Laser Directed Energy Deposition of an AlMgScZr-Alloy in High-Speed Process Regimes
Abstract
1. Introduction
2. Materials and Methods
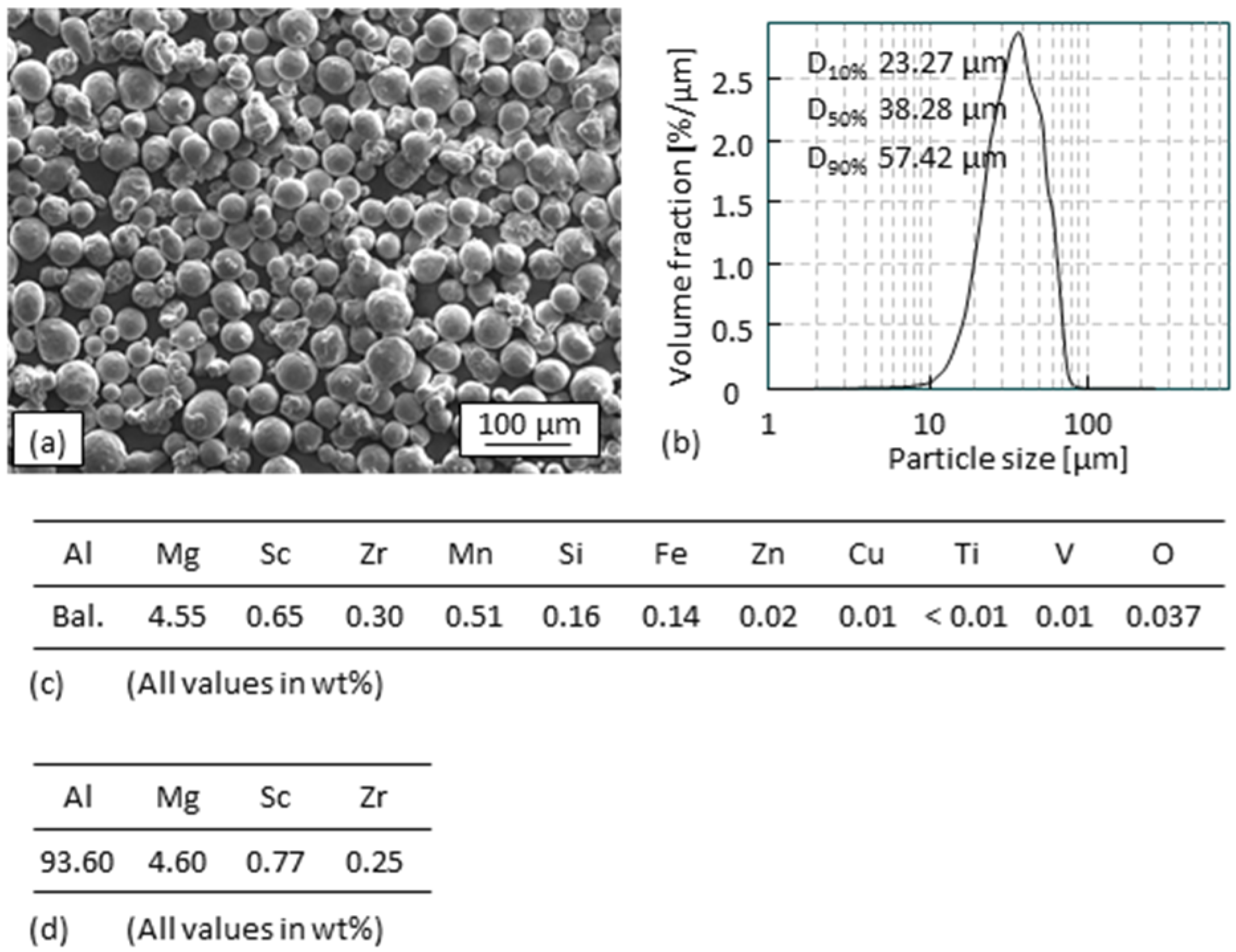
2.1. Powder
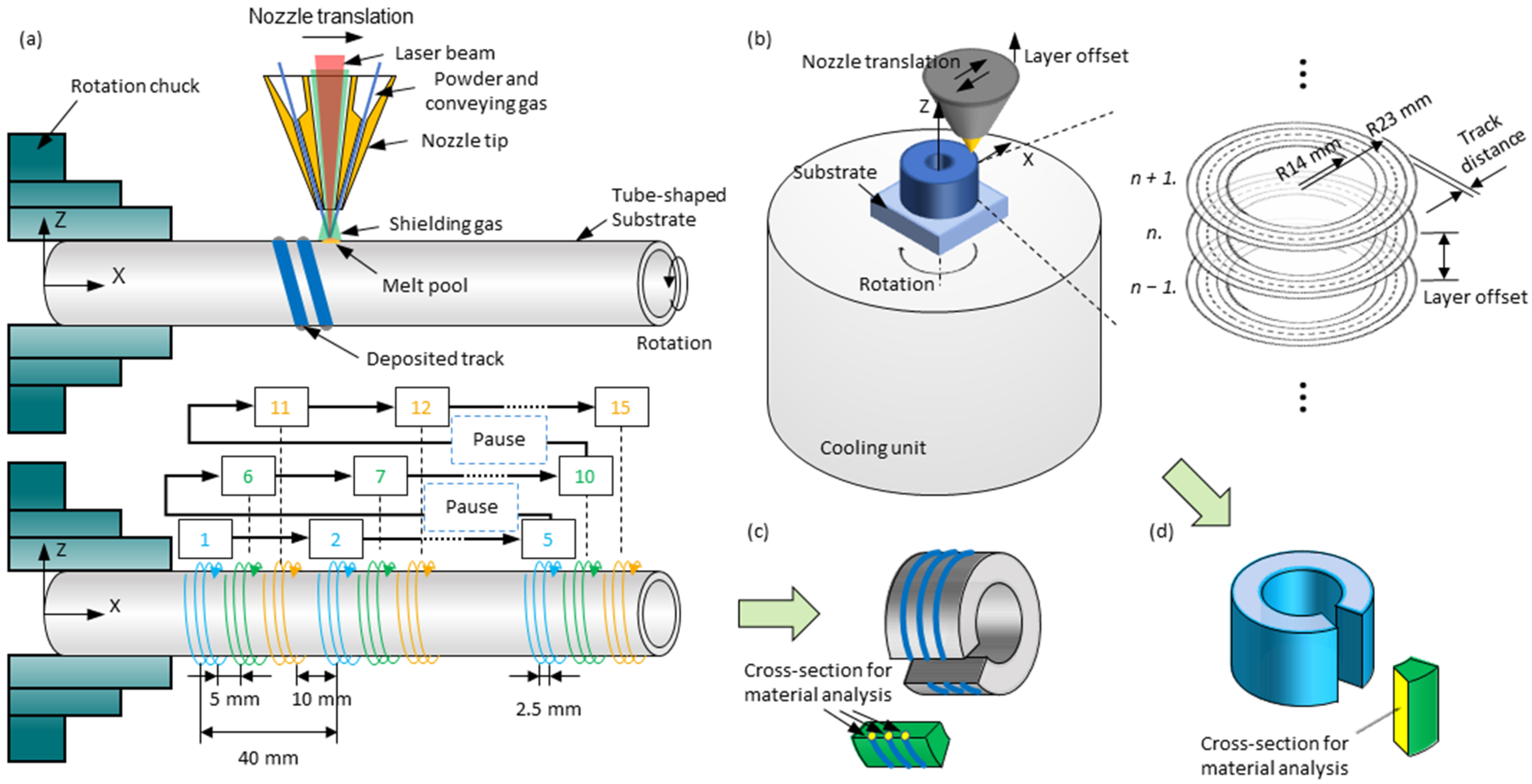
2.2. Experimental Setup
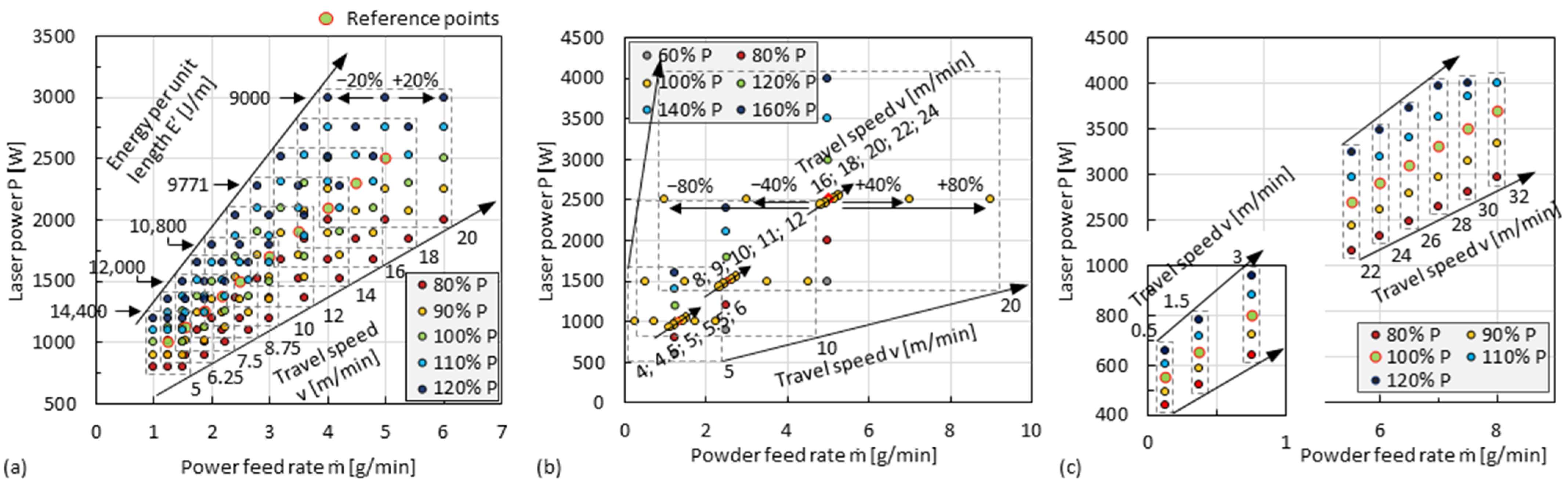
2.3. Process Variables
