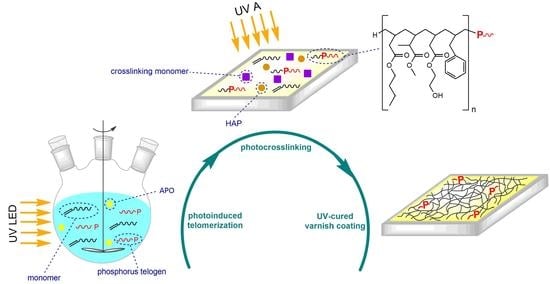
Phosphorus-Containing Telomers as UV-Curable Binders of Solvent-Free Varnish Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- n-butyl acrylate (BA; BASF, Ludwigshafen, Germany),
- methyl methacrylate (MMA; Sigma Aldrich, Steinheim, Germany),
- 2-hydroxyethyl acrylate (HEA; Across Organics, Geel, Belgium),
- styrene (STY; Sigma Aldrich, Steinheim, Germany).
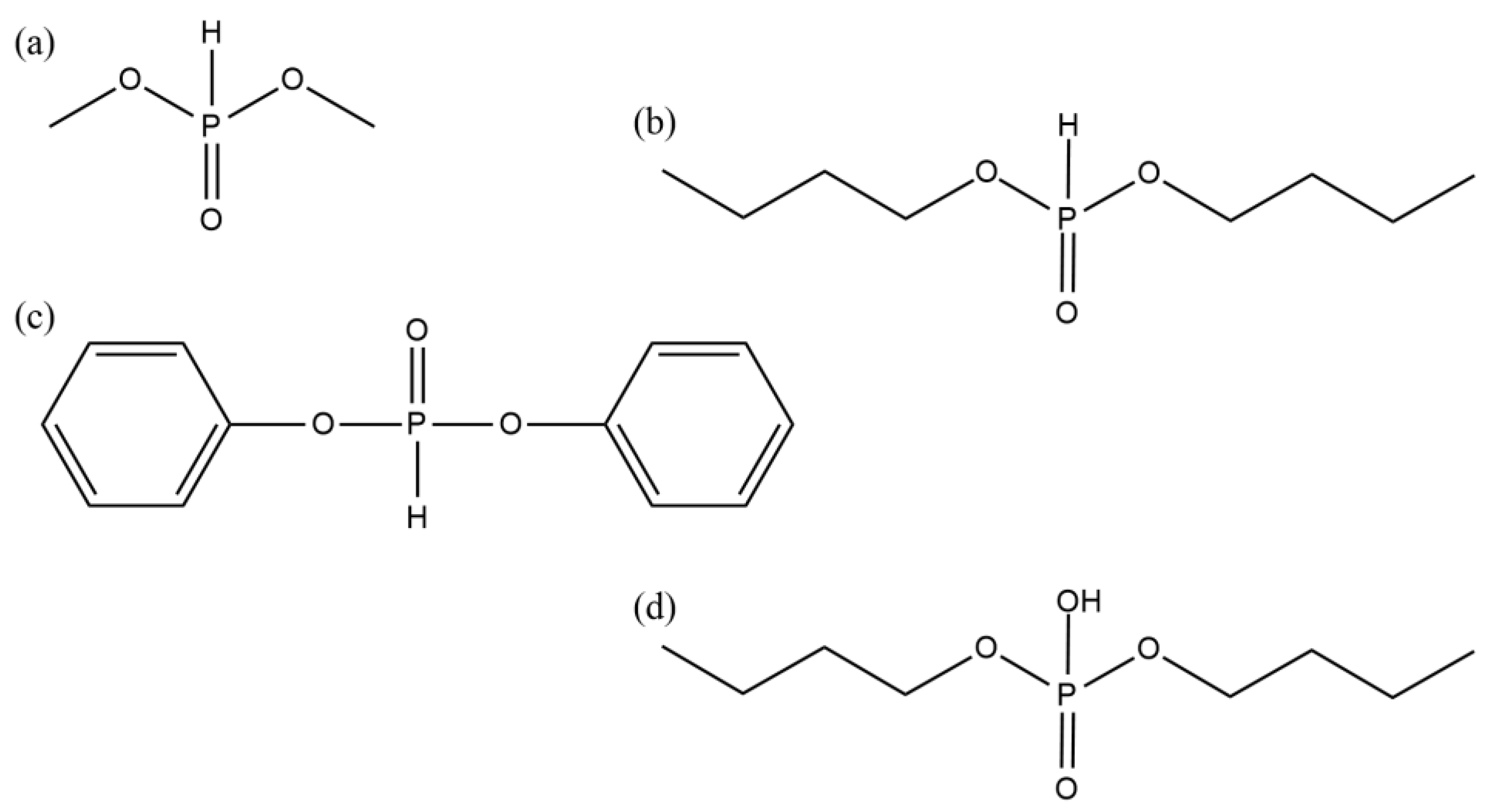
- dimethyl phosphite (DMPh; Sigma Aldrich, Steinheim, Germany),
- dibutyl phosphite (DBPh; Sigma Aldrich, Steinheim, Germany),
- diphenyl phosphite (DPPh; Sigma Aldrich, Steinheim, Germany),
- dibutyl phosphate (DBP; Sigma Aldrich, Steinheim, Germany).
