Thermal Treatment Impact on the Mechanical Properties of Mg3Si2O5(OH)4 Nanoscrolls
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoscrolls Synthesis and Analysis
2.2. Sample Preparation for the AFM Experiment
2.3. AFM Experiment and Its Treatment
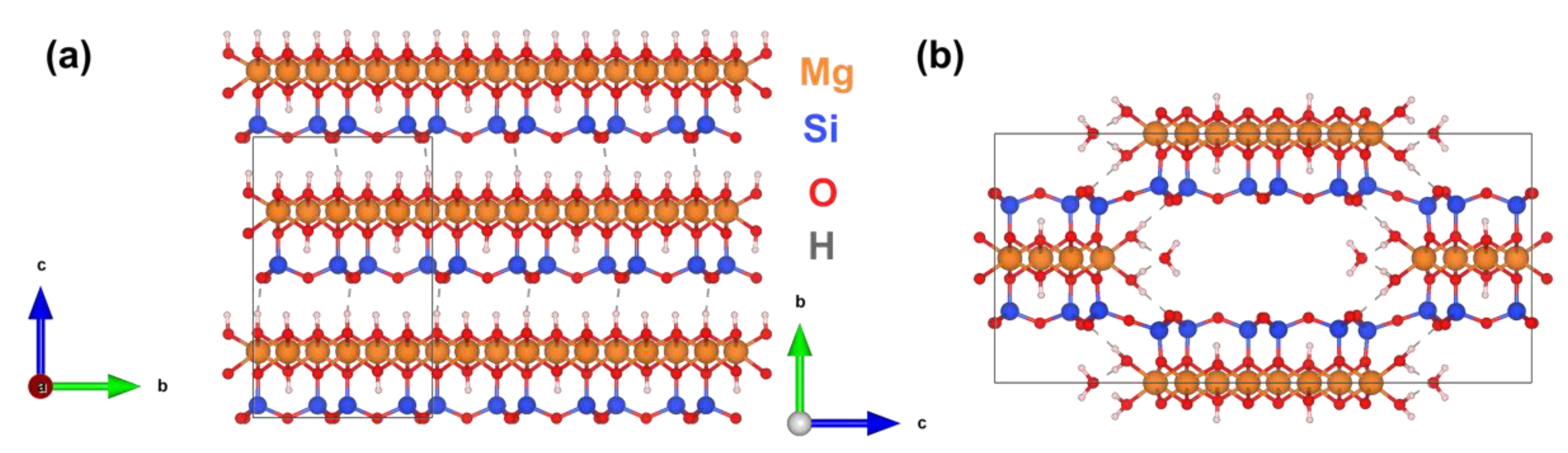
2.4. DFT Modeling of Mechanical Properties
3. Results and Discussion
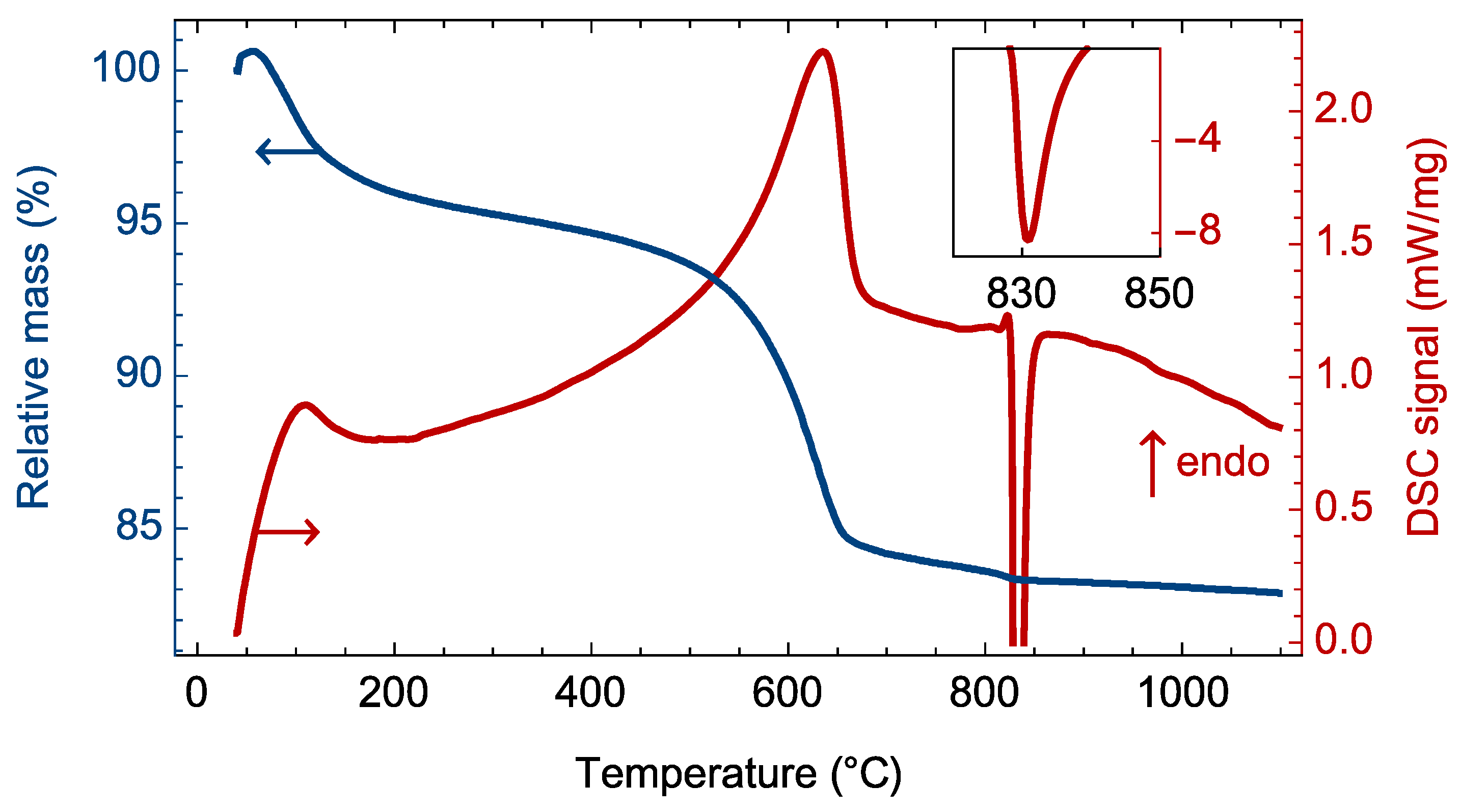
3.1. Phase Transitions during the Heat Treatment
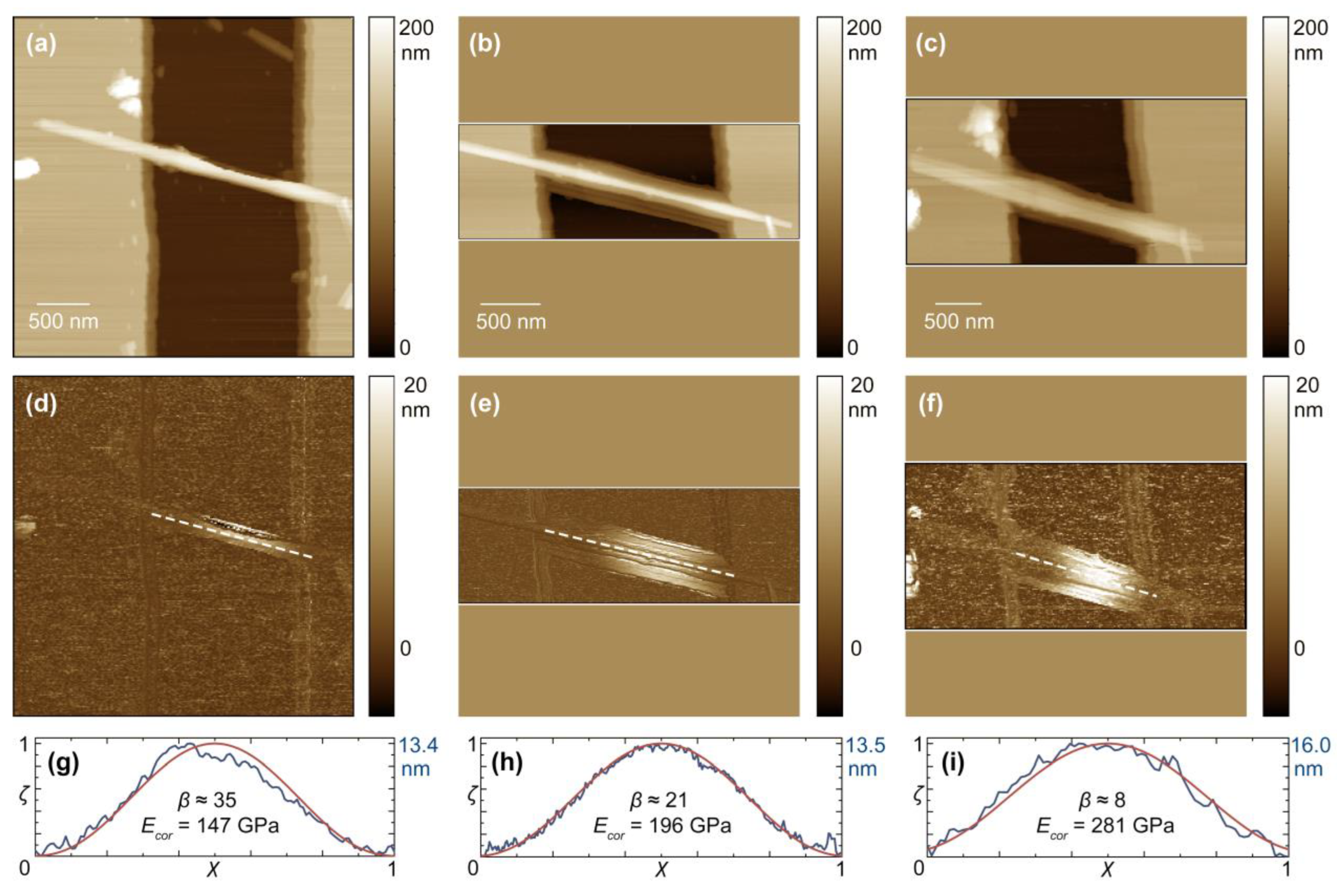
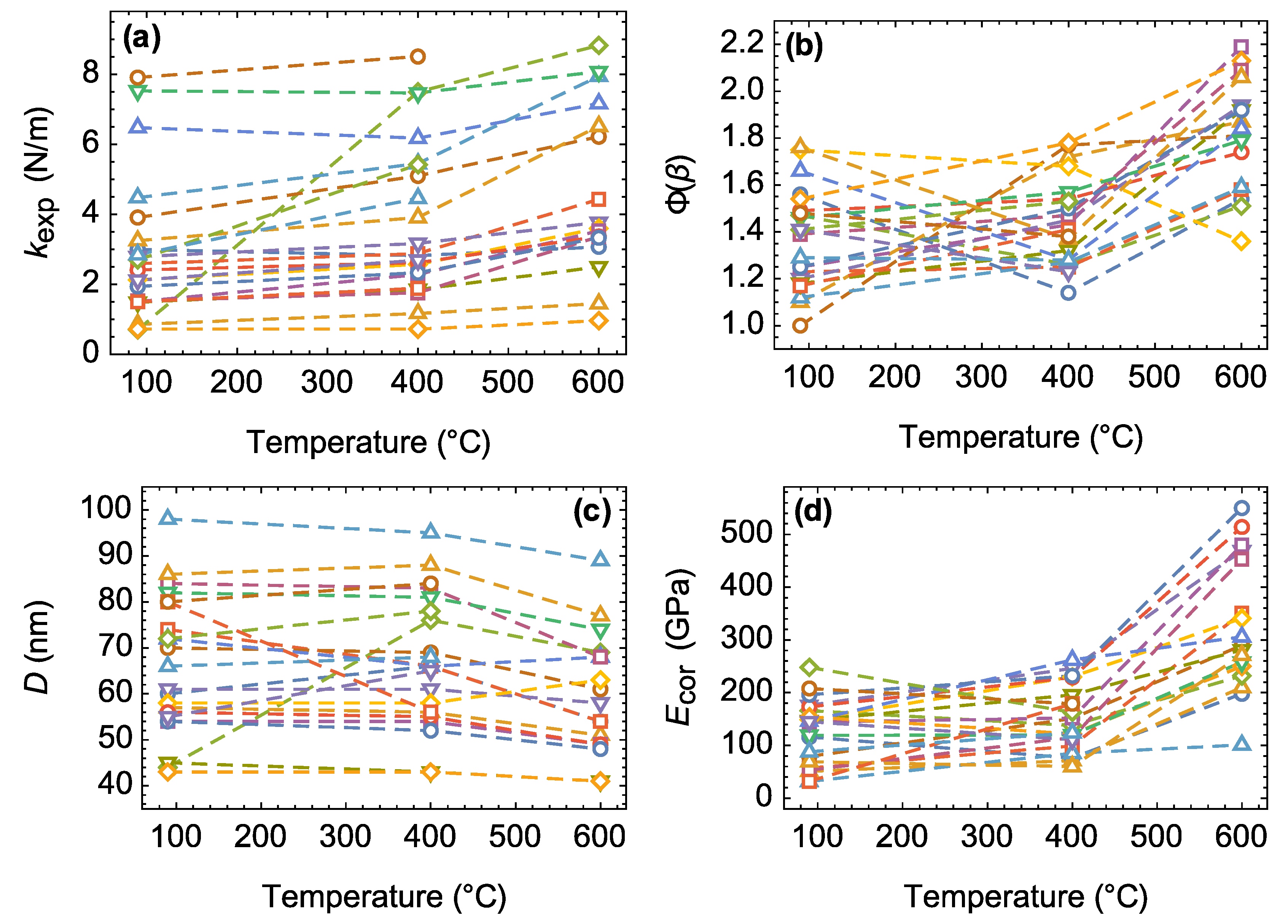
3.2. Alteration of Individual Nanobridge Parameters
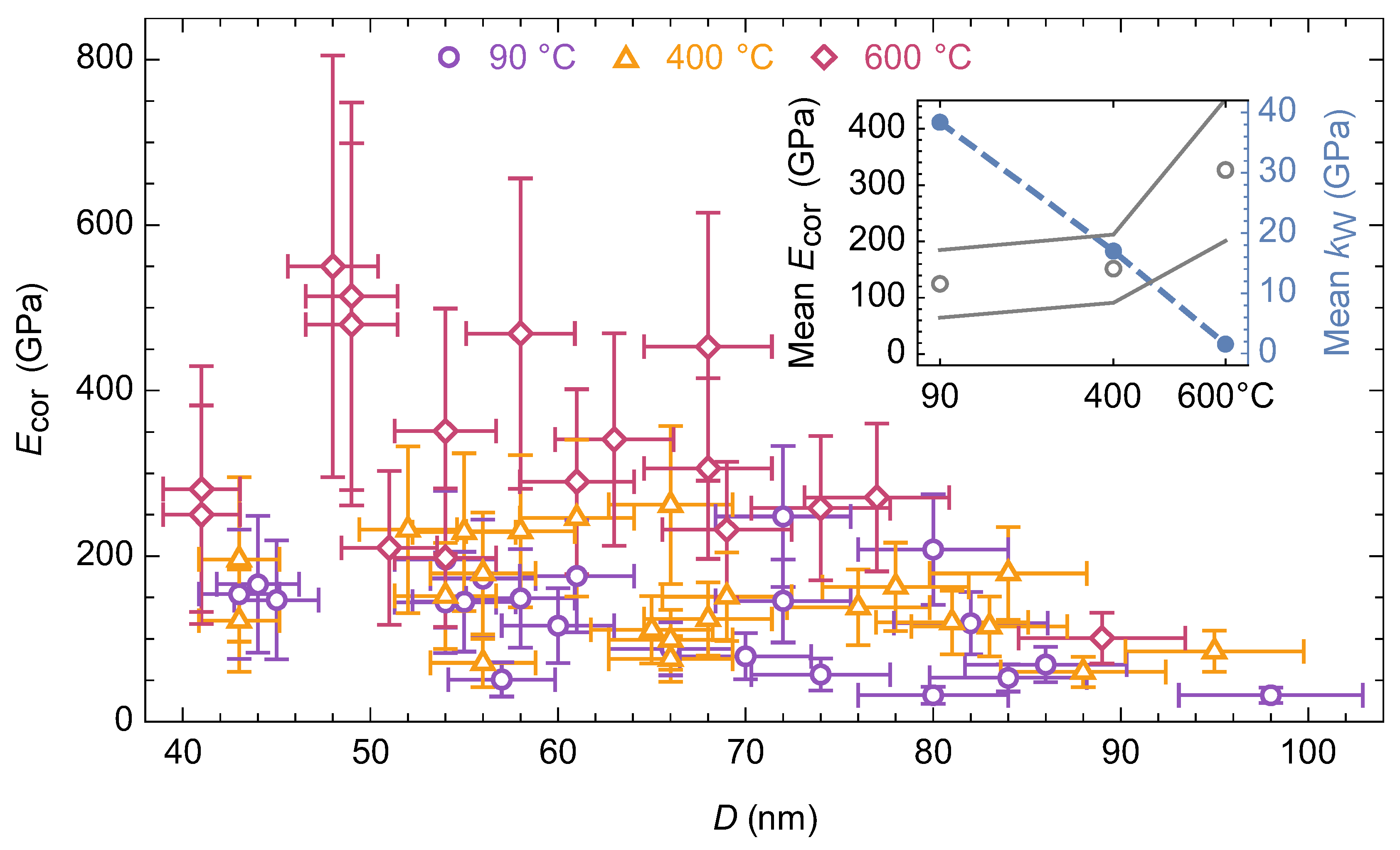
3.3. Changes in Mechanical Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yah, W.O.; Yamamoto, K.; Jiravanichanun, N.; Otsuka, H.; Takahara, A. Imogolite Reinforced Nanocomposites: Multifaceted Green Materials. Materials 2010, 3, 1709–1745. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, Y. Nanocarbon Reinforced Rubber Nanocomposites: Detailed Insights about Mechanical, Dynamical Mechanical Properties, Payne, and Mullin Effects. Nanomaterials 2018, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Crosby, A.J.; Lee, J. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Mozharov, A.; Berdnikov, Y.; Solomonov, N.; Novikova, K.; Nadoyan, I.; Shkoldin, V.; Golubok, A.; Kislov, D.; Shalin, A.; Petrov, M.; et al. Nanomass Sensing via Node Shift Tracing in Vibrations of Coupled Nanowires Enhanced by Fano Resonances. ACS Appl. Nano Mater. 2021, 4, 11989–11996. [Google Scholar] [CrossRef]