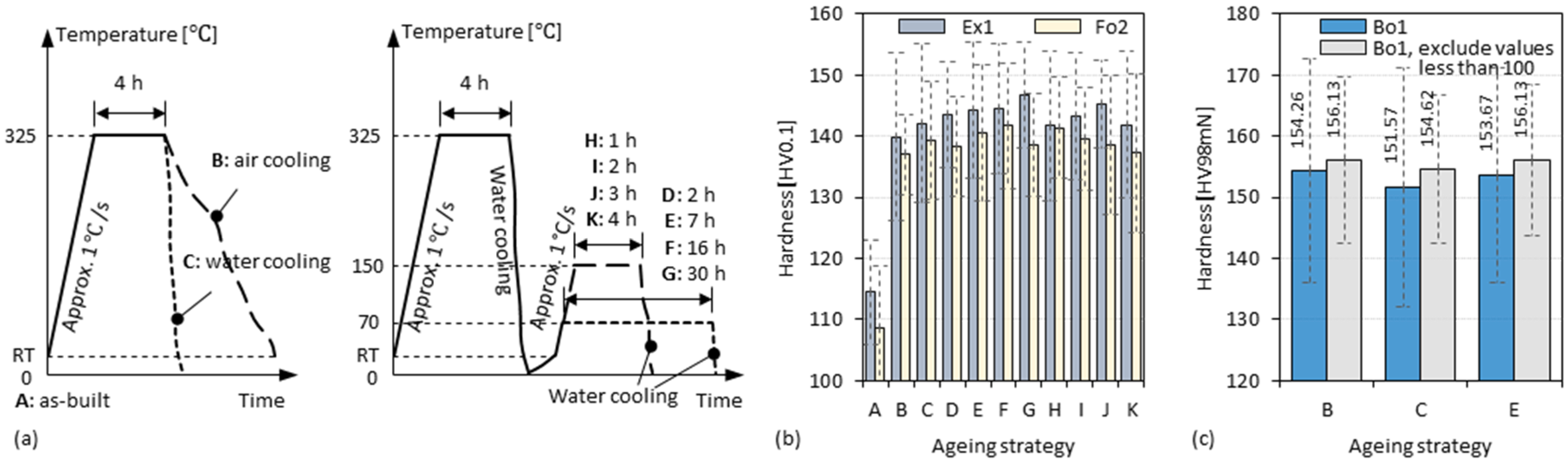
2.4. Heat Treatment
2.5. Material Analysis
3. Results
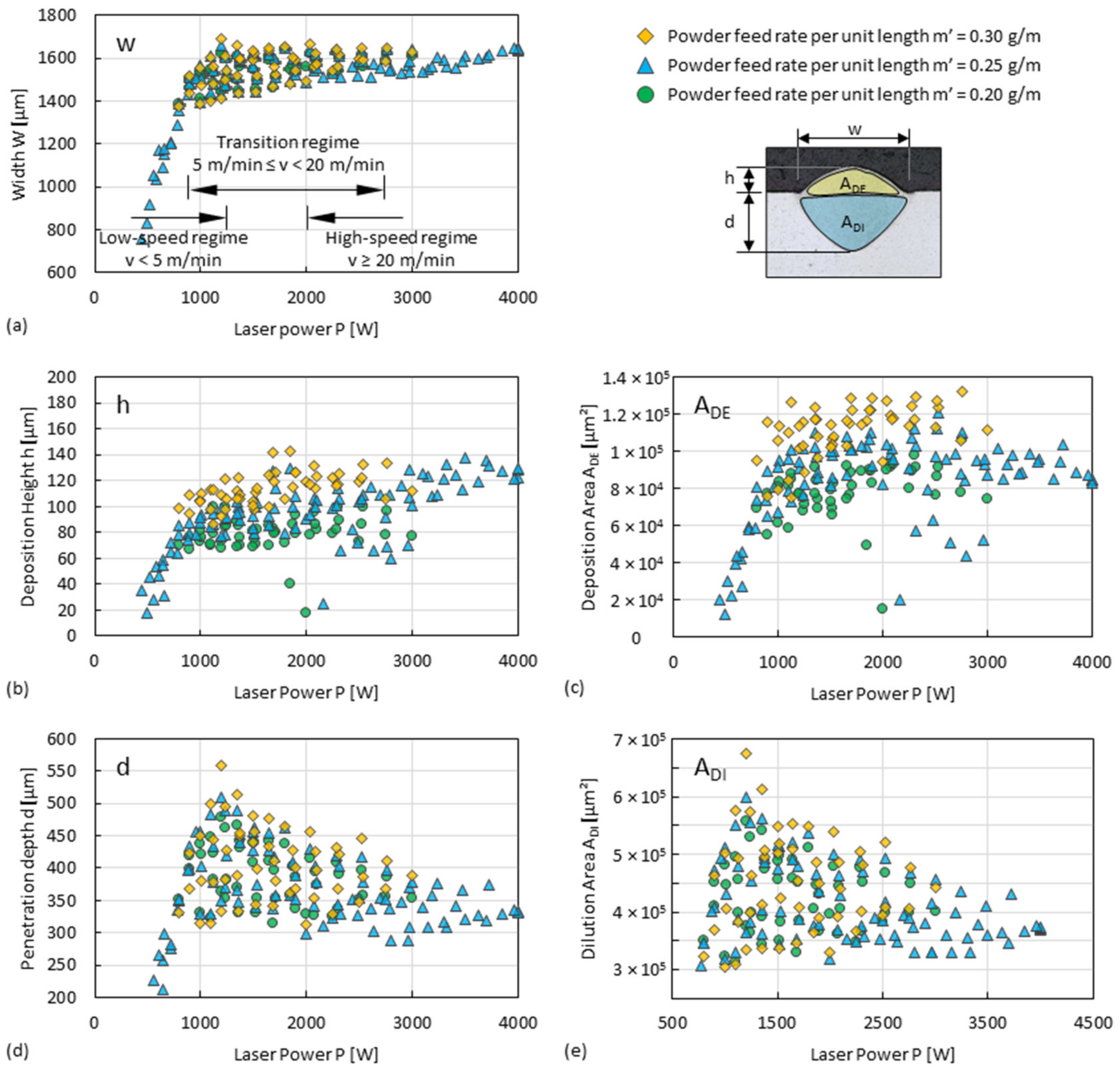
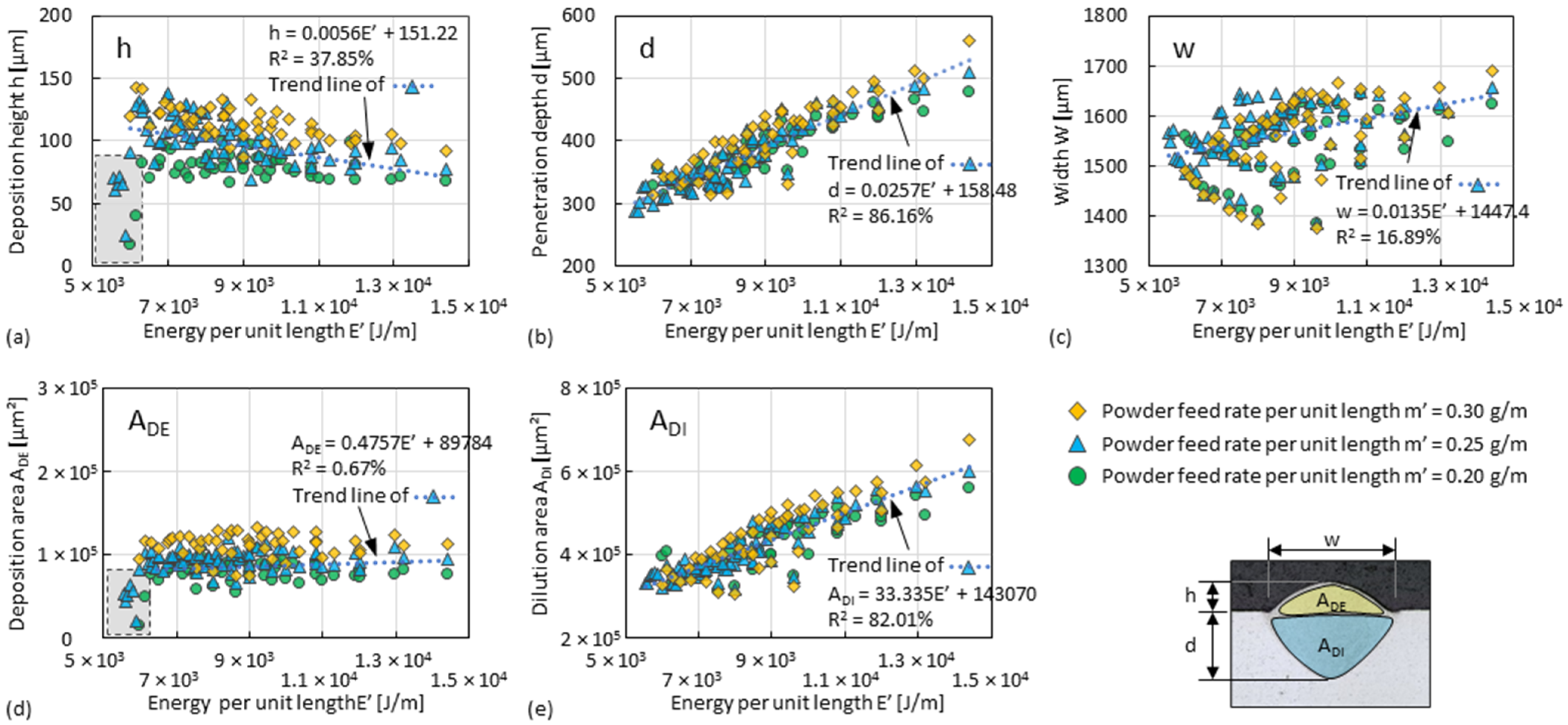
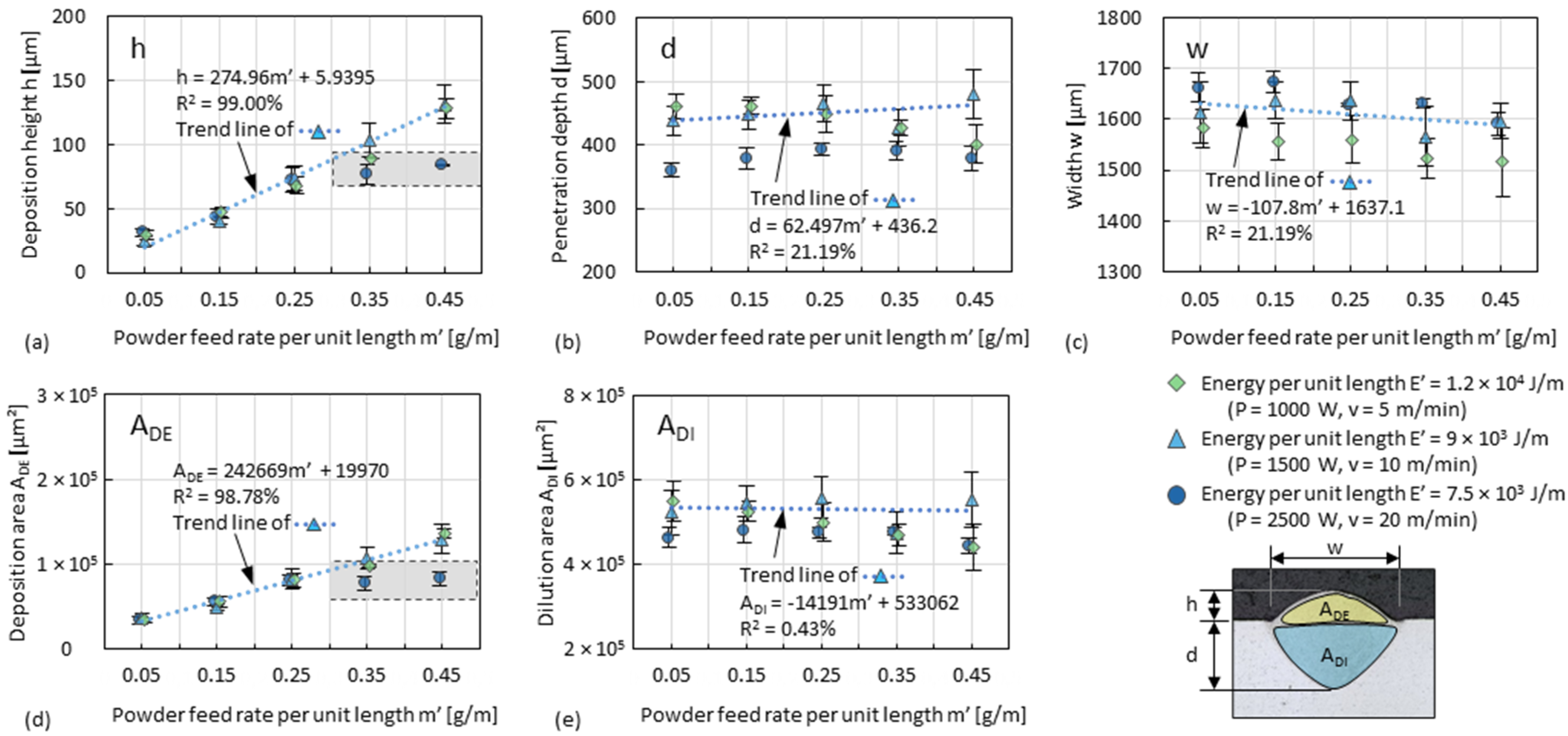
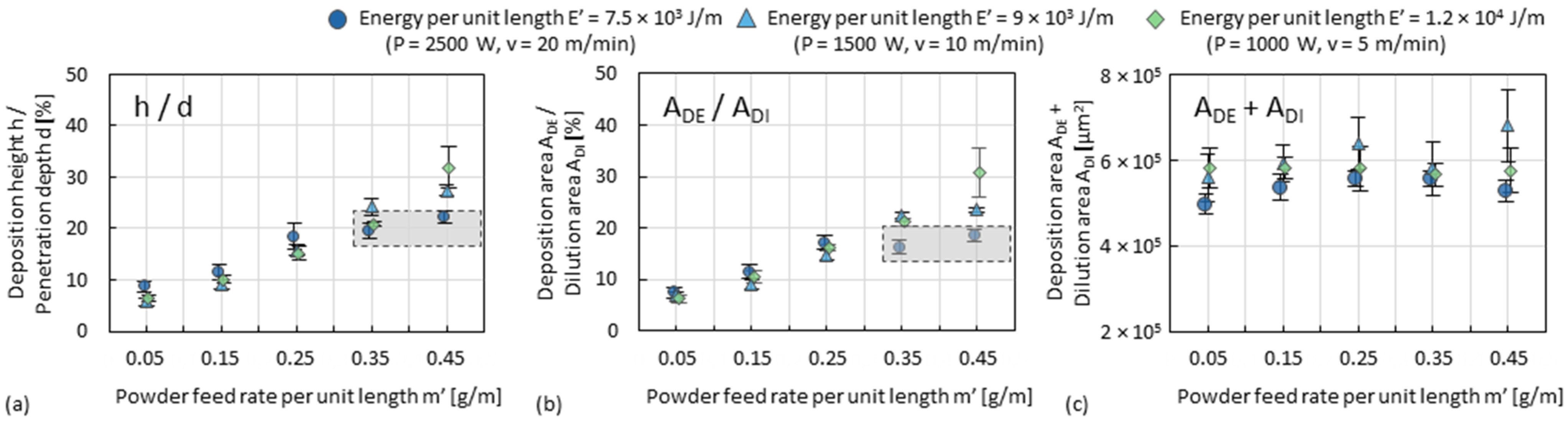
3.1. Dilution Zone of Single Tracks