2.2. P-Telomers Syrups and Coatings Preparation
2.3. Characterization of P-Telomers Syrups
2.4. Characterization of the Varnishes
3. Results and Discussion
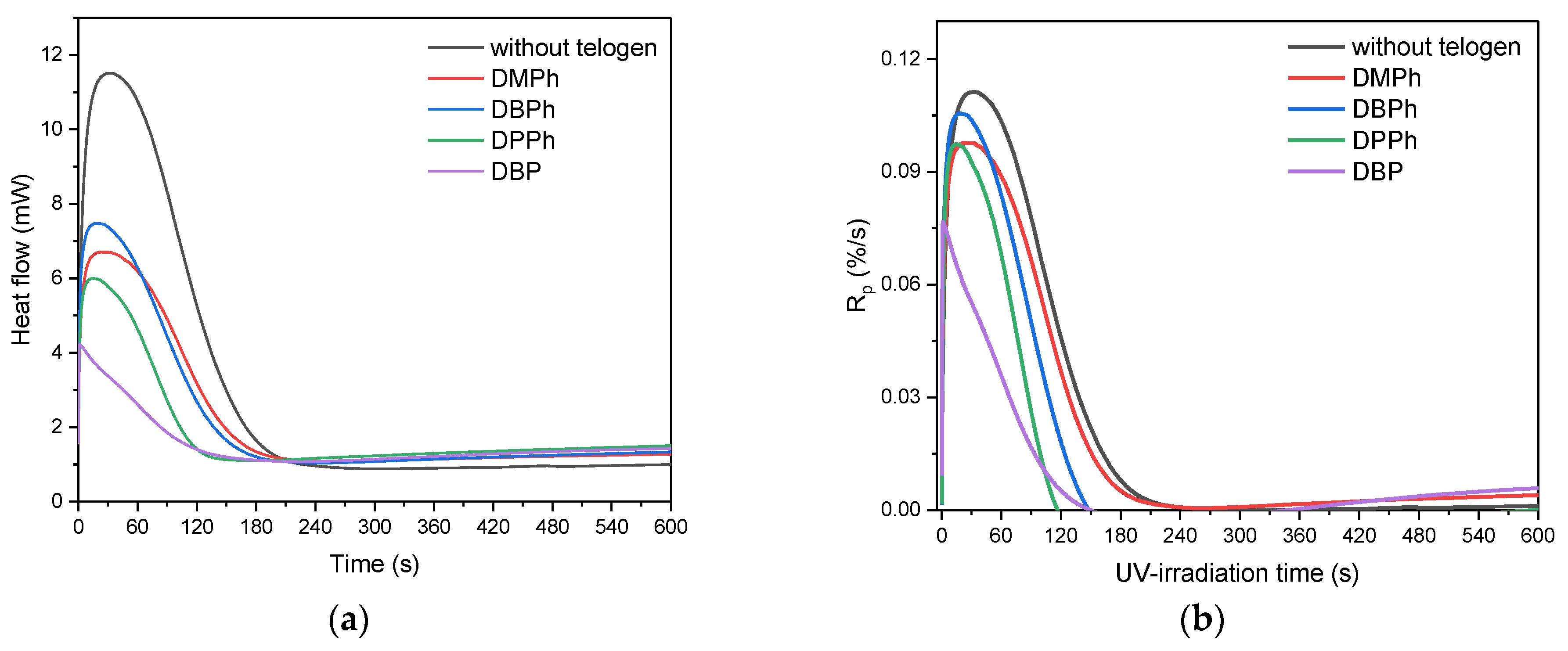
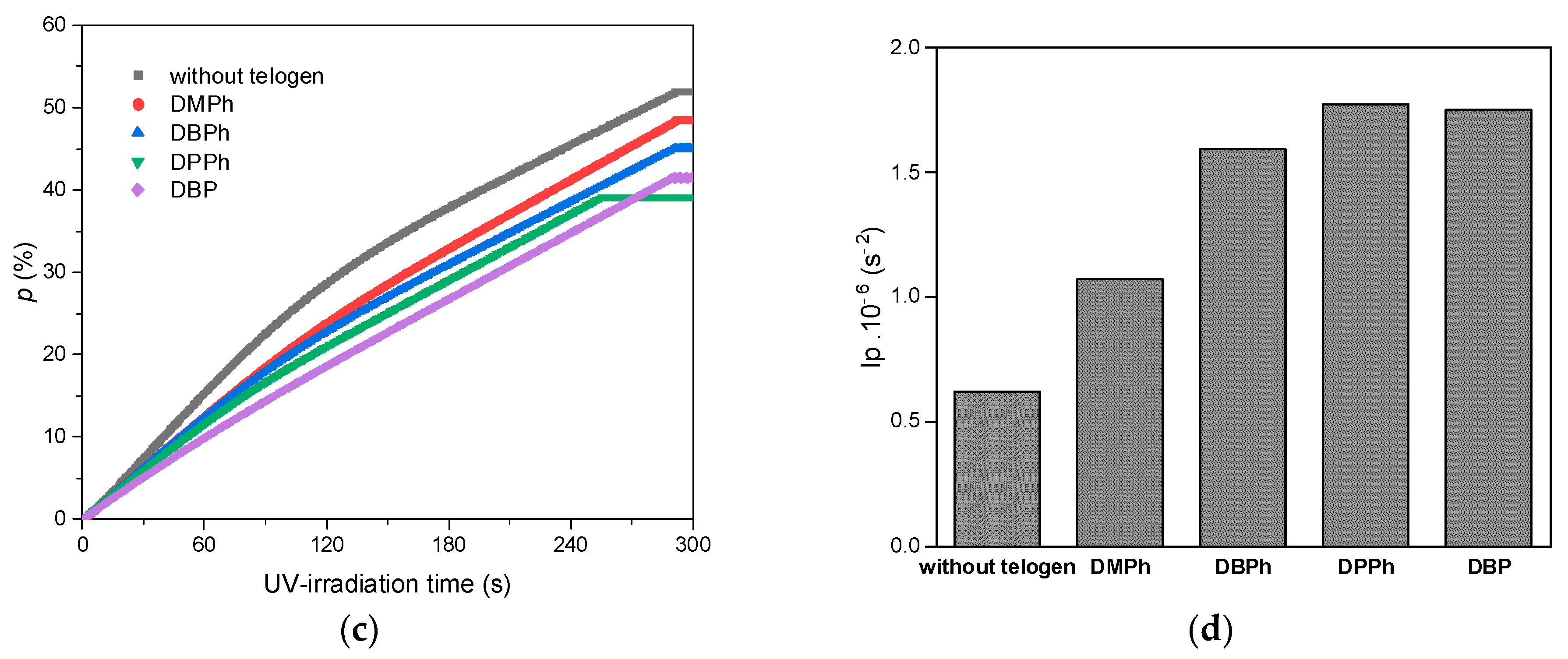
3.1. Kinetics of UV-Phototelomerization Process
3.2. Properties of P-Telomers and P-Telomers Syrups
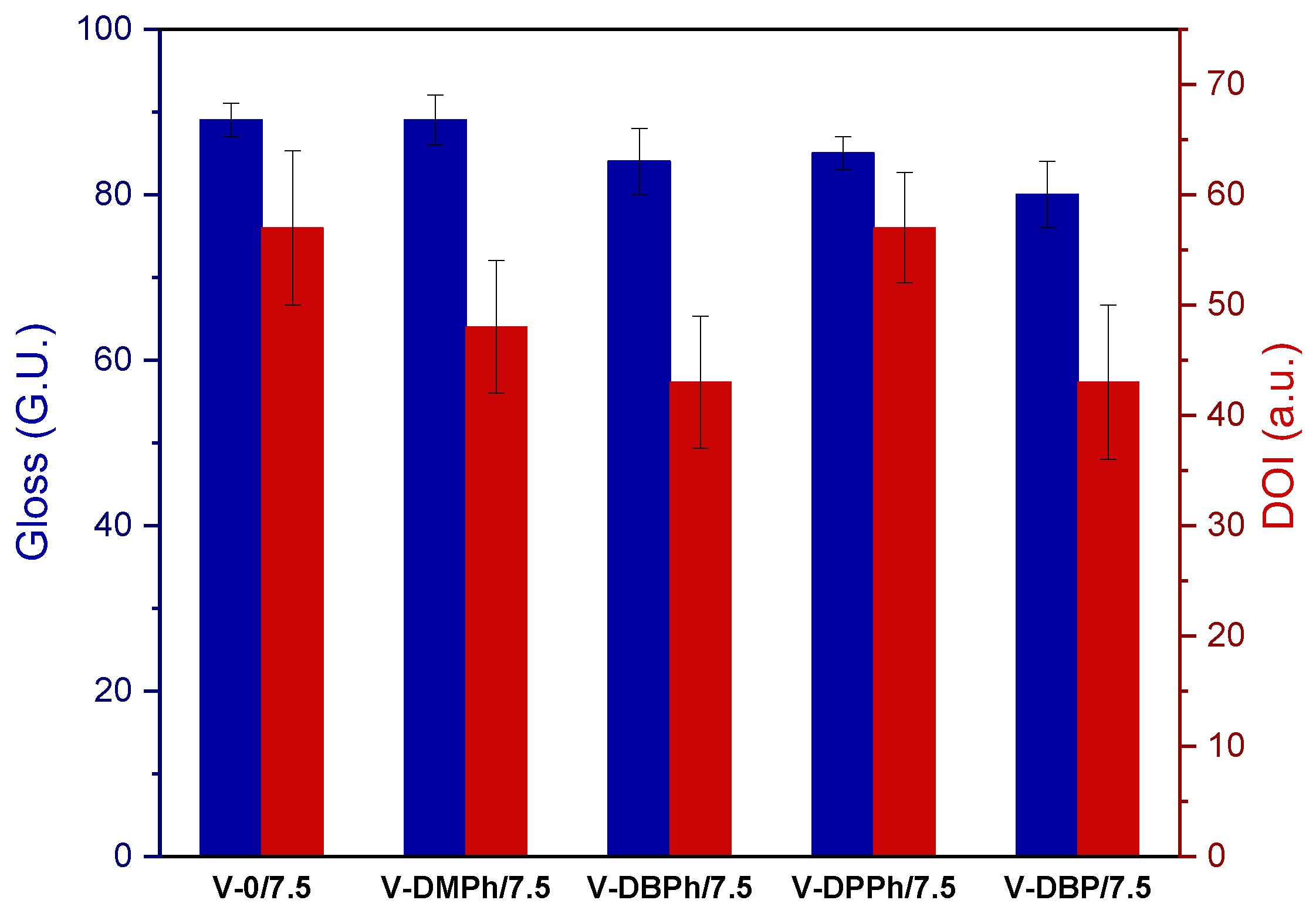
3.3. Properties of UV-Cured Varnish Coatings
4. Conclusions
- P-telogen/APO initiating systems showed higher efficiency in the process than the APO photoinitiator itself (higher photoinitiation index), which resulted in a lower rate of telomerization reaction than in the case of photopolymerization and a slightly lower monomer conversion.