- Efremov, Y.M.; Suter, D.M.; Timashev, P.S.; Raman, A. 3D Nanomechanical Mapping of Subcellular and Sub-Nuclear Structures of Living Cells by Multi-Harmonic AFM with Long-Tip Microcantilevers. Sci. Rep. 2022, 12, 529. [Google Scholar] [CrossRef]
- Brás, M.M.; Radmacher, M.; Sousa, S.R.; Granja, P.L. Melanoma in the Eyes of Mechanobiology. Front. Cell Dev. Biol. 2020, 8, 54. [Google Scholar] [CrossRef]
- Plakhova, V.B.; Penniyaynen, V.A.; Rogachevskii, I.V.; Podzorova, S.A.; Khalisov, M.M.; Ankudinov, A.V.; Krylov, B.V. Dual Mechanism of Modulation of NaV1.8 Sodium Channels by Ouabain. Can. J. Physiol. Pharmacol. 2020, 98, 785–802. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef]
- Khalisov, M.M.; Lebedev, V.A.; Poluboyarinov, A.S.; Garshev, A.V.; Khrapova, E.K.; Krasilin, A.A.; Ankudinov, A.V. Young’s Modulus of Phyllosilicate Nanoscrolls Measured by the AFM and by the in-Situ TEM Indentation. Nanosyst. Phys. Chem. Math. 2021, 12, 118–127. [Google Scholar] [CrossRef]
- Schuh, C.A. Nanoindentation Studies of Materials. Mater. Today 2006, 9, 32–40. [Google Scholar] [CrossRef]
- Ilinov, A.; Kuronen, A. Size-Dependent Elastic Properties of Oxidized Silicon Nanorods. J. Appl. Phys. 2014, 116, 204305. [Google Scholar] [CrossRef]
- Petrova, H.; Perez-Juste, J.; Zhang, Z.; Zhang, J.; Kosel, T.; Hartland, G.V. Crystal Structure Dependence of the Elastic Constants of Gold Nanorods. J. Mater. Chem. 2006, 16, 3957. [Google Scholar] [CrossRef]