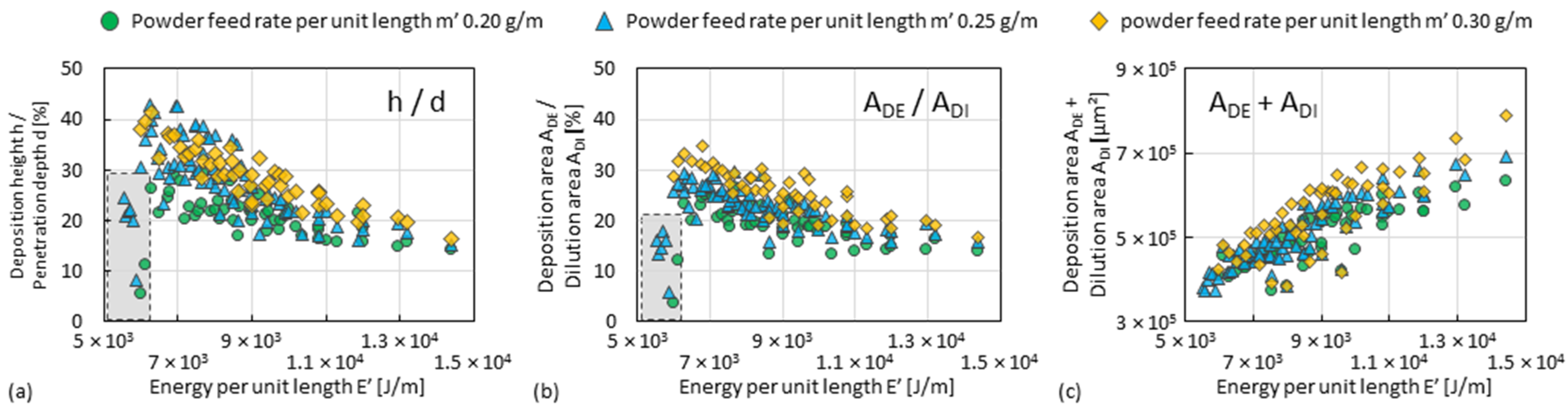
3.2. Deposition Zone of Single Tracks
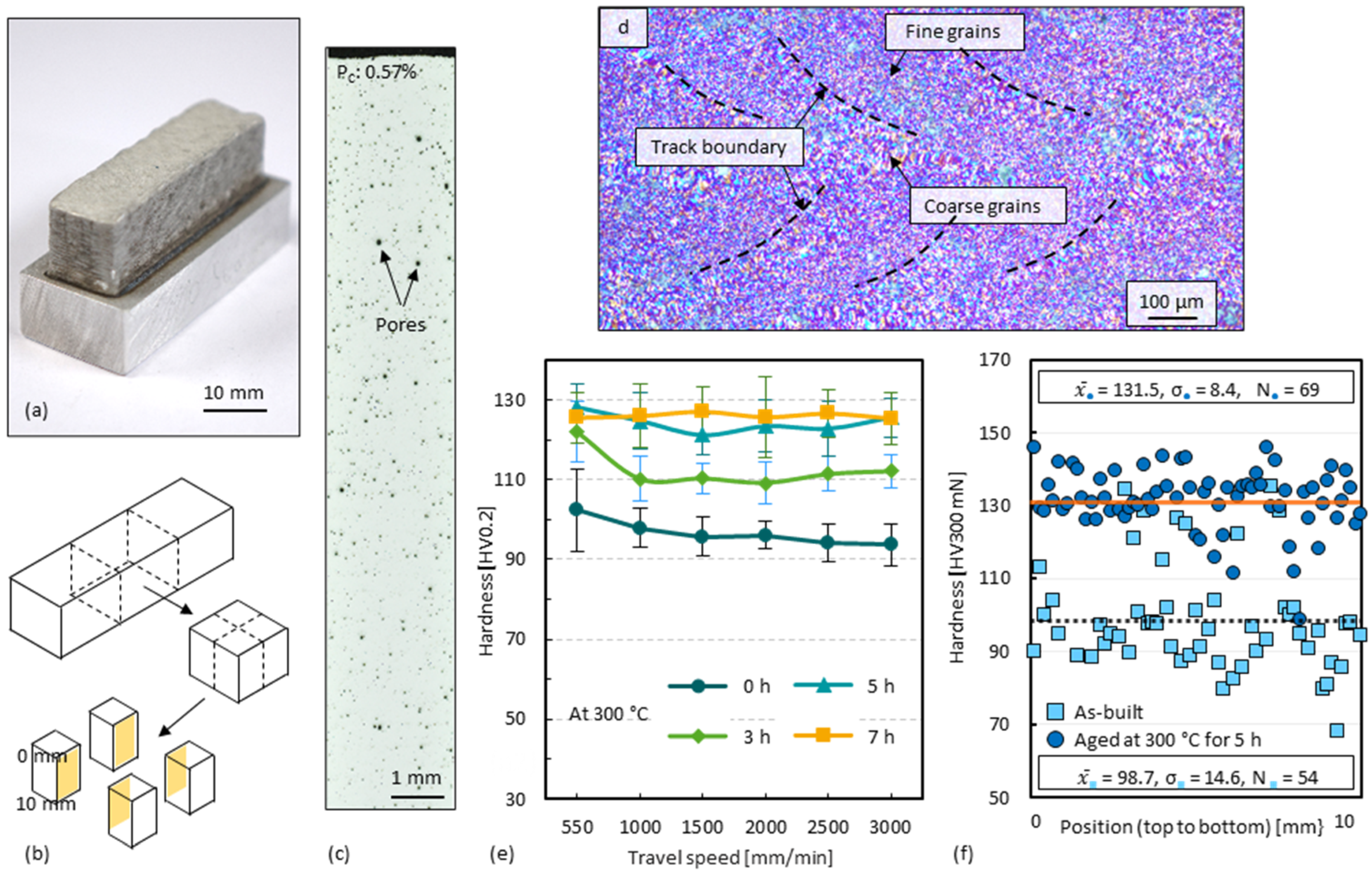
3.3. Ageing of 3D-Parts
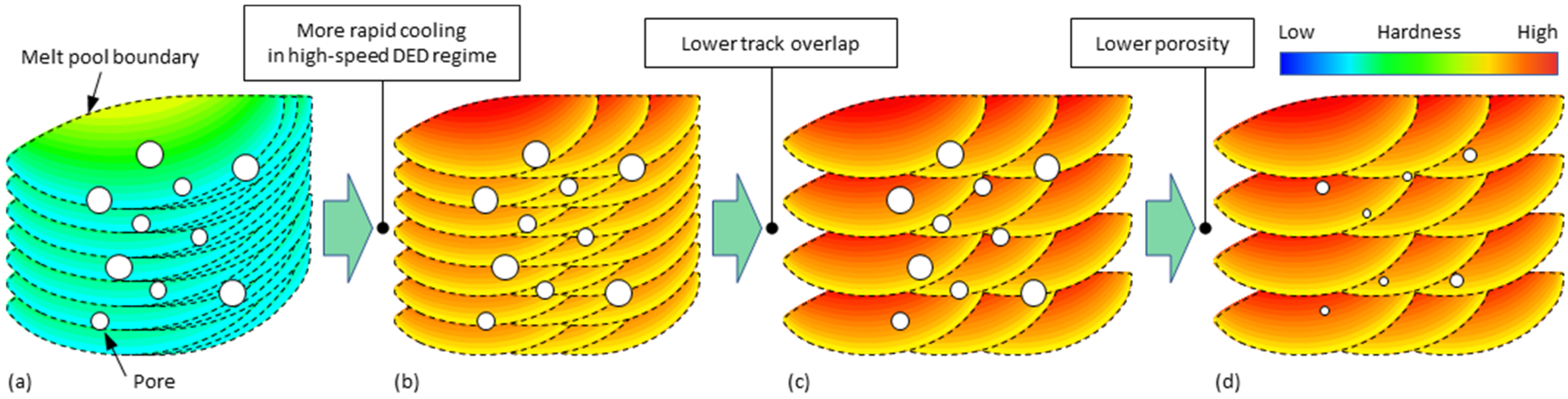
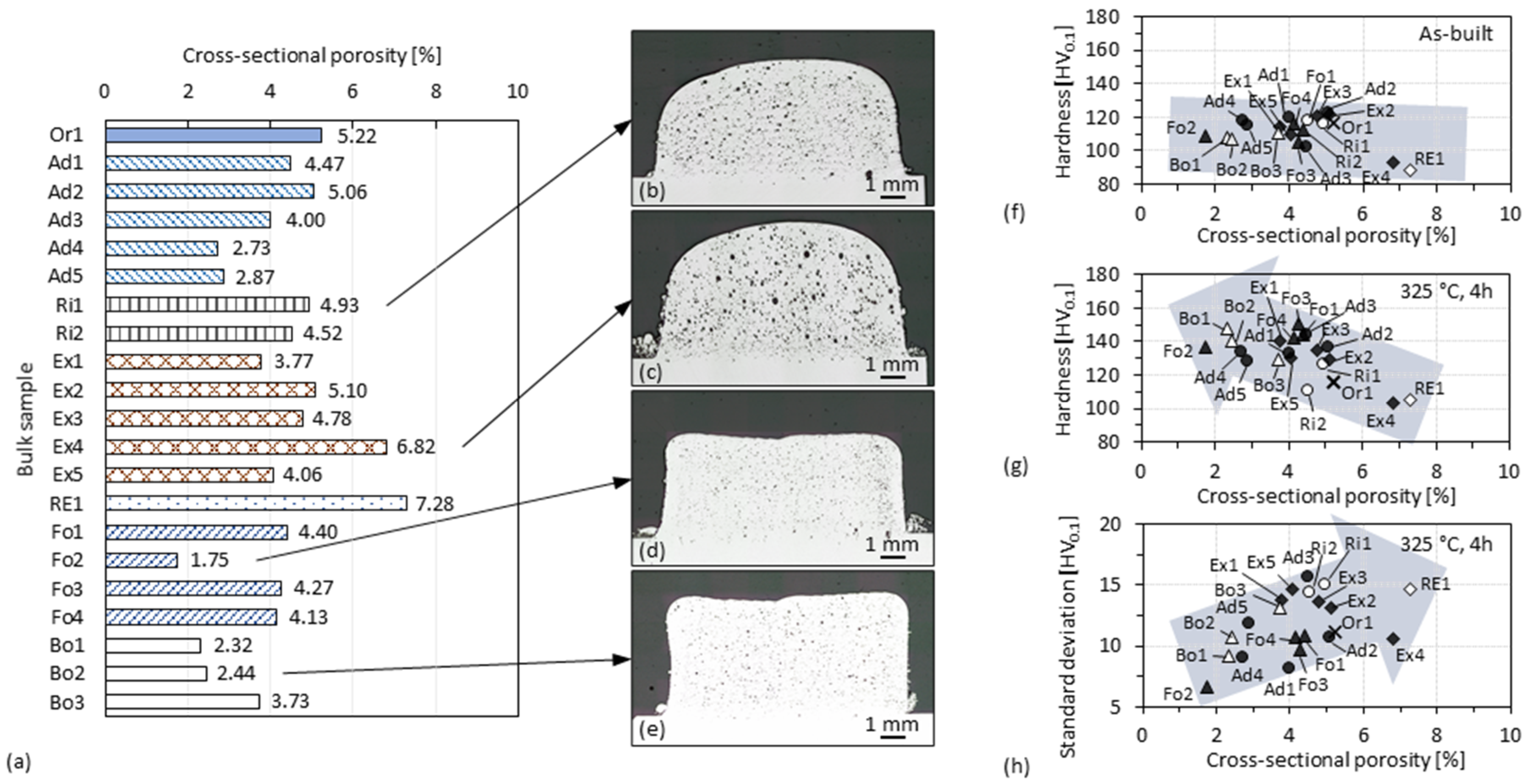
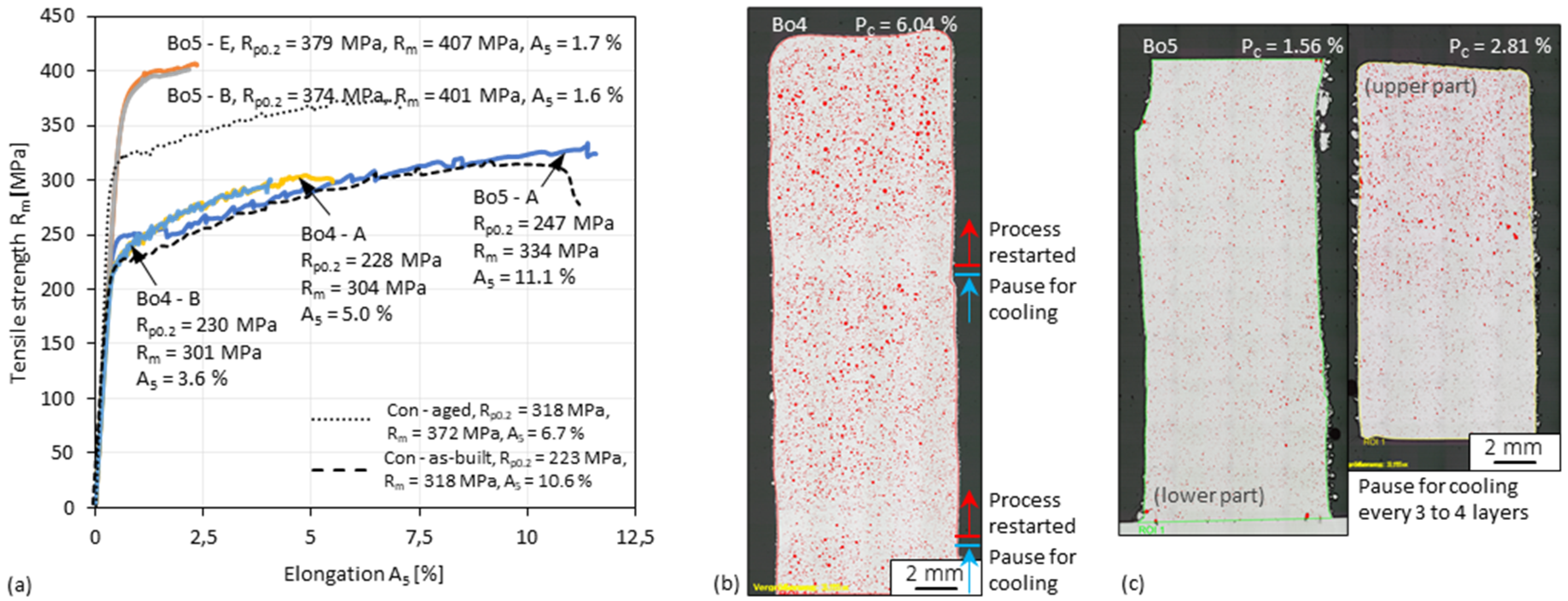
3.4. Cross-Sectional Porosity of 3D-Parts