- Chemical modification of P-telomer syrups with a crosslinking monomer significantly improved the hardness and adhesion of the obtained coatings. The highest hardness values were reported for UV-cured varnish coating with dimethyl phosphite (DMPh) telomers (111 a.u.), while the strongest adhesive bond to the glass surface was observed in the case of diphenyl phosphite (DPPh) telomers (8.2 MPa).
- The most satisfactory scratch, water, and solvent resistance characterized UV-cured varnish coatings based on DMPh telomers.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Liu, J.; Cheng, F.; Jiao, X.; Fan, Y.; Lai, G.; Hua, X.; Yang, X. UV cured transparent coatings with good thermal stability prepared from a liquid polyhedral oligomeric silsesquioxane with allyl sulphur−containing carbosilane substitutes. Prog. Org. Coat. 2020, 148, 105886. [Google Scholar] [CrossRef]
- Shu, P.; Ai, L.; Kong, Y.; Ji, H.; Lu, Y.; Zhang, J.; Song, W. UV-cured organic–inorganic composites for highly durable and flexible antireflection coatings. Appl. Surf. Sci. 2022, 584, 152600. [Google Scholar] [CrossRef]
- Pendar, M.-R.; Rodrigues, F.; Páscoa, J.C.; Lima, R. Review of Coating and Curing Processes: Evaluation in Automotive Industry. Phys. Fluids 2022, 34, 101301. [Google Scholar] [CrossRef]
- Ribas-Massonis, A.; Cicujano, M.; Duran, J.; Besalú, E.; Poater, A. Free-Radical Photopolymerization for Curing Products for Refinish Coatings Market. Polymers 2022, 14, 2856. [Google Scholar] [CrossRef] [PubMed]
- Scotton, R.S.; Guerrini, L.M.; Oliveira, M.P. Evaluation of solvent-based and UV-curing inkjet inks on the adhesion and printing quality of different aircraft surfaces coating. Prog. Org. Coat. 2021, 158, 106389. [Google Scholar] [CrossRef]
- Zareanshahraki, F.; Asemani, H.R.; Skuza, J.; Mannari, V. Synthesis of non-isocyanate polyurethanes and their application in radiation-curable aerospace coatings. Prog. Org. Coat. 2020, 138, 105394. [Google Scholar] [CrossRef]
- Mao, H.; Qiang, S.; Xu, Y.; Wang, C. Synthesis of polymeric dyes based on UV curable multifunctional waterborne polyurethane for textile coating. New J. Chem. 2017, 41, 619–627. [Google Scholar] [CrossRef]