- Cuenot, S.; Frétigny, C.; Demoustier-Champagne, S.; Nysten, B. Surface Tension Effect on the Mechanical Properties of Nanomaterials Measured by Atomic Force Microscopy. Phys. Rev. B 2004, 69, 165410. [Google Scholar] [CrossRef]
- Krasilin, A.A.; Khrapova, E.K.; Maslennikova, T.P. Cation Doping Approach for Nanotubular Hydrosilicates Curvature Control and Related Applications. Crystals 2020, 10, 654. [Google Scholar] [CrossRef]
- Kis, A.; Kasas, S.; Babić, B.; Kulik, A.J.; Benoît, W.; Briggs, G.A.D.; Schönenberger, C.; Catsicas, S.; Forró, L. Nanomechanics of Microtubules. Phys. Rev. Lett. 2002, 89, 248101. [Google Scholar] [CrossRef]
- Vatankhah, C.; Badehian, H.A. Structural, Elastic, and Optical Properties of Silicon Carbide Nanotubes Using DFT. Solid State Commun. 2022, 344, 114672. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Zak, A.; Tenne, R.; Goodilin, E.A.; Solntsev, K.A. Nanocomposites Based on Tubular and Onion Nanostructures of Molybdenum and Tungsten Disulfides: Inorganic Design, Functional Properties and Applications. Russ. Chem. Rev. 2018, 87, 251–271. [Google Scholar] [CrossRef]
- Sade, H.; Moshkovich, A.; Lellouche, J.-P.; Rapoport, L. Testing of WS2 Nanoparticles Functionalized by a Humin-Like Shell as Lubricant Additives. Lubricants 2018, 6, 3. [Google Scholar] [CrossRef]