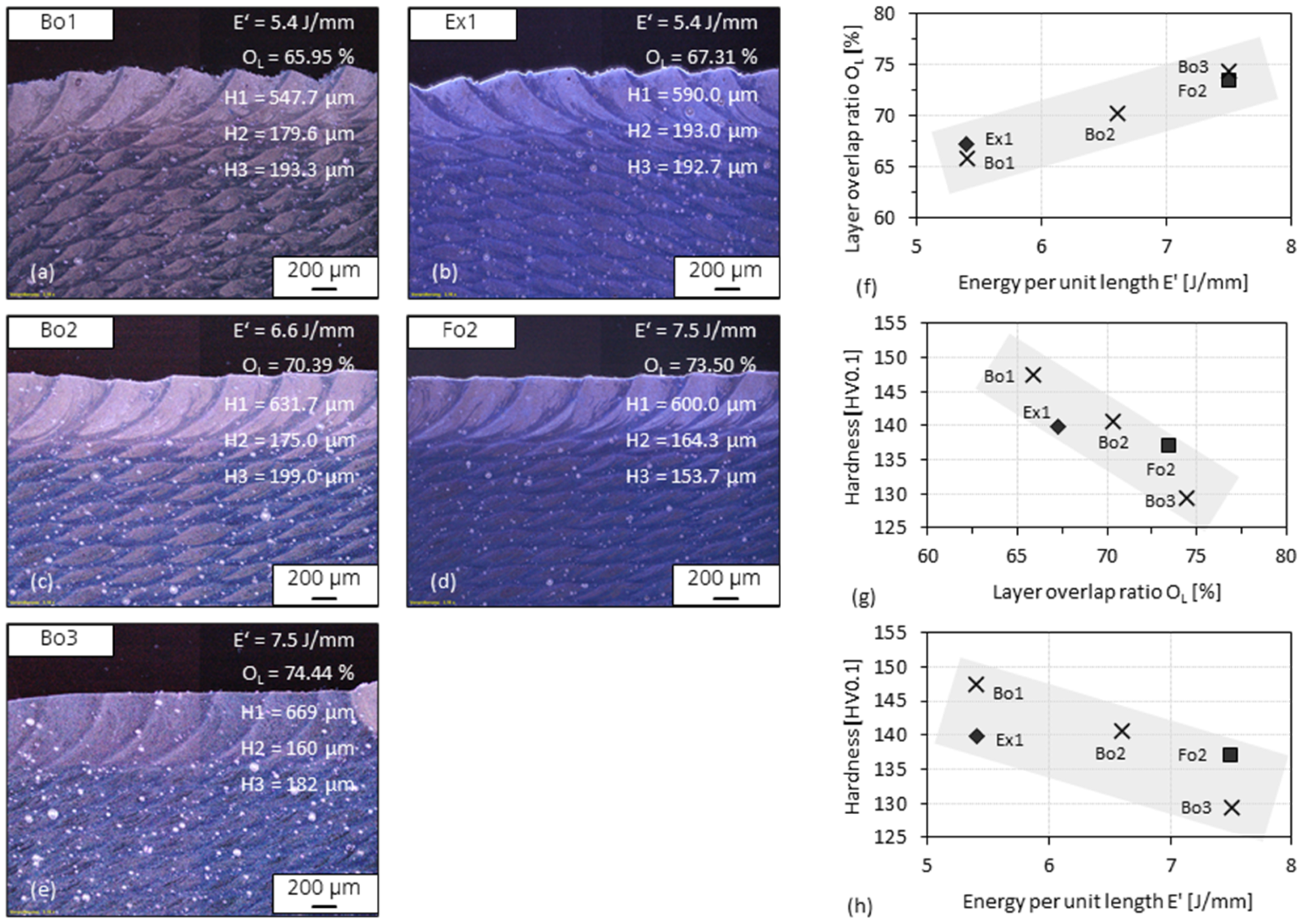
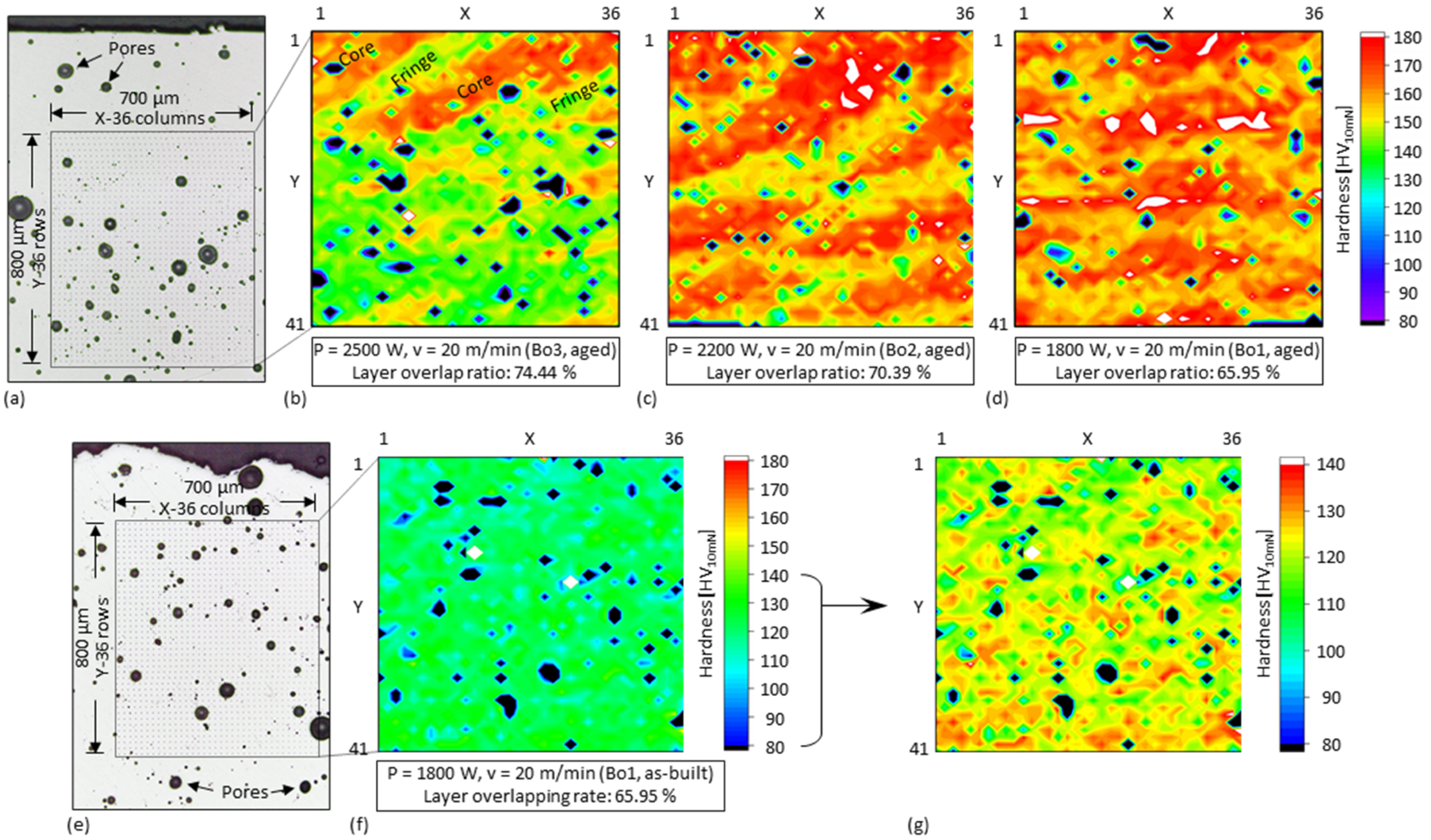
3.5. Layer Overlap Ratio of 3D-Parts
3.6. Double-Stage Ageing of 3D-Parts
3.7. Tensile Test of 3D-Parts
4. Discussion
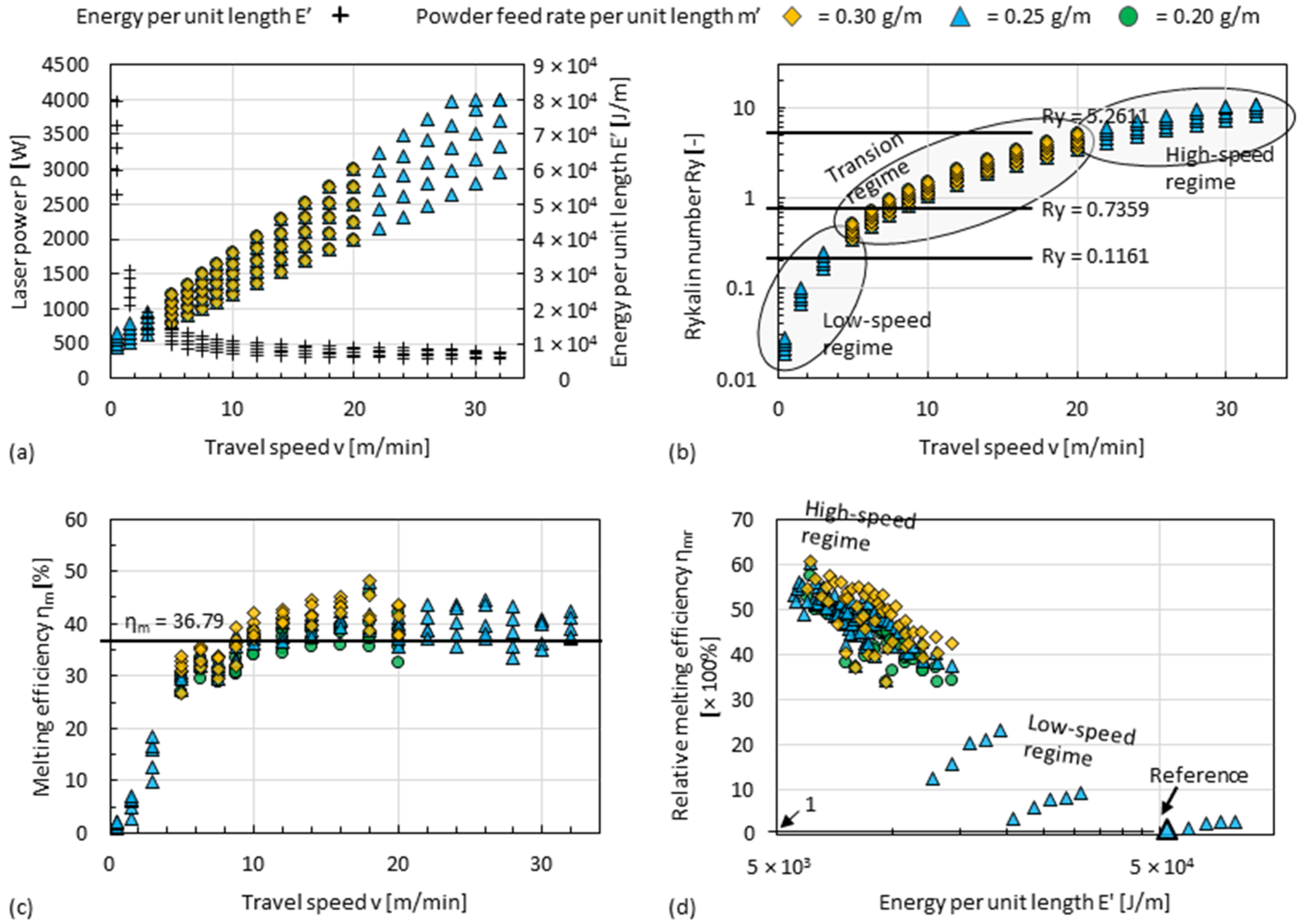
4.1. Process Regimes of DED
4.2. Ageing Response
5. Conclusions
- Cooling rates of Al alloys in high-speed regimes (travel speed ≥20 m/min) can theoretically reach up to about 2.34 × 105 K/s. This is an increase of 1–2 orders of magnitude compared to that of conventional DED, thus increasing the overall precipitation strengthening potential of every single track.