- Bahria, H.; Erbil, Y. UV technology for use in textile dyeing and printing: Photocured applications. Dye. Pigment 2016, 134, 442–447. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Kunwong, D.; Sumanochitraporn, N.; Kaewpirom, S. Curing Behavior of a UV-Curable Coating Based on Urethane Acrylate Oligomer: The Influence of Reactive Monomers. Songklanakarin J. Sci. Technol. 2011, 33, 201–207. [Google Scholar]
- Wang, Z.; Liang, H.; Yang, H.; Xiong, L.; Zhou, J.; Huang, S.; Zhao, C.; Zhong, J.; Fan, X. UV-curable self-healing polyurethane coating based on thiol-ene and Diels-Alder double click reactions. Prog. Org. Coat. 2019, 137, 105282. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Jiao, C.; Song, L. Preparation and thermal properties of a novel flame-retardant coating. Polym. Degrad. Stab. 2007, 92, 1141–1150. [Google Scholar] [CrossRef]
- Khudyakov, I. Fast photopolymerization of acrylate coatings: Achievements and problems. Prog. Org. Coat. 2018, 121, 151–159. [Google Scholar] [CrossRef]
- Ajekwene, K.K. Properties and Applications of Acrylates. In Acrylate Polymers for Advanced Applications; Serrano-Aroca, Á., Deb, S., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Qiao, H.; Liang, Y.; Xu, G.; Wang, Y.; Yang, J.; Hu, J. Preparation, characterization and flame retardancy of phosphorus-containing poly-styrene-acrylate emulsion. Des. Monomers Polym. 2019, 22, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.; Nan, Y.; Zhang, Y.; Huang, J.; Liu, J. Synthesis and properties of phosphorus-containing cardanol-based acrylates for flame-retardant UV/EB-cured coatings. J. Coat. Technol. Res. 2021, 18, 1353–1364. [Google Scholar] [CrossRef]
- Phalak, G.; Patil, D.; Patil, A.; Mhaske, S. Synthesis of acrylated cardanol diphenyl phosphate for UV curable flame-retardant coating application. Eur. Polym. J. 2019, 121, 109320. [Google Scholar] [CrossRef]
- Chougrani, K.; Boutevin, B.; David, G.; Seabrook, S.; Loubat, C. Acrylate based anticorrosion films using novel bis-phosphonic methacrylates. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7972–7984. [Google Scholar] [CrossRef]