- Stefanov, M.; Enyashin, A.N.; Heine, T.; Seifert, G. Nanolubrication: How Do MoS2-Based Nanostructures Lubricate? J. Phys. Chem. C 2008, 112, 17764–17767. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Krasilin, A.A.; Khalisov, M.M.; Khrapova, E.K.; Kunkel, T.S.; Kozlov, D.A.; Anuchin, N.M.; Enyashin, A.N.; Ankudinov, A.V. Surface Tension and Shear Strain Contributions to the Mechanical Behavior of Individual Mg-Ni-Phyllosilicate Nanoscrolls. Part. Part. Syst. Charact. 2021, 38, 2100153. [Google Scholar] [CrossRef]
- Piperno, S.; Kaplan-Ashiri, I.; Cohen, S.R.; Popovitz-Biro, R.; Wagner, H.D.; Tenne, R.; Foresti, E.; Lesci, I.G.; Roveri, N. Characterization of Geoinspired and Synthetic Chrysotile Nanotubes by Atomic Force Microscopy and Transmission Electron Microscopy. Adv. Funct. Mater. 2007, 17, 3332–3338. [Google Scholar] [CrossRef]
- Lourenço, M.P.; de Oliveira, C.; Oliveira, A.F.; Guimarães, L.; Duarte, H.A. Structural, Electronic, and Mechanical Properties of Single-Walled Chrysotile Nanotube Models. J. Phys. Chem. C 2012, 116, 9405–9411. [Google Scholar] [CrossRef]
- Guimarães, L.; Enyashin, A.N.; Seifert, G.; Duarte, H.A. Structural, Electronic, and Mechanical Properties of Single-Walled Halloysite Nanotube Models. J. Phys. Chem. C 2010, 114, 11358–11363. [Google Scholar] [CrossRef]
- Lecouvet, B.; Horion, J.; D’Haese, C.; Bailly, C.; Nysten, B. Elastic Modulus of Halloysite Nanotubes. Nanotechnology 2013, 24, 105704. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yudin, V.E.; Otaigbe, J.U.; Korytkova, E.N.; Nazarenko, S. Gas Barrier Behavior of Polyimide Films Filled with Synthetic Chrysotile Nanotubes. J. Polym. Sci. B Polym. Phys. 2013, 51, 1184–1193. [Google Scholar] [CrossRef]