- Since single tracks of AlMgScZr alloys are observed to have soft fringes and hard cores on their cross-sections, decreasing the amount of remelted area when tracks overlap can increase the percentage of the hard zones. This can be achieved by decreasing the energy per unit length E’ (= Laser power P/travel speed v) and increasing the powder feed rate per unit length m’ (= powder feed rate ṁ/travel speed v), ultimately obtaining a high deposition-to-dilution ratio of 30%, which allows one to fabricate bulk 3D-parts having an average hardness of nearly 150 HV0.1 and a tensile strength of 407 MPa (with E’ = 5400 J/m, m’ = 0.25 g/m and ageing at 325 °C for 4 h).
- Concepts that have better cooling and Ar shielding conditions are proven effective, and these are beneficial for reducing the porosity and improving the mechanical performance of the material. Notwithstanding that the current experimental setup does not allow for more precise studies regarding the material oxidation during the high-speed DED process, the signs of trends observed in this work suggest there would be a fruitful area for further work.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Raw Data of Single Tracks
Appendix B. Specimens Fabricated with Conventional DED
References
- GDA Gesamtverband der Aluminiumindustrie e.V. Technische Merkblätter: W01 Der Werkstoff Aluminium, 6th ed.; Aluminum Deutschland e.V.: Dusseldorf, Germany, 2004. [Google Scholar]
- Knipling, K.E.; Dunand, D.C.; Seidman, D.N. Criteria for developing castable, creep-resistant aluminum-based alloys—A review. Z. Metallk. 2006, 97, 246–265. [Google Scholar] [CrossRef]
- Marquis, E.; Seidman, D. Nanoscale structural evolution of Al3Sc precipitates in Al(Sc) alloys. Acta Mater. 2001, 49, 1909–1919. [Google Scholar] [CrossRef]
- Toropova, L.; Eskin, D.; Kharakterova, M.; Dobatkina, T. Advanced Aluminum Alloys Containing Scandium; Routledge: London, UK, 2017. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Calderwood, F.W. The Alt-Sc (aluminum-scandium) system. Bull. Alloy Phase Diagr. 1989, 10, 34–36. [Google Scholar] [CrossRef]
- Murray, J.L. The Al-Sc (aluminum-scandium) system. J. Phase Equilibria Diffus. 1998, 19, 380–384. [Google Scholar] [CrossRef]
- Norman, A.; Prangnell, P.; McEwen, R. The solidification behaviour of dilute aluminium–scandium alloys. Acta Mater. 1998, 46, 5715–5732. [Google Scholar] [CrossRef]
- Yin, Z.; Pan, Q.; Zhang, Y.; Jiang, F. Effect of minor Sc and Zr on the microstructure and mechanical properties of Al–Mg based alloys. Mater. Sci. Eng. A 2000, 280, 151–155. [Google Scholar] [CrossRef]
- Fuller, C.B.; Seidman, D.N. Temporal evolution of the nanostructure of Al(Sc,Zr) alloys: Part II-coarsening of Al(ScZr) precipitates. Acta Mater. 2005, 53, 5415–5428. [Google Scholar] [CrossRef]
- Harada, Y.; Dunand, D. Microstructure of Al3Sc with ternary transition-metal additions. Mater. Sci. Eng. A 2002, 329–331, 686–695. [Google Scholar] [CrossRef]
- Riddle, Y.W.; Sanders, T.H. A study of coarsening, recrystallization, and morphology of microstructure in Al-Sc-(Zr)-(Mg) alloys. Met. Mater. Trans. A 2004, 35, 341–350. [Google Scholar] [CrossRef]
- Clouet, E.; Laé, L.; Epicier, T.; Lefebvre, W.; Nastar, M.; Deschamps, A. Complex precipitation pathways in multicomponent alloys. Nat. Mater. 2006, 5, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.L. The Al−Mg (Aluminum−Magnesium) system. Bull. Alloy Phase Diagr. 1982, 3, 60–74. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N.; Dunand, D.C. Effect of Mg addition on the creep and yield behavior of an Al–Sc alloy. Acta Mater. 2003, 51, 4751–4760. [Google Scholar] [CrossRef]
- Murray, J.; Peruzzi, A.; Abriata, J.P. The Al-Zr (aluminum-zirconium) system. Bull. Alloy Phase Diagr. 1992, 13, 277–291. [Google Scholar] [CrossRef]
- Marquis, E.A.; Seidman, D.N. Coarsening kinetics of nanoscale Al3Sc precipitates in an Al–Mg–Sc alloy. Acta Mater. 2005, 53, 4259–4268. [Google Scholar] [CrossRef]
- Xu, P.; Jiang, F.; Tang, Z.; Yan, N.; Jiang, J.; Xu, X.; Peng, Y. Coarsening of Al3Sc precipitates in Al-Mg-Sc alloys. J. Alloys Compd. 2018, 781, 209–215. [Google Scholar] [CrossRef]
- Costa, S.; Puga, H.; Barbosa, J.; Pinto, A. The effect of Sc additions on the microstructure and age hardening behaviour of as cast Al–Sc alloys. Mater. Des. 2012, 42, 347–352. [Google Scholar] [CrossRef]
- Davydov, V.; Rostova, T.; Zakharov, V.; Filatov, Y.; Yelagin, V. Scientific principles of making an alloying addition of scandium to aluminium alloys. Mater. Sci. Eng. A 2000, 280, 30–36. [Google Scholar] [CrossRef]
- Lohar, A.; Mondal, B.; Panigrahi, S. Influence of cooling rate on the microstructure and ageing behavior of as-cast Al–Sc–Zr alloy. J. Mater. Process. Technol. 2010, 210, 2135–2141. [Google Scholar] [CrossRef]
- Taendl, J.; Orthacker, A.; Amenitsch, H.; Kothleitner, G.; Poletti, C. Influence of the degree of scandium supersaturation on the precipitation kinetics of rapidly solidified Al-Mg-Sc-Zr alloys. Acta Mater. 2016, 117, 43–50. [Google Scholar] [CrossRef]
- Deane, K.; Kampe, S.L.; Swenson, D.; Sanders, P.G. Precipitate Evolution and Strengthening in Supersaturated Rapidly Solidified Al-Sc-Zr Alloys. Met. Mater. Trans. A 2017, 48, 2030–2039. [Google Scholar] [CrossRef]
- Parker, B.A.; Zhou, Z.F.; Nolle, P. The effect of small additions of scandium on the properties of aluminium alloys. J. Mater. Sci. 1995, 30, 452–458. [Google Scholar] [CrossRef]
- Kendig, K.L.; Miracle, D.B. Strengthening mechanisms of an Al-Mg-Sc-Zr alloy. Acta Mater. 2002, 50, 4165–4175. [Google Scholar] [CrossRef]
- Meiners, W.; Wissenbach, K.; Gasser, A. Selective Laser Sintering at Melting Temperature. U.S. Patent 6215093B1, 1997. [Google Scholar]
- Buchbinder, D.; Meiners, W.; Brandl, E.; Palm, F.; Müller-Lohmeier, K.; Wolter, M.; Over, C.; Moll, W.; Weber, J.; Skrynecki, N.; et al. Generative Fertigung von Aluminiumbauteilen für die Serienproduktion-AluGenerativ. Abschlussbericht (BMBF 01RI0639A-D, 02.2007–01.2010). 2010. Available online: http://edok01.tib.uni-hannover.de/edoks/e01fb11/667761012.pdf (accessed on 23 November 2022).