- El Asri, Z.; Chougrani, K.; Negrell-Guirao, C.; David, G.; Boutevin, B.; Loubat, C. An efficient process for synthesizing and hydrolyzing a phosphonated methacrylate: Investigation of the adhesive and anticorrosive properties. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 4794–4803. [Google Scholar] [CrossRef]
- Millet, F.; Auvergne, R.; Caillol, S.; David, G.; Manseri, A.; Pébère, N. Improvement of corrosion protection of steel by incorporation of a new phosphonated fatty acid in a phosphorus-containing polymer coating obtained by UV curing. Prog. Org. Coat. 2014, 77, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Wang, C.; Du, C.; Li, X.; Wang, X. A corrosion-resistance waterborne polyacrylate coatings based on novel phosphate esters polymeric surfactant. J. Appl. Polym. Sci. 2022, 139, 52267. [Google Scholar] [CrossRef]
- Yeniad, B.; Albayrak, A.Z.; Olcum, N.C.; Avci, D. Synthesis and photopolymerizations of new phosphonated monomers for dental applications. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2290–2299. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. Recent Developments of New Components for Dental Adhesives and Composites. Macromol. Mater. Eng. 2007, 292, 245–271. [Google Scholar] [CrossRef]
- Mehdawi, I.; Neel, E.A.A.; Valappil, S.P.; Palmer, G.; Salih, V.; Pratten, J.; Spratt, D.A.; Young, A.M. Development of remineralizing, antibacterial dental materials. Acta Biomater. 2009, 5, 2525–2539. [Google Scholar] [CrossRef] [PubMed]
- Kemal, E.; Adesanya, K.O.; Deb, S. Phosphate based 2-hydroxyethyl methacrylate hydrogels for biomedical applications. J. Mater. Chem. 2011, 21, 2237–2245. [Google Scholar] [CrossRef]
- DelDonno, T.A. Phosphorus-Containing Polymer Compositions Containing Water-Soluble Polyvalent Metal Compounds. U.S. Patent 5,191,029, 2 March 1993. [Google Scholar]
- Bohling, J.C.; Trejo-O’Reilly, J.A. Polymeric Bead Composition Comprising Polymerized Phosphorus-Containing Acid Monomer. U.S. Patent 9,493,585 B2, 15 November 2016. [Google Scholar]
- Verardi, C.A.; Donaldson, S.F.; Lamers, P.H.; Morrow, K.; Mauer, G.W., III; Barncyk, S.V. Coating Compositions Containing Phosphorus Acid Functional Polyol Polymers and Coatings Formed Therefrom. Patent WO2020093010A1, 7 May 2020. [Google Scholar]