- Song, K.; Polak, R.; Chen, D.; Rubner, M.F.; Cohen, R.E.; Askar, K.A. Spray-Coated Halloysite–Epoxy Composites: A Means to Create Mechanically Robust, Vertically Aligned Nanotube Composites. ACS Appl. Mater. Interfaces 2016, 8, 20396–20406. [Google Scholar] [CrossRef]
- Qi, R.; Guo, R.; Zheng, F.; Liu, H.; Yu, J.; Shi, X. Controlled Release and Antibacterial Activity of Antibiotic-Loaded Electrospun Halloysite/Poly(Lactic-Co-Glycolic Acid) Composite Nanofibers. Colloids Surf. B Biointerfaces 2013, 110, 148–155. [Google Scholar] [CrossRef]
- Naumenko, E.; Fakhrullin, R. Halloysite Nanoclay/Biopolymers Composite Materials in Tissue Engineering. Biotechnol. J. 2019, 14, 1900055. [Google Scholar] [CrossRef]
- Sivaiah, M.V.; Petit, S.; Beaufort, M.F.; Eyidi, D.; Barrault, J.; Batiot-Dupeyrat, C.; Valange, S. Nickel Based Catalysts Derived from Hydrothermally Synthesized 1:1 and 2:1 Phyllosilicates as Precursors for Carbon Dioxide Reforming of Methane. Microporous Mesoporous Mater. 2011, 140, 69–80. [Google Scholar] [CrossRef]
- Bian, Z.; Suryawinata, I.Y.; Kawi, S. Highly Carbon Resistant Multicore-Shell Catalyst Derived from Ni-Mg Phyllosilicate Nanotubes@silica for Dry Reforming of Methane. Appl. Catal. B 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, P.; Li, W. Supported Ni Catalyst on a Natural Halloysite Derived Silica–Alumina Composite Oxide with Unexpected Coke-Resistant Stability for Steam-CO2 Dual Reforming of Methane. RSC Adv. 2016, 6, 49487–49496. [Google Scholar] [CrossRef]
