In Situ Electrodeposition of Pb and Ag Applied on Fluorine Doped Tin Oxide Substrates: Comparative Experimental and Theoretical Study
Abstract
:1. Introduction
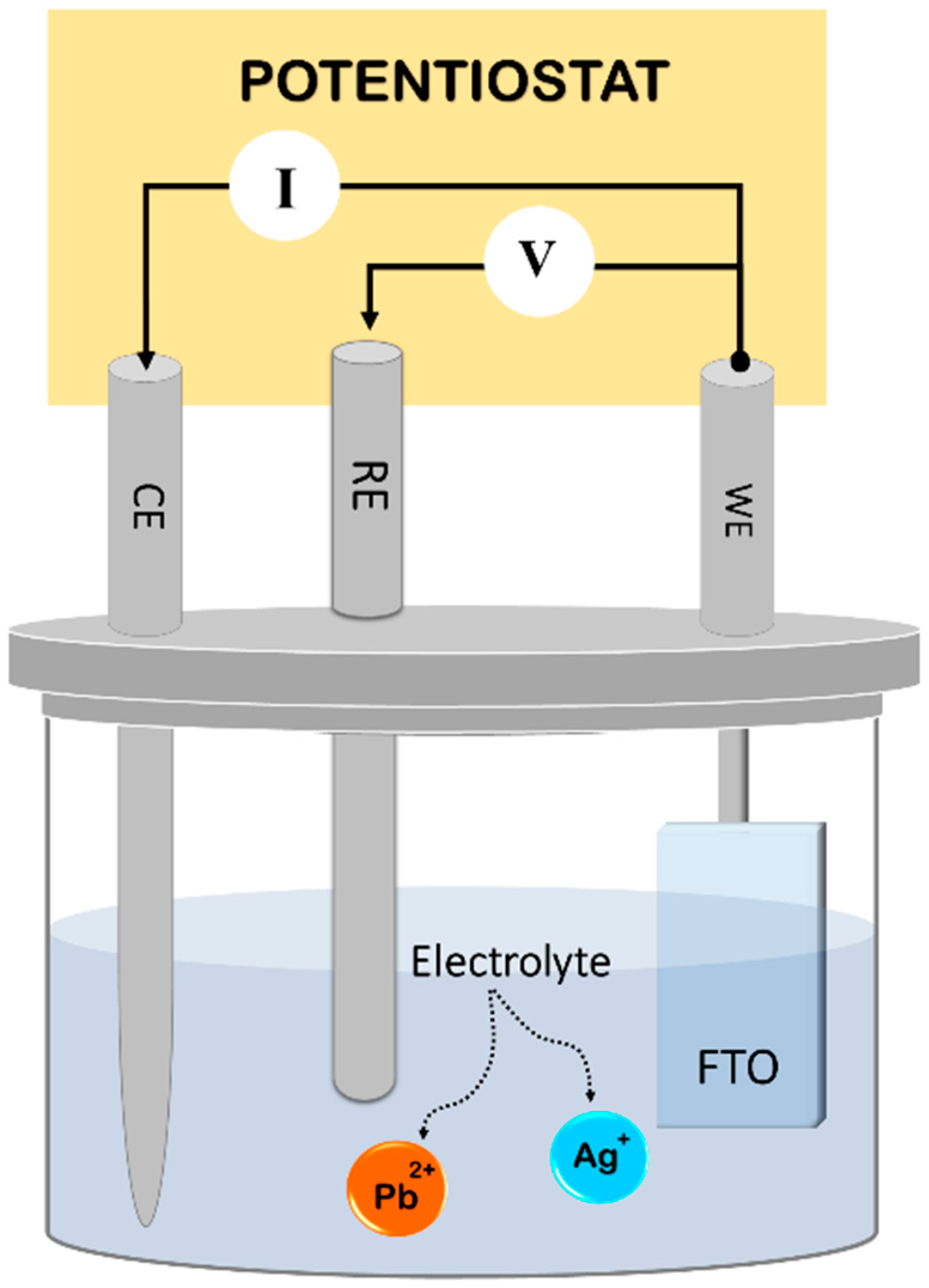
2. Materials and Methods
3. Results and Discussion
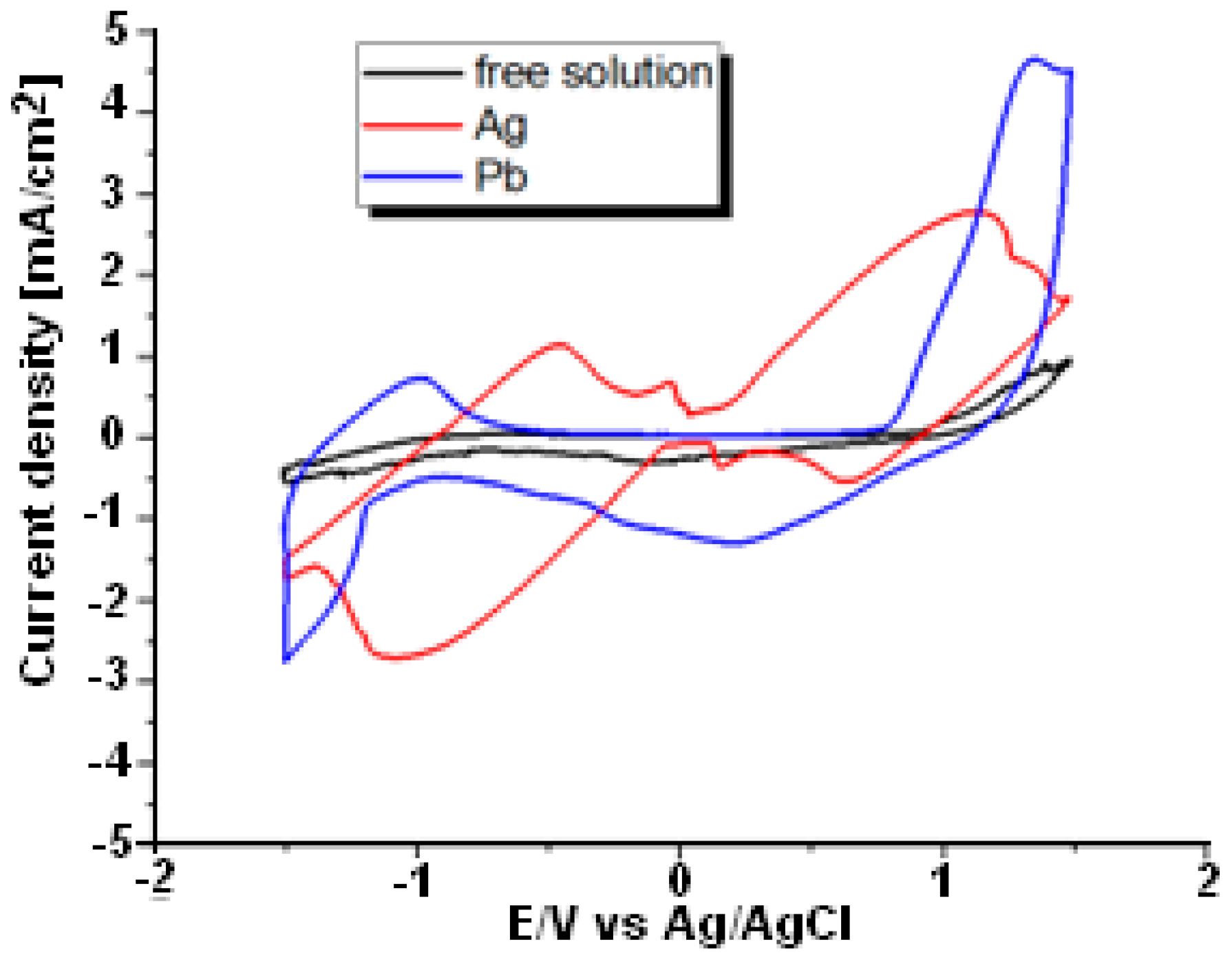
3.1. Cyclic Voltammetry
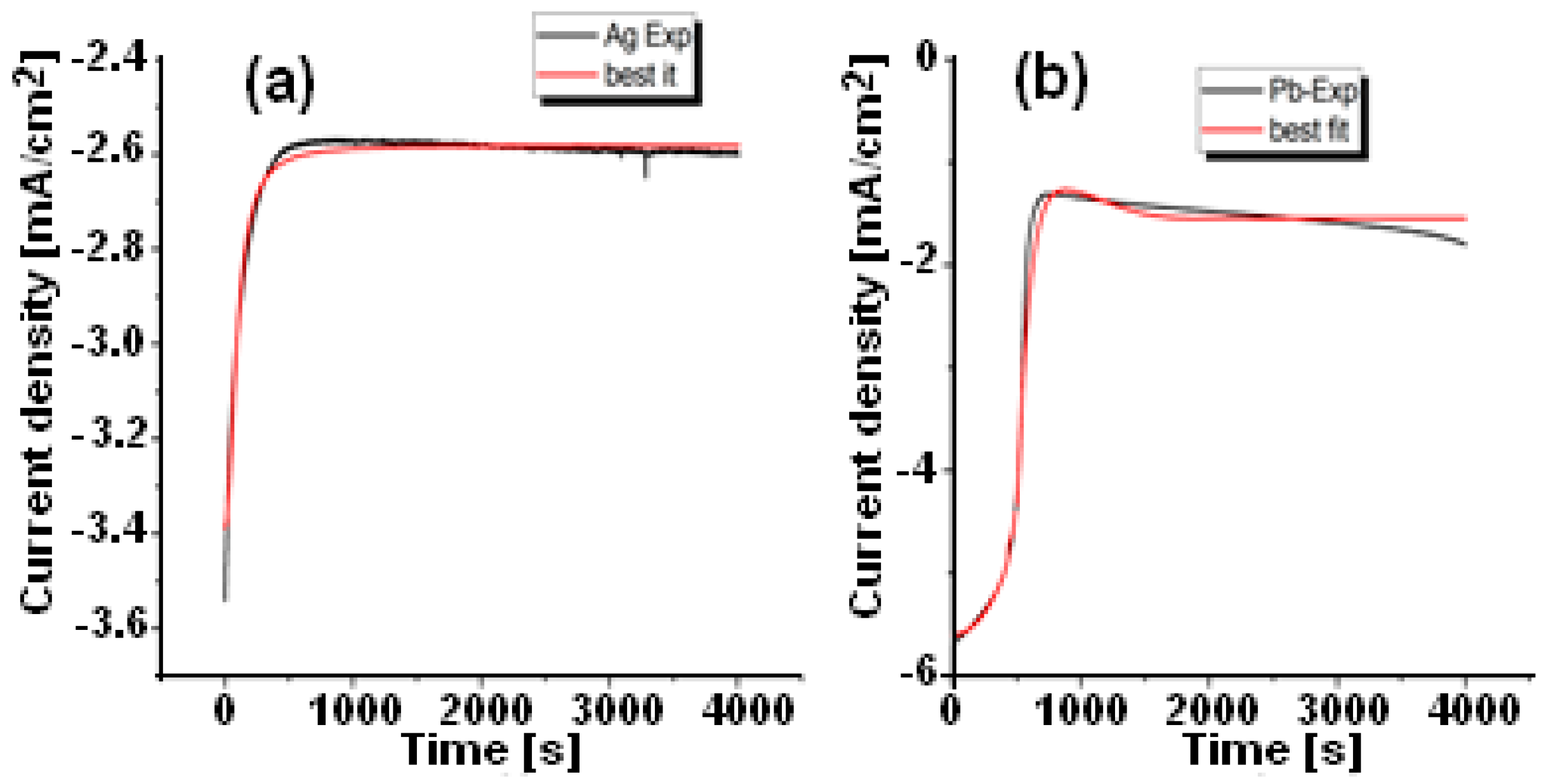
3.2. Chronoamperograms Analysis
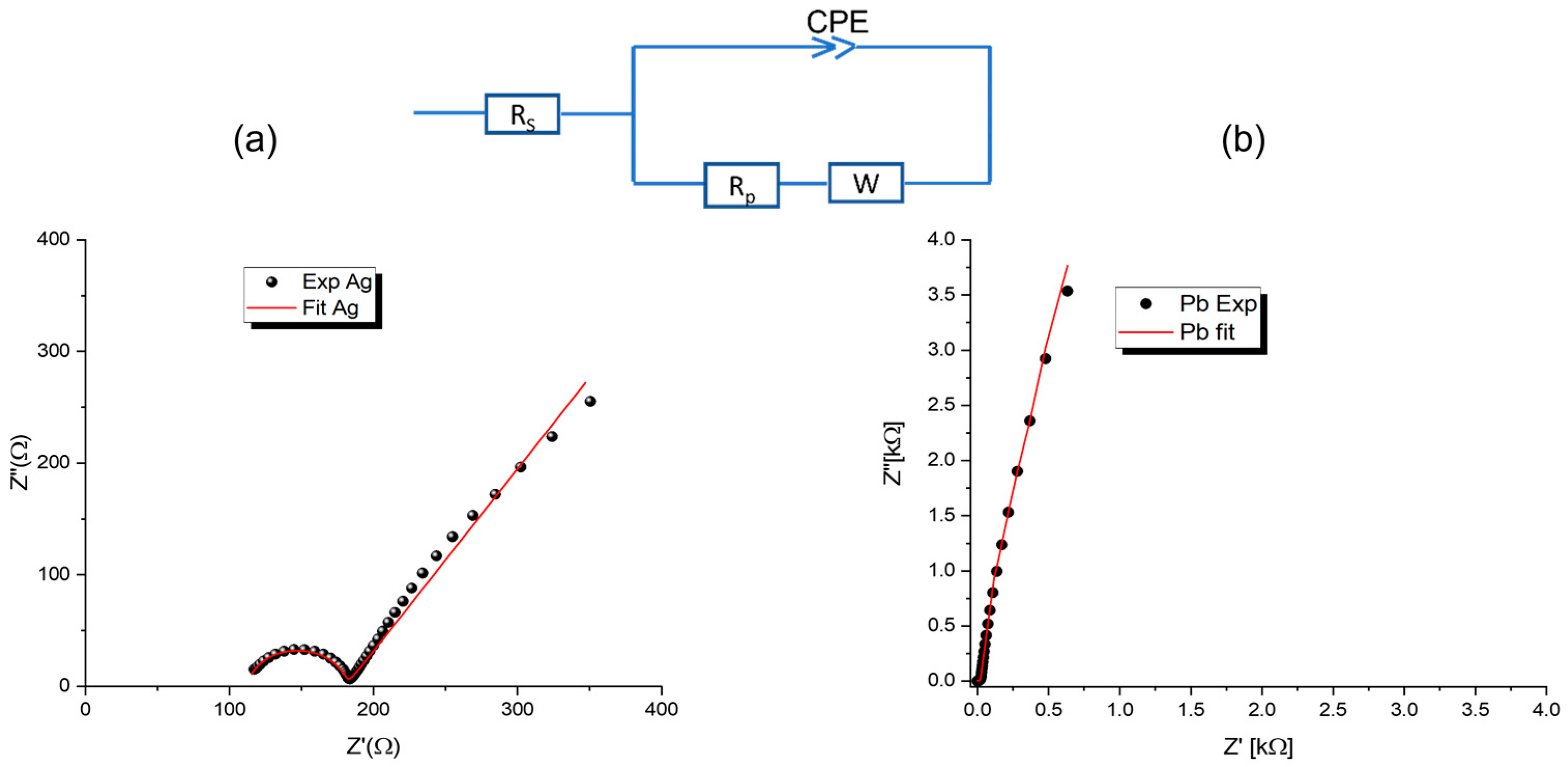
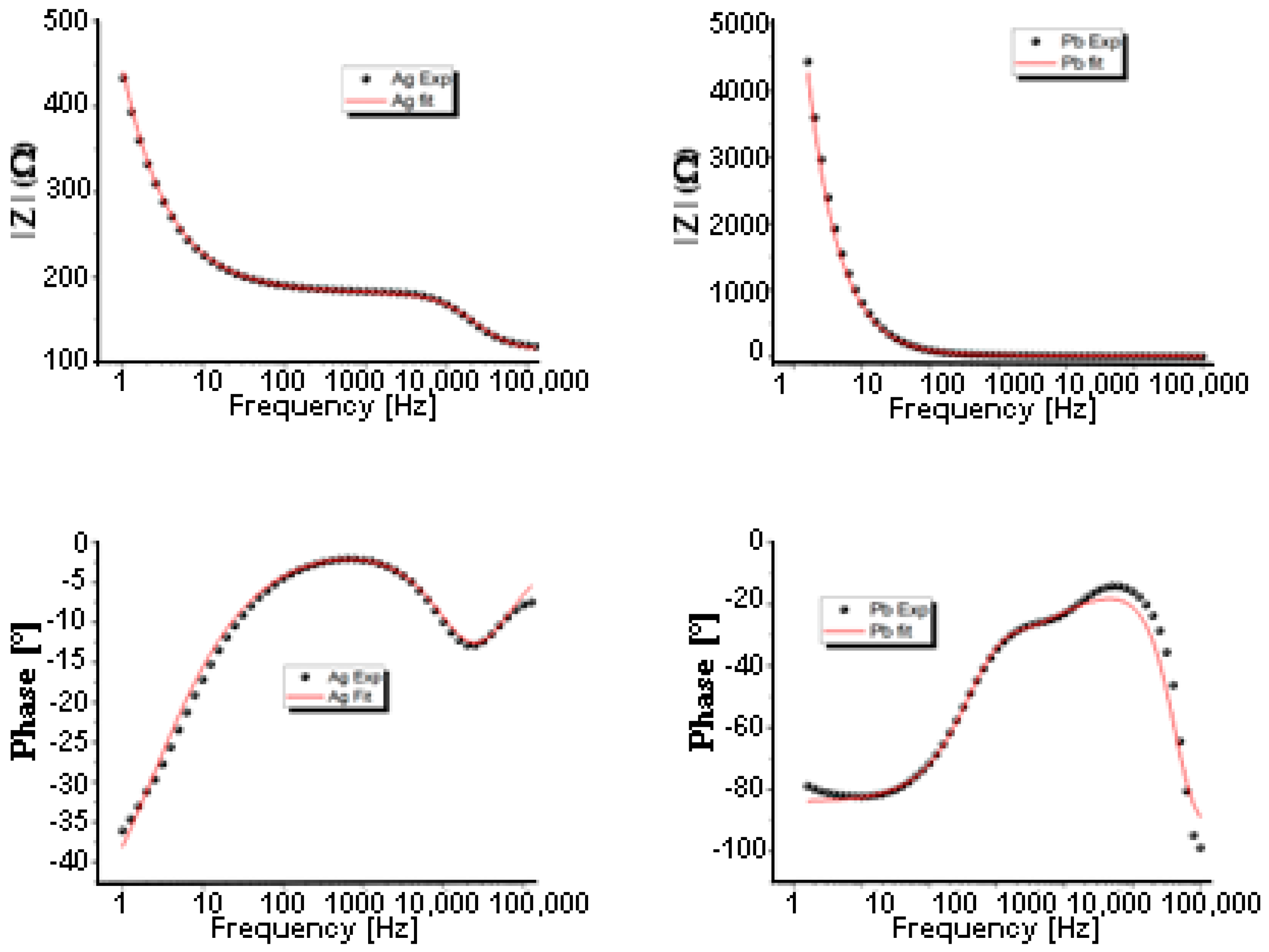
3.3. Impedance Spectroscopy
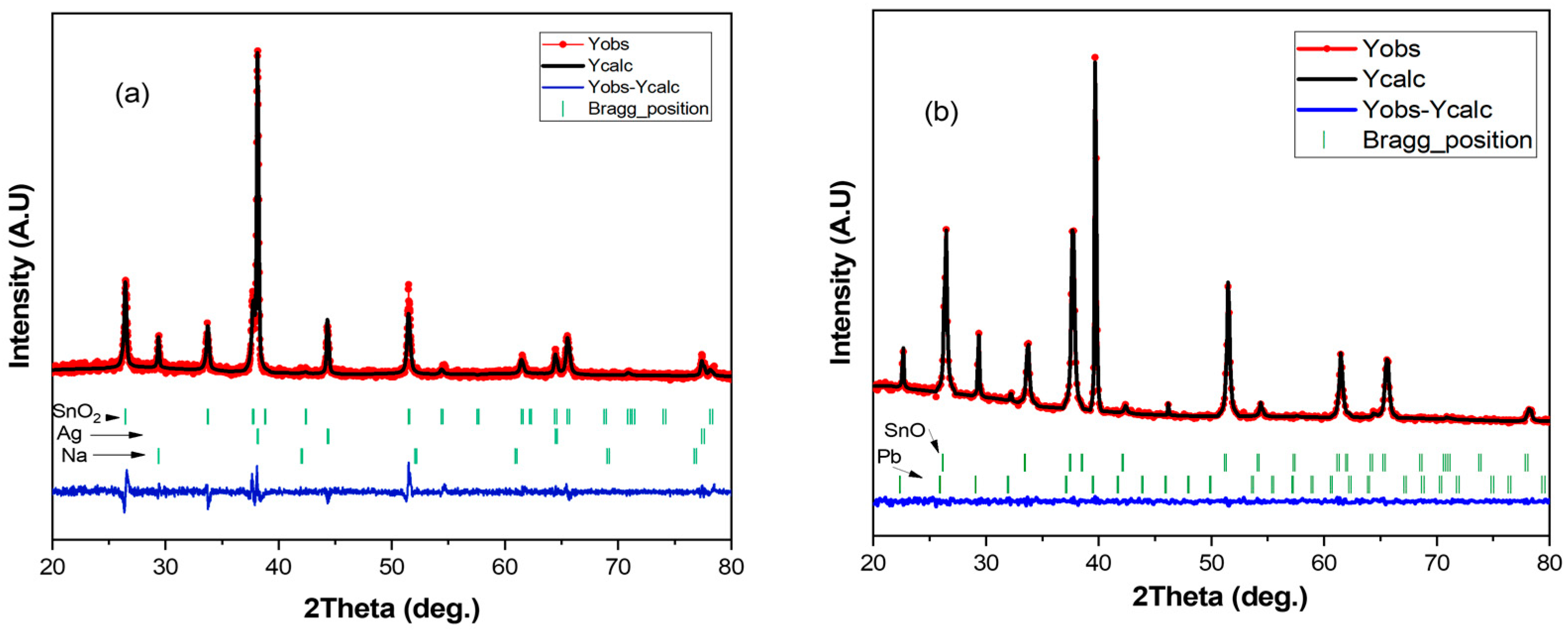