- Xiaojie, L.; Fangcao, W.; Wei, W.; Jingcheng, L. Photocuring Phosphorus-Nitrogen Flame-Retardant Acrylic Resin and Photocuring Coating Prepared from Same. Patent CN113603864B, 17 May 2022. [Google Scholar]
- Kun, W.; Yue, L.; Jun, S.; Mangeng, L. Phosphorus-Containing Polyurethane-Acrylic Resin Flame-Retardant Coating with Semi-Interpenetrating Network Structure and Preparation Method and Application Thereof. Patent CN113122121A, 16 July 2021. [Google Scholar]
- Kawaguchi, A.; Takeuchi, K. Phosphorus-Containing Acrylic Resin and Method for Producing the Same, Acrylic Resin Composition, Resin Film, Prepreg, Metal Foil with Resin, Metal Foil-Clad Laminate, and Printed Wiring Board. Patent JP2011225853, 10 November 2011. [Google Scholar]
- Gordon, R.; Loftus, R. Telomerization. In Encyclopedia Polymer Science Technology; Kirk, R.E., Othmer, D.F., Eds.; Wiley: New York, NY, USA, 1989; p. 533. [Google Scholar]
- Chung, I. Monte Carlo simulation of free radical telomerization. Polymer 2000, 41, 5643–5651. [Google Scholar] [CrossRef]
- Hauptschein, M.; Braid, M.; Lawlor, F. Thermal Syntheses of Telomers of Fluorinated Olefins. I. Perfluoropropene1. J. Am. Chem. Soc. 1957, 79, 2549–2553. [Google Scholar] [CrossRef]
- Améduri, B.; Boutevin, B. Telomerisation Reactions of Fluorinated Alkenes. In Organofluorine Chemistry: Fluorinated Alkenes and Reactive Intermediates; Chambers, R.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 165–233. [Google Scholar]
- Starks, C.M. Free Radical Telomerization; Academic Press: New York, NY, USA; London, UK, 1974; pp. 198–204. [Google Scholar]
- Kabatc, J. The influence of a radical structure on the kinetics of photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1575–1589. [Google Scholar] [CrossRef]
- Kazicyna, L.A.; Kupletska, N.B. Metody Spektroskopowe Wyznaczania Struktury Związków Organicznych (Spectroscopic Methods for Determining the Structure of Organic Compounds); PWN: Warsaw, Poland, 1976. [Google Scholar]
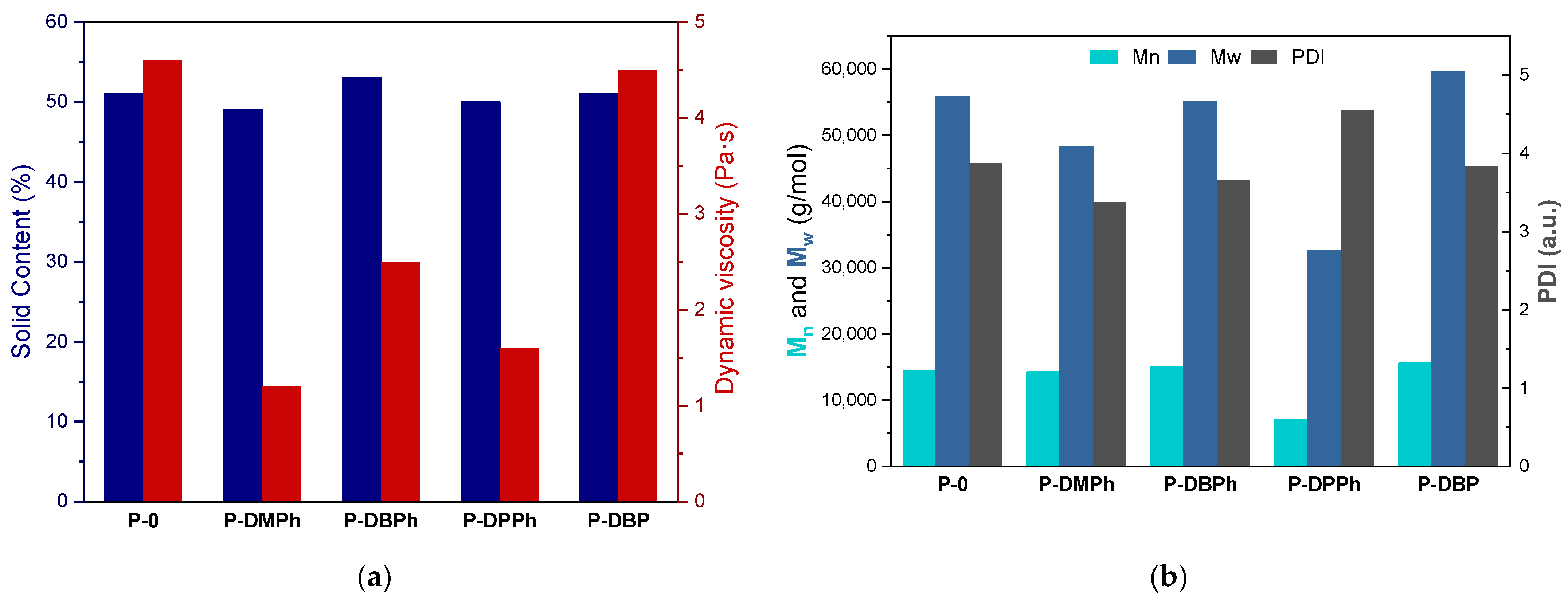
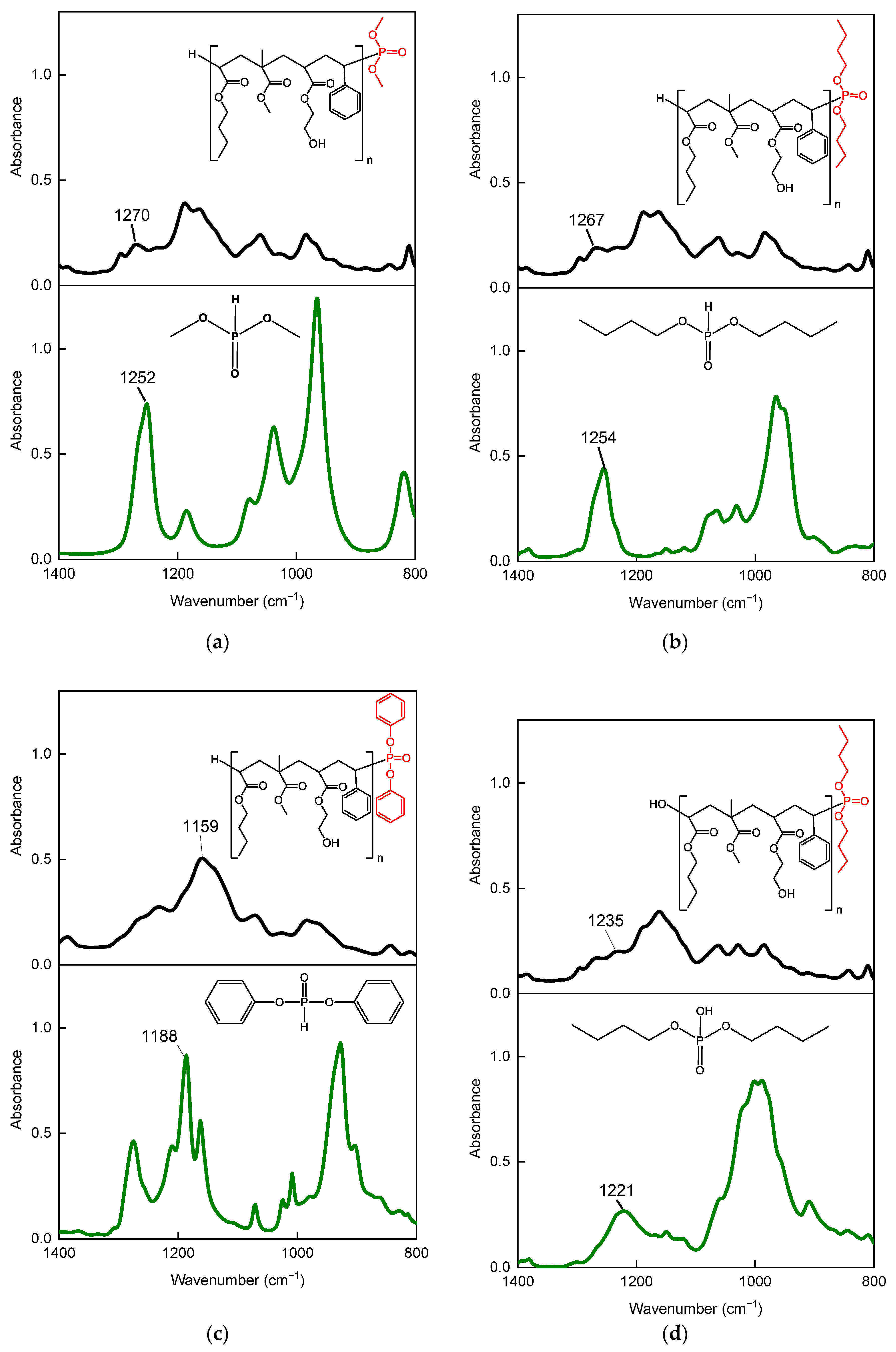