- Maslennikova, T.P.; Korytkova, E.N. Regularities of the Filling of Mg3Si2O5(OH)4 Hydrosilicate Nanotubes with Solutions of Sodium Hydroxide and Sodium Chloride. Glass Phys. Chem. 2011, 37, 418–425. [Google Scholar] [CrossRef]
- Lvov, Y.M.; DeVilliers, M.M.; Fakhrullin, R.F. The Application of Halloysite Tubule Nanoclay in Drug Delivery. Expert Opin. Drug Deliv. 2016, 13, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Bediako, E.G.; Nyankson, E.; Dodoo-Arhin, D.; Agyei-Tuffour, B.; Łukowiec, D.; Tomiczek, B.; Yaya, A.; Efavi, J.K. Modified Halloysite Nanoclay as a Vehicle for Sustained Drug Delivery. Heliyon 2018, 4, e00689. [Google Scholar] [CrossRef]
- Ankudinov, A.V.; Khalisov, M.M. Contact Stiffness Measurements with an Atomic Force Microscope. Tech. Phys. 2020, 65, 1866–1872. [Google Scholar] [CrossRef]
- Khrapova, E.K.; Ugolkov, V.L.; Straumal, E.A.; Lermontov, S.A.; Lebedev, V.A.; Kozlov, D.A.; Kunkel, T.S.; Nominé, A.; Bruyere, S.; Ghanbaja, J.; et al. Thermal Behavior of Mg−Ni-phyllosilicate Nanoscrolls and Performance of the Resulting Composites in Hexene-1 and Acetone Hydrogenation. ChemNanoMat 2021, 7, 257–269. [Google Scholar] [CrossRef]
- Bloise, A.; Catalano, M.; Gualtieri, A. Effect of Grinding on Chrysotile, Amosite and Crocidolite and Implications for Thermal Treatment. Minerals 2018, 8, 135. [Google Scholar] [CrossRef]