3.4. SEM Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sangkarak, S.; Phetrak, A.; Kittipongvises, S.; Kitkaew, D.; Phihusut, D.; Lohwacharin, J. Adsorptive Performance of Activated Carbon Reused from Household Drinking Water Filter for Hexavalent Chromium-Contaminated Water. J. Environ. Manag. 2020, 272, 111085. [Google Scholar] [CrossRef] [PubMed]
- Wood, D.M.; Greenland, B.W.; Acton, A.L.; Rodríguez-Llansola, F.; Murray, C.A.; Cardin, C.J.; Miravet, J.F.; Escuder, B.; Hamley, I.W.; Hayes, W. PH-Tunable Hydrogelators for Water Purification: Structural Optimisation and Evaluation. Chem.–A Eur. J. 2012, 18, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Savage, N.; Diallo, M.S. Nanomaterials and Water Purification: Opportunities and Challenges. J. Nanopart. Res. 2005, 7, 331–342. [Google Scholar] [CrossRef]
- Ambashta, R.D.; Sillanpää, M. Water Purification Using Magnetic Assistance: A Review. J. Hazard. Mater. 2010, 180, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water Purification by Using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Rebey, A.; Hamdi, R.; Hammami, B. Analysis of Growth Mechanisms and Microstructure Evolution of Pb+2 Minor Concentrations by Electrodeposition Technique. Eur. Phys. J. Plus 2022, 137, 295. [Google Scholar] [CrossRef]
- Hamdi, R.; Rached, A.; Massoudi, I.; Al-Zuraie, R.; Al-Hamadah, K.; Al-Otaibi, A.; Flemban, T.; Alonizan, N.; Ghrib, T. Electrodeposition Study of Silver: Nucleation Process and Theoretical Analysis. J. Electron. Mater. 2021, 50, 5507–5513. [Google Scholar] [CrossRef]
- Wong, S.M.; Abrantes, L.M. Lead Electrodeposition from Very Alkaline Media. Electrochim. Acta 2005, 51, 619–626. [Google Scholar] [CrossRef]