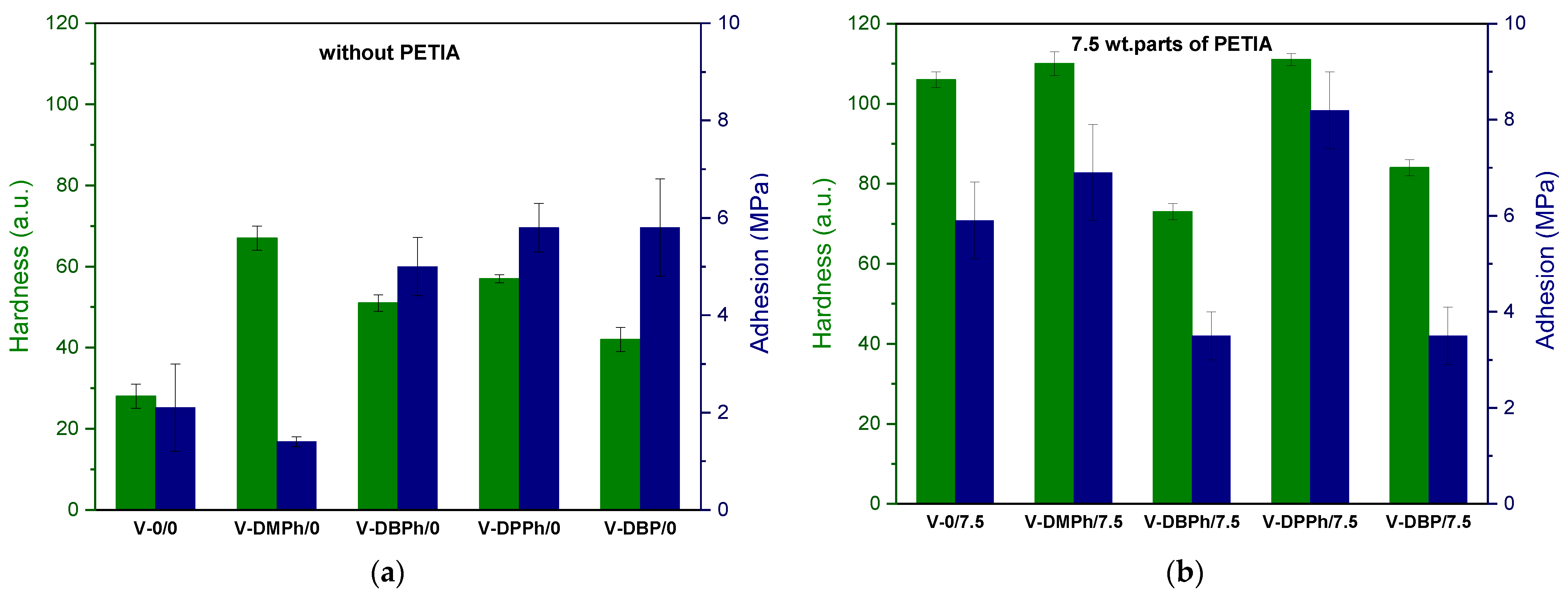
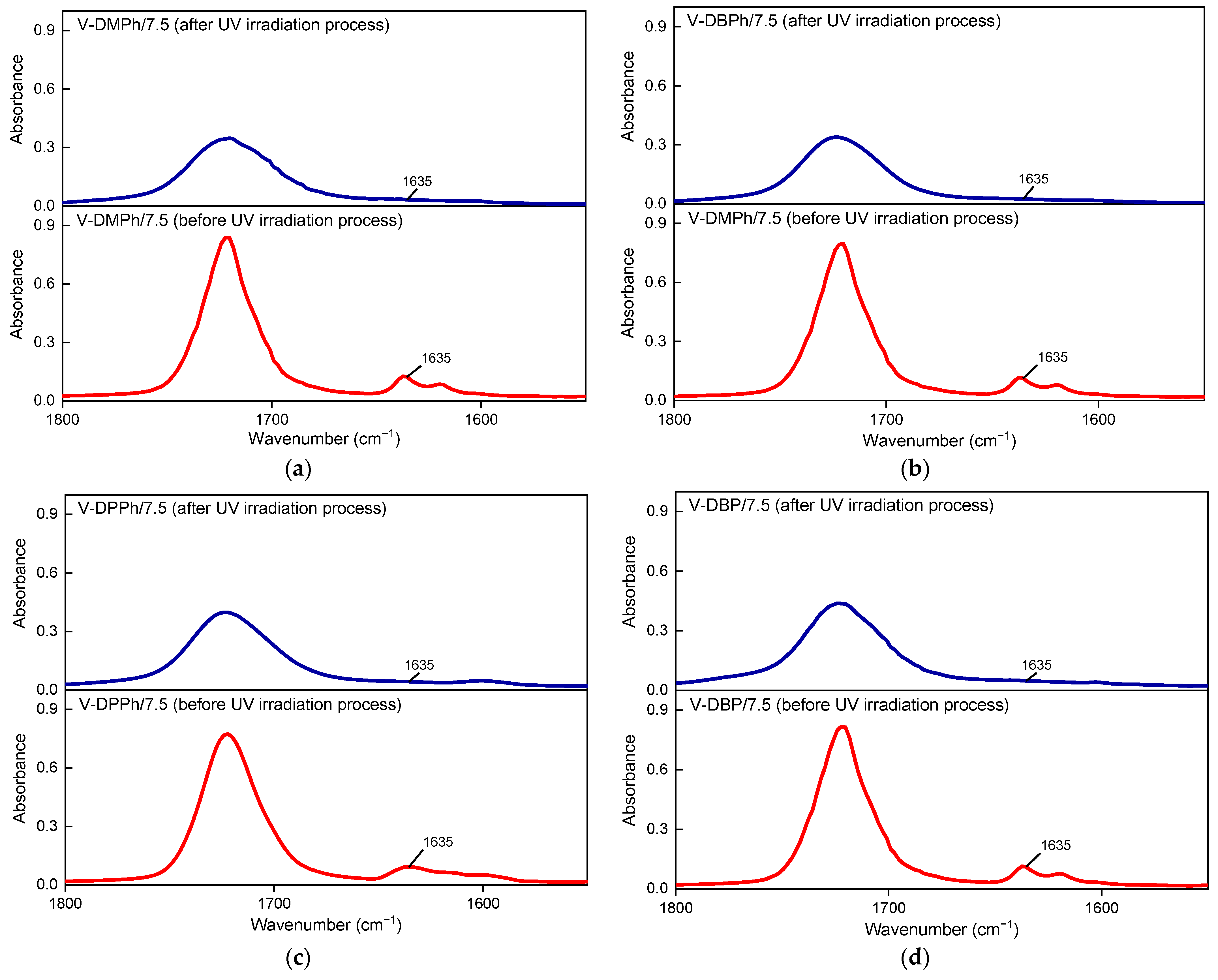
| P-Telomers Syrup | Comonomers [wt%] | Telogen | APO b | Varnish Symbol | PETIA c | HAP c | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | MMA | HEA | STY | Symbol | Dose a (mole) | Dose b (wt. Parts) | |||||
| P-0 | 40 | 35 | 15 | 10 | - | 0 | 0 | 0.75 | V-0/0 | 0 | 1 |
| V-0/7.5 | 7.5 | ||||||||||
| P-DMPh | DMPh | 0.006 | 1.65 | V-DMPh/0 | 0 | ||||||
| V-DMPh/7.5 | 7.5 | ||||||||||
| P-DBPh | DBPh | 2.90 | V-DBPh/0 | 0 | |||||||
| V-DBPh/7.5 | 7.5 | ||||||||||
| P-DPPh | DPPh | 3.50 | V-DPPh/0 | 0 | |||||||
| V-DPPh/7.5 | 7.5 | ||||||||||
| P-DBP | DBP | 3.15 | V-DBP/0 | 0 | |||||||
| V-DBP/7.5 | 7.5 | ||||||||||
| Varnish Symbol | Scratch Resistance 1 (g) | Effect of Distilled Water 2 | Effect of Organic Solvents 2 | ||
|---|---|---|---|---|---|
| Acetone | Propan-2-ol | MEK | |||
| V-0/7.5 | 350 | Blisters 5(S2) | Blisters 4(S3) | Blisters 4(S3) | Blisters 4(S3) |
| V-DMPh/7.5 | 350 | Blisters 4(S2) | Blisters 5(S2) | Blisters 3(S2) | Blisters 5(S2) |
| V-DBPh/7.5 | 200 | Blisters 5(S2) | Blisters 5(S3) | Blisters 5(S3) | Blisters 5(S3) |
| V-DPPh/7.5 | 100 | Matt coating, blisters 5(S2) | Blisters 4(S3) | Blisters 4(S3) | Blisters 4(S3) |
| V-DBP/7.5 | 300 | Matt coating, blisters 5(S2) | Cracking without preferential direction 5(S4) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraśkiewicz, A.; Kowalczyk, A. Phosphorus-Containing Telomers as UV-Curable Binders of Solvent-Free Varnish Coatings. Materials 2022, 15, 8991. https://doi.org/10.3390/ma15248991
Kraśkiewicz A, Kowalczyk A. Phosphorus-Containing Telomers as UV-Curable Binders of Solvent-Free Varnish Coatings. Materials. 2022; 15(24):8991. https://doi.org/10.3390/ma15248991
Chicago/Turabian StyleKraśkiewicz, Agata, and Agnieszka Kowalczyk. 2022. "Phosphorus-Containing Telomers as UV-Curable Binders of Solvent-Free Varnish Coatings" Materials 15, no. 24: 8991. https://doi.org/10.3390/ma15248991
APA StyleKraśkiewicz, A., & Kowalczyk, A. (2022). Phosphorus-Containing Telomers as UV-Curable Binders of Solvent-Free Varnish Coatings. Materials, 15(24), 8991. https://doi.org/10.3390/ma15248991