- Zaremba, T.; Krząkała, A.; Piotrowski, J.; Garczorz, D. Study on the Thermal Decomposition of Chrysotile Asbestos. J. Therm. Anal. Calorim. 2010, 101, 479–485. [Google Scholar] [CrossRef]
- Duce, C.; Vecchio Ciprioti, S.; Ghezzi, L.; Ierardi, V.; Tinè, M.R. Thermal Behavior Study of Pristine and Modified Halloysite Nanotubes. J. Therm. Anal. Calorim. 2015, 121, 1011–1019. [Google Scholar] [CrossRef]
- Ankudinov, A.V. A New Algorithm for Measuring the Young’s Modulus of Suspended Nanoobjects by the Bending-Based Test Method of Atomic Force Microscopy. Semiconductors 2019, 53, 1891–1899. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of Atomic-force Microscope Tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An Open-Source Software for SPM Data Analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Ankudinov, A.; Dunaevskiy, M.; Khalisov, M.; Khrapova, E.; Krasilin, A. AFM Bending Tests of a Suspended Rod-Shaped Object: Accounting for Object Fixing Conditions. Phys. Rev. E 2022, under review. [Google Scholar]
- Gere, J.M.; Timoshenko, S.P. Mechanics of Materials; PWS Pub Co: Boston, MA, USA, 1990; p. 807. [Google Scholar]
- Ordejón, P.; Artacho, E.; Soler, J.M. Self-Consistent Order-N Density-Functional Calculations for Very Large Systems. Phys. Rev. B 1996, 53, R10441–R10444. [Google Scholar] [CrossRef]
- García, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerdá, J.I.; Corsetti, F.; et al. Siesta: Recent Developments and Applications. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef]
