39 | 15.91 | 45.90 | 2.40 |
| CD15 | 11.38 | 34.17 | 19.81 | 42.00 | 1.93 |
| CD20 | 10.80 | 32.10 | 18.10 | 39.40 | 2.10 |
| After firing at 1060 °C | |||||
| CS | 2.96 | 14.50 | 6.34 | 47.10 | 5.86 |
| CD5 | 4.77 | 21.01 | 9.59 | 52.35 | 3.76 |
| CD10 | 4.93 | 22.47 | 9.68 | 54.88 | 3.66 |
| CD15 | 6.97 | 26.07 | 13.09 | 58.90 | 2.84 |
| CD20 | 5.10 | 24.10 | 12.30 | 55.1 | 3.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranckevičienė, J.; Pundienė, I. Use of Magnesium Silicate Contaminated with Organic Compounds in Ceramic Materials as a Pore Modifier. Materials 2022, 15, 8833. https://doi.org/10.3390/ma15248833
Pranckevičienė J, Pundienė I. Use of Magnesium Silicate Contaminated with Organic Compounds in Ceramic Materials as a Pore Modifier. Materials. 2022; 15(24):8833. https://doi.org/10.3390/ma15248833
Chicago/Turabian StylePranckevičienė, Jolanta, and Ina Pundienė. 2022. "Use of Magnesium Silicate Contaminated with Organic Compounds in Ceramic Materials as a Pore Modifier" Materials 15, no. 24: 8833. https://doi.org/10.3390/ma15248833