- Schmidtke, K.; Palm, F.; Hawkins, A.; Emmelmann, C. Process and Mechanical Properties: Applicability of a Scandium modified Al-alloy for Laser Additive Manufacturing. Phys. Procedia 2011, 12, 369–374. [Google Scholar] [CrossRef]
- Spierings, A.B.; Dawson, K.; Heeling, T.; Uggowitzer, P.J.; Schäublin, R.; Palm, F.; Wegener, K. Microstructural features of Sc- and Zr-modified Al-Mg alloys processed by selective laser melting. Mater. Des. 2017, 115, 52–63. [Google Scholar] [CrossRef]
- Spierings, A.; Dawson, K.; Kern, K.; Palm, F.; Wegener, K. SLM-processed Sc- and Zr- modified Al-Mg alloy: Mechanical properties and microstructural effects of heat treatment. Mater. Sci. Eng. A 2017, 701, 264–273. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, F.; Rometsch, P.; Li, J.; Mata, J.; Weyland, M.; Bourgeois, L.; Sui, M.; Wu, X. Precipitation kinetics, microstructure evolution and mechanical behavior of a developed Al–Mn–Sc alloy fabricated by selective laser melting. Acta Mater. 2020, 193, 239–251. [Google Scholar] [CrossRef]
- Li, Y.; Gu, D. Parametric analysis of thermal behavior during selective laser melting additive manufacturing of aluminum alloy powder. Mater. Des. 2014, 63, 856–867. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, D.; Yang, J.; Dai, D.; Zhao, T.; Hong, C.; Gasser, A.; Poprawe, R. Selective laser melting of rare earth element Sc modified aluminum alloy: Thermodynamics of precipitation behavior and its influence on mechanical properties. Addit. Manuf. 2018, 23, 1–12. [Google Scholar] [CrossRef]
- Gasser, A.; Backes, G.; Kelbassa, I.; Weisheit, A.; Wissenbach, K. Laser Additive Manufacturing. Laser Tech. J. 2010, 7, 58–63. [Google Scholar] [CrossRef]
- Gu, D.; Shi, X.; Poprawe, R.; Bourell, D.L.; Setchi, R.; Zhu, J. Material-structure-performance integrated laser-metal additive manufacturing. Science 2021, 372. [Google Scholar] [CrossRef]
- Javidani, M.; Arreguin-Zavala, J.; Danovitch, J.; Tian, Y.; Brochu, M. Additive Manufacturing of AlSi10Mg Alloy Using Direct Energy Deposition: Microstructure and Hardness Characterization. J. Therm. Spray Technol. 2016, 26, 587–597. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Z.; Zeng, X. A comparison on metallurgical behaviors of 316L stainless steel by selective laser melting and laser cladding deposition. Mater. Sci. Eng. A 2017, 685, 265–273. [Google Scholar] [CrossRef]
- Dinda, G.; Dasgupta, A.; Mazumder, J. Evolution of microstructure in laser deposited Al–11.28%Si alloy. Surf. Coatings Technol. 2012, 206, 2152–2160. [Google Scholar] [CrossRef]
- Awd, M.; Tenkamp, J.; Hirtler, M.; Siddique, S.; Bambach, M.; Walther, F. Comparison of Microstructure and Mechanical Properties of Scalmalloy® Produced by Selective Laser Melting and Laser Metal Deposition. Materials 2018, 11, 17. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, X.; Tang, Y.; Kang, N.; Gao, X.; Shi, S.; Huang, W. Laser-based directed energy deposition of novel Sc/Zr-modified Al-Mg alloys: Columnar-to-equiaxed transition and aging hardening behavior. J. Mater. Sci. Technol. 2020, 69, 168–179. [Google Scholar] [CrossRef]
- Zhao, T.; Dahmen, M.; Cai, W.; Alkhayat, M.; Schaible, J.; Albus, P.; Zhong, C.; Hong, C.; Biermann, T.; Zhang, H.; et al. Laser metal deposition for additive manufacturing of AA5024 and nanoparticulate TiC modified AA5024 alloy composites prepared with balling milling process. Opt. Laser Technol. 2020, 131, 106438. [Google Scholar] [CrossRef]
- Zhao, T.; Cai, W.; Dahmen, M.; Schaible, J.; Hong, C.; Gasser, A.; Weisheit, A.; Biermann, T.; Kelbassa, I.; Zhang, H.; et al. Ageing response of an Al-Mg-Mn-Sc-Zr alloy processed by laser metal deposition in thin-wall structures. Vacuum 2018, 158, 121–125. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Y.; Xu, T.; Bakir, M.; Cai, W.; Wang, M.; Dahmen, M.; Zheng, Q.; Wei, X.; Hong, C.; et al. Some factors affecting porosity in directed energy deposition of AlMgScZr-alloys. Opt. Laser Technol. 2021, 143, 107337. [Google Scholar] [CrossRef]
- Rosenthal, D. The theory of moving sources of heat and its application of metal treatments. Trans. ASME 1946, 68, 859–866. [Google Scholar]
- Narender, K.; Rao, A.S.M.; Rao, K.G.K.; Krishna, N.G. Temperature Dependent Density and Thermal Expansion of Wrought Aluminum Alloys 5070, 5083 and 5483 by Gamma Ray Attenuation Technique. J. Adv. Phys. 2014, 3, 23–28. [Google Scholar] [CrossRef]
- Bergström, D.; Powell, J.; Kaplan, A.F.H. Absorptance of nonferrous alloys to Nd:YLF and Nd:YAG laser light at room temperature. Appl. Opt. 2007, 46, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Elagin, V.I.; Zakharov, V.V.; Rostova, T.D. Some features of decomposition for the solid solution of scandium in aluminum. Met. Sci. Heat Treat. 1983, 25, 546–549. [Google Scholar] [CrossRef]
- Kaiser, M.; Datta, S.; Roychowdhury, A.; Banerjee, M.K. Effect of scandium on the microstructure and ageing behaviour of cast Al–6Mg alloy. Mater. Charact. 2008, 59, 1661–1666. [Google Scholar] [CrossRef]
- Koutny, D.; Skulina, D.; Pantelejev, L.; Paloušek, D.; Lenczowski, B.; Palm, F.; Nick, A. Processing of Al-Sc aluminum alloy using SLM technology. Procedia CIRP 2018, 74, 44–48. [Google Scholar] [CrossRef]
- Starink, M.; Zahra, A.-M. β′ and β precipitation in an Al–Mg alloy studied by DSC and TEM. Acta Mater. 1998, 46, 3381–3397. [Google Scholar] [CrossRef]
- Nebti, S.; Hamana, D.; Cizeron, G. Calorimetric study of pre-precipitation and precipitation in Al-Mg alloy. Acta Metall. Mater. 1995, 43, 3583–3588. [Google Scholar] [CrossRef]