- Shapiro, A.A.; Bonner, J.K.; Ogunseitan, O.A.; Saphores, J.-D.; Schoenung, J.M. Implications of Pb-Free Microelectronics Assembly in Aerospace Applications. IEEE Trans. Compon. Packag. Technol. 2006, 29, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Inberg, A.; Shacham-Diamand, Y.; Rabinovich, E.; Golan, G.; Croitoru, N. Electroless-Deposited Ag–W Films for Microelectronics Applications. Thin Solid Film. 2001, 389, 213–218. [Google Scholar] [CrossRef]
- Bhalothia, D.; Shuan, L.; Wu, Y.-J.; Yan, C.; Wang, K.-W.; Chen, T.-Y. A Highly Mismatched NiO2-to-Pd Hetero-Structure as an Efficient Nanocatalyst for the Hydrogen Evolution Reaction. Sustain. Energy Fuels 2020, 4, 2541–2550. [Google Scholar] [CrossRef]
- Lee, H.; Lee, J.-H.; Hwang, Y.-H.; Kim, Y. Cyclic Voltammetry Study of Electrodeposition of CuGaSe2 Thin Films on ITO-Glass Substrates. Curr. Appl. Phys. 2014, 14, 18–22. [Google Scholar] [CrossRef]
- González-García, J.; Iniesta, J.; Expósito, E.; García-García, V.; Montiel, V.; Aldaz, A. Early Stages of Lead Dioxide Electrodeposition on Rough Titanium. Thin Solid Film. 1999, 352, 49–56. [Google Scholar] [CrossRef]
- Popov, K.; Stojilković, E.; Radmilović, V.; Pavlović, M. Morphology of Lead Dendrites Electrodeposited by Square-Wave Pulsating Overpotential. Powder Technol. 1997, 93, 55–61. [Google Scholar] [CrossRef]
- Hamann, C.H.; Hamnett, A.; Vielstich, W. Electrochemistry, 2nd, Completely Revised and Updated Edition. Available online: https://www.wiley.com/en-us/Electrochemistry%2C+2nd%2C+Completely+Revised+and+Updated+Edition-p-9783527310692 (accessed on 12 December 2020).