- Predel, B. Al-Mg (Aluminum-Magnesium). In Ac-Ag … Au-Zr; Martienssen, W., Predel, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–5. [Google Scholar] [CrossRef]
- Kurnsteiner, P.; Bajaj, P.; Gupta, A.; Benjamin, W.; Weisheit, A.; Li, X.; Leinebach, C.; Gault, B.; Jagle, E.; Raabe, D. Control of thermally stable core-shell nano-precipitates in additively manufactured Al-Sc-Zr alloys. Addit. Manuf. 2020, 32, 100910. [Google Scholar] [CrossRef]
- Deillon, L.; Jensch, F.; Palm, F.; Bambach, M. A new high strength Al–Mg–Sc alloy for laser powder bed fusion with calcium addition to effectively prevent magnesium evaporation. J. Mater. Process. Technol. 2021, 300, 117416. [Google Scholar] [CrossRef]
- Mendez, P.F.; Lu, Y.; Wang, Y. Scaling Analysis of a Moving Point Heat Source in Steady-State on a Semi-Infinite Solid. J. Heat Transf. 2018, 140. [Google Scholar] [CrossRef]
- Fuerschbach, P.W.; Eisler, G.R. Determination of Material Properties for Welding Models by Means of Arc Weld Experiments. In Proceedings of the 6th International Trends in Welding Research, Phoenix, AZ, USA, 15–19 April 2002. [Google Scholar]
- Wang, Y.; Lu, Y.; Mendez, P.F. Scaling expressions of characteristic values for a moving point heat source in steady state on a semi-infinite solid. Int. J. Heat Mass Transf. 2019, 135, 1118–1129. [Google Scholar] [CrossRef]
- Pirch, N.; Linnenbrink, S.; Gasser, A.; Schleifenbaum, H. Laser-aided directed energy deposition of metal powder along edges. Int. J. Heat Mass Transf. 2019, 143. [Google Scholar] [CrossRef]
- Pirch, N.; Linnenbrink, S.; Gasser, A.; Wissenbach, K.; Poprawe, R. Analysis of track formation during laser metal deposition. J. Laser Appl. 2017, 29, 022506. [Google Scholar] [CrossRef]
- Tabor, D. Indentation hardness: Fifty years on a personal view. Philos. Mag. A 1996, 74, 1207–1212. [Google Scholar] [CrossRef]





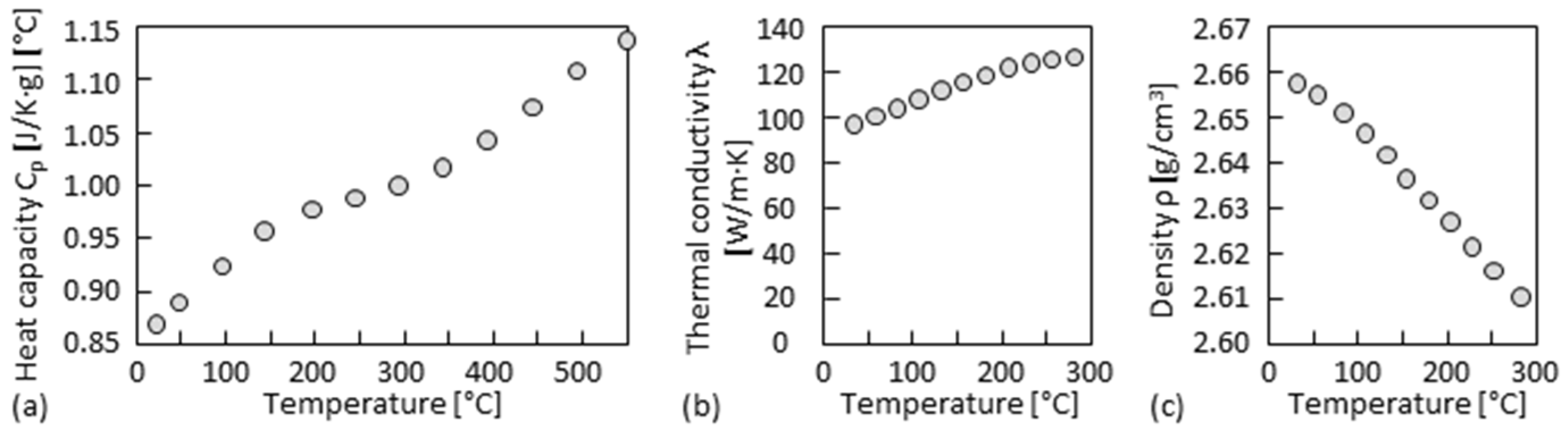
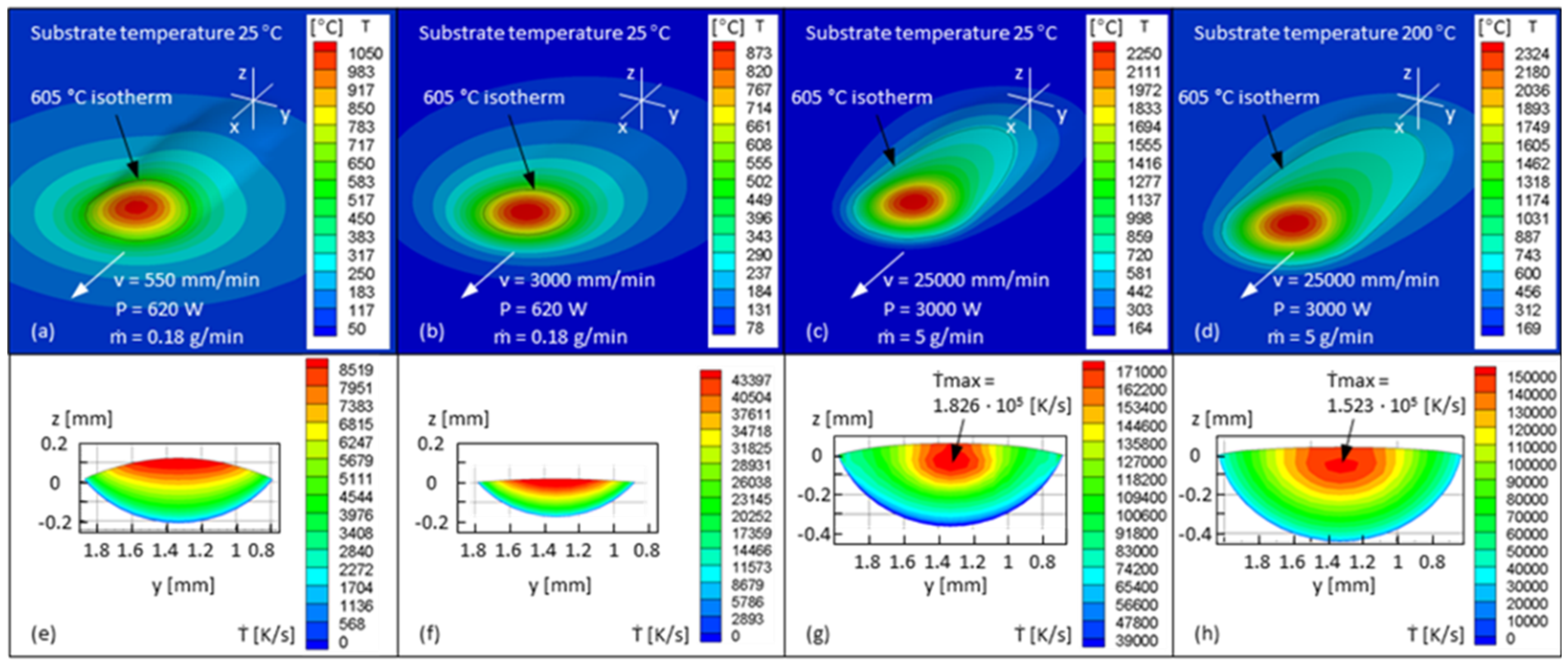
| Sample | Shielding and Exhausting Measure | P [W] | v [m/min] | Vs [L/min] | Vad [L/min] |
|---|---|---|---|---|---|
| Or1 | Nozzle’s original shielding | 2500 | 20 | 10 | - |
| Ad1 | With an additional shielding gas flow | 1800 | 20 | 20 | 10 |
| Ad2 | 2500 | 20 | 20 | 10 | |
| Ad3 | 2500 | 25 | 20 | 10 | |
| Ad4 | 3000 | 25 | 20 | 10 | |
| Ad5 | 3500 | 25 | 20 | 10 | |
| Ri1 | With a shielding sleeve | 2500 | 20 | 10 | - |
| Ri2 | 2500 | 20 | 10 | - | |
| Ex1 | With an active smoke exhaust flue | 1800 | 20 | 10 | - |
| Ex2 | 2500 | 20 | 10 | - | |
| Ex3 | 2500 | 20 | 10 | - | |
| Ex4 | 2500 | 20 | 10 | - | |
| Ex5 | 2500 | 20 | 10 | - | |
| RE1 | With a shielding sleeve | 2500 | 20 | 10 | - |
| and an active smoke | |||||
| Exhaust flue | |||||
| Fo1 | Covered with an Al curtain filled with Ar | 1800 | 20 | 10 | - |
| Fo2 | 2500 | 20 | 10 | - | |
| Fo3 | 1800 | 20 | 10 | - | |
| Fo4 | 2500 | 20 | 20 | - | |
| Bo1 | Covered with a protective box filled with Ar | 1800 | 20 | 10 | - |
| Bo2 | 2200 | 20 | 10 | - | |
| Bo3 | 2500 | 20 | 10 | - | |
| Bo4 | 1800 | 20 | 10 | - | |
| Bo5 | 1800 | 20 | 10 | - |
| Material | Process | Optimum Heat Treatment | Best Mechanical Properties Achieved | Source |
|---|---|---|---|---|
| Al–6Mg–0.2Sc | Casting | 1 h at 300 °C | Hardness: 69 HV5 | [48] |
| Al–6Mg–0.4Sc | Hardness: 76 HV5 | |||
| Al–6Mg–0.6Sc | Hardness: 81 HV5 | |||
| Al–4Mg–0.4Sc–0.12Zr | Casting | 3 h at 325 °C | Hardness: 95 HV0.1 | [22] |
| & electron beam | Hardness: 110 HV0.1 | |||
| Al–4.5Mg–0.66Sc–0.37Zr | LPBF | 4 h at 325 °C | Hardness: 177 HV0.3 | [28] |
| UTS: 530 MPa, A: 14 % | ||||
| Al–4.6Mg–0.66Sc–0.42Zr | LPBF | 4 h at 400 °C | Hardness: 137 HB | [30] |
| 5 h at 325 °C | Hardness: 145 HB | |||
| 4 h at 325 °C – 350 °C | UTS: 515 MPa (vertically built) | |||
| UTS: 530 MPa (horizontally built) | ||||
| Al–2.9Mg–1.11Sc–0.42Zr | LPBF | 1 h at 540 °C, water quenching, | UTS = 541.3 MPa, A: 0.7% | [49] |
| then 2 h at 325 °C | ||||
| Al–4.2Mg–0.4Sc–0.2Zr | DED | 5 h at 300 °C | Hardness: 129 HV0.2 | [41] |
| UTS: 347 MPa, A: 5.1% | ||||
| Al–4.82Mg–0.25Sc–0.12Zr | DED | 4 h at 325 °C | Hardness: 97.9 HV0.2 | [40] |
| Al–4.87Mg–0.35Sc–0.16Zr | Hardness: 100 HV0.2 | |||
| Al–4.86Mg–0.50Sc–0.21Zr | Hardness: 105 HV0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Chen, T.; Wang, Y.; Wang, M.; Bakir, M.; Dahmen, M.; Cai, W.; Hong, C.; Schopphoven, T.; Pirch, N.; et al. Laser Directed Energy Deposition of an AlMgScZr-Alloy in High-Speed Process Regimes. Materials 2022, 15, 8951. https://doi.org/10.3390/ma15248951
Zhao T, Chen T, Wang Y, Wang M, Bakir M, Dahmen M, Cai W, Hong C, Schopphoven T, Pirch N, et al. Laser Directed Energy Deposition of an AlMgScZr-Alloy in High-Speed Process Regimes. Materials. 2022; 15(24):8951. https://doi.org/10.3390/ma15248951
Chicago/Turabian StyleZhao, Tong, Teng Chen, Yuhan Wang, Mengjie Wang, Maha Bakir, Marius Dahmen, Wangcan Cai, Chen Hong, Thomas Schopphoven, Norbert Pirch, and et al. 2022. "Laser Directed Energy Deposition of an AlMgScZr-Alloy in High-Speed Process Regimes" Materials 15, no. 24: 8951. https://doi.org/10.3390/ma15248951
APA StyleZhao, T., Chen, T., Wang, Y., Wang, M., Bakir, M., Dahmen, M., Cai, W., Hong, C., Schopphoven, T., Pirch, N., Brucki, M., Gasser, A., & Häfner, C. L. (2022). Laser Directed Energy Deposition of an AlMgScZr-Alloy in High-Speed Process Regimes. Materials, 15(24), 8951. https://doi.org/10.3390/ma15248951