- Basile, A.; Bhatt, A.I.; O’Mullane, A.P.; Bhargava, S.K. An Investigation of Silver Electrodeposition from Ionic Liquids: Influence of Atmospheric Water Uptake on the Silver Electrodeposition Mechanism and Film Morphology. Electrochim. Acta 2011, 56, 2895–2905. [Google Scholar] [CrossRef] [Green Version]
- Bhalothia, D.; Tsai, D.-L.; Wang, S.-P.; Yan, C.; Chan, T.-S.; Wang, K.-W.; Chen, T.-Y.; Chen, P.-C. Ir-Oxide Mediated Surface Restructure and Corresponding Impacts on Durability of Bimetallic NiOx@Pd Nanocatalysts in Oxygen Reduction Reaction. J. Alloys Compd. 2020, 844, 156160. [Google Scholar] [CrossRef]
- Bhalothia, D.; Chou, J.-P.; Yan, C.; Hu, A.; Yang, Y.-T.; Chen, T.-Y. Programming ORR Activity of Ni/NiO X@ Pd Electrocatalysts via Controlling Depth of Surface-Decorated Atomic Pt Clusters. ACS Omega 2018, 3, 8733–8744. [Google Scholar] [CrossRef] [Green Version]
- Hamdi, R. Synthesis and Physicochemical Studies of Double Fluorinated Hydrogen-Bonded Liquid Crystals (n-OBAFF). J. Mol. Liq. 2022, 349, 118201. [Google Scholar] [CrossRef]
- Tsui, L.; Zafferoni, C.; Lavacchi, A.; Innocenti, M.; Vizza, F.; Zangari, G. Electrocatalytic Activity and Operational Stability of Electrodeposited Pd–Co Films towards Ethanol Oxidation in Alkaline Electrolytes. J. Power Sources 2015, 293, 815–822. [Google Scholar] [CrossRef]
- Nikolić, N.D.; Maksimović, V.M.; Branković, G. Morphological and Crystallographic Characteristics of Electrodeposited Lead from a Concentrated Electrolyte. RSC Adv. 2013, 3, 7466–7471. [Google Scholar] [CrossRef]
- Cherevko, S.; Xing, X.; Chung, C.H. Hydrogen Template Assisted Electrodeposition of Sub-Micrometer Wires Composing Honeycomb-like Porous Pb Films. Appl. Surf. Sci. 2011, 257, 8054–8061. [Google Scholar] [CrossRef]


| Parameters | Pb | Ag |
|---|---|---|
| log k0, mol cm−2 s−1 | −3.537 | −3.801 |
| log k′, mol cm−2 s−1 | −4.331 | −4.887 |
| log A3D, nuclei cm−2 s−1 | 10.600 | 11.126 |
| Pb | Ag | |
|---|---|---|
| RS (Ω) | 0.393 | 112.943 |
| Rp (Ω) | 6.1334 | 67.992 |
| CPE-T (F) | 6.094 × 10−10 | 1.9075 × 10−7 |
| CPE-P | 0.967 | 0.959 |
| W-R (Ω) | 40.68 | 0.1051 |
| W-T (F) | 69.089 × 10−5 | 7.078 × 10−7 |
| W-P | 0.467 | 0.325 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebey, A.; Hamdi, R.; Massoudi, I.; Hammami, B. In Situ Electrodeposition of Pb and Ag Applied on Fluorine Doped Tin Oxide Substrates: Comparative Experimental and Theoretical Study. Materials 2022, 15, 8865. https://doi.org/10.3390/ma15248865
Rebey A, Hamdi R, Massoudi I, Hammami B. In Situ Electrodeposition of Pb and Ag Applied on Fluorine Doped Tin Oxide Substrates: Comparative Experimental and Theoretical Study. Materials. 2022; 15(24):8865. https://doi.org/10.3390/ma15248865
Chicago/Turabian StyleRebey, Ahmed, Ridha Hamdi, Imen Massoudi, and Bechir Hammami. 2022. "In Situ Electrodeposition of Pb and Ag Applied on Fluorine Doped Tin Oxide Substrates: Comparative Experimental and Theoretical Study" Materials 15, no. 24: 8865. https://doi.org/10.3390/ma15248865