- Moreno, J.; Soler, J.M. Optimal Meshes for Integrals in Real- and Reciprocal-Space Unit Cells. Phys. Rev. B 1992, 45, 13891–13898. [Google Scholar] [CrossRef]
- Falini, G.; Foresti, E.; Gazzano, M.; Gualtieri, A.F.; Leoni, M.; Lesci, I.G.; Roveri, N. Tubular-Shaped Stoichiometric Chrysotile Nanocrystals. Chemistry 2004, 10, 3043–3049. [Google Scholar] [CrossRef]
- Post, J.E.; Bish, D.L.; Heaney, P.J. Synchrotron Powder X-Ray Diffraction Study of the Structure and Dehydration Behavior of Sepiolite. Am. Mineral. 2007, 92, 91–97. [Google Scholar] [CrossRef]
- Krasilin, A.A.; Danilovich, D.P.; Yudina, E.B.; Bruyere, S.; Ghanbaja, J.; Ivanov, V.K. Crystal Violet Adsorption by Oppositely Twisted Heat-Treated Halloysite and Pecoraite Nanoscrolls. Appl. Clay Sci. 2019, 173, 1–11. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Krasilin, A.A. The Influence of Edge Specific Surface Energy on the Direction of Hydrosilicate Layers Scrolling. Nanosyst. Phys. Chem. Math. 2021, 12, 623–629. [Google Scholar] [CrossRef]
- Prishchenko, D.A.; Zenkov, E.V.; Mazurenko, V.V.; Fakhrullin, R.F.; Lvov, Y.M.; Mazurenko, V.G. Molecular Dynamics of the Halloysite Nanotubes. Phys. Chem. Chem. Phys. 2018, 20, 5841–5849. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Khrapova, E.; Kozlov, D.; Krasilin, A.; Gusarov, V. Structure Refinement, Microstrains and Crystallite Sizes of Mg-Ni-Phyllosilicate Nanoscroll Powders. J. Appl. Crystallogr. 2022, 55, 484–502. [Google Scholar] [CrossRef]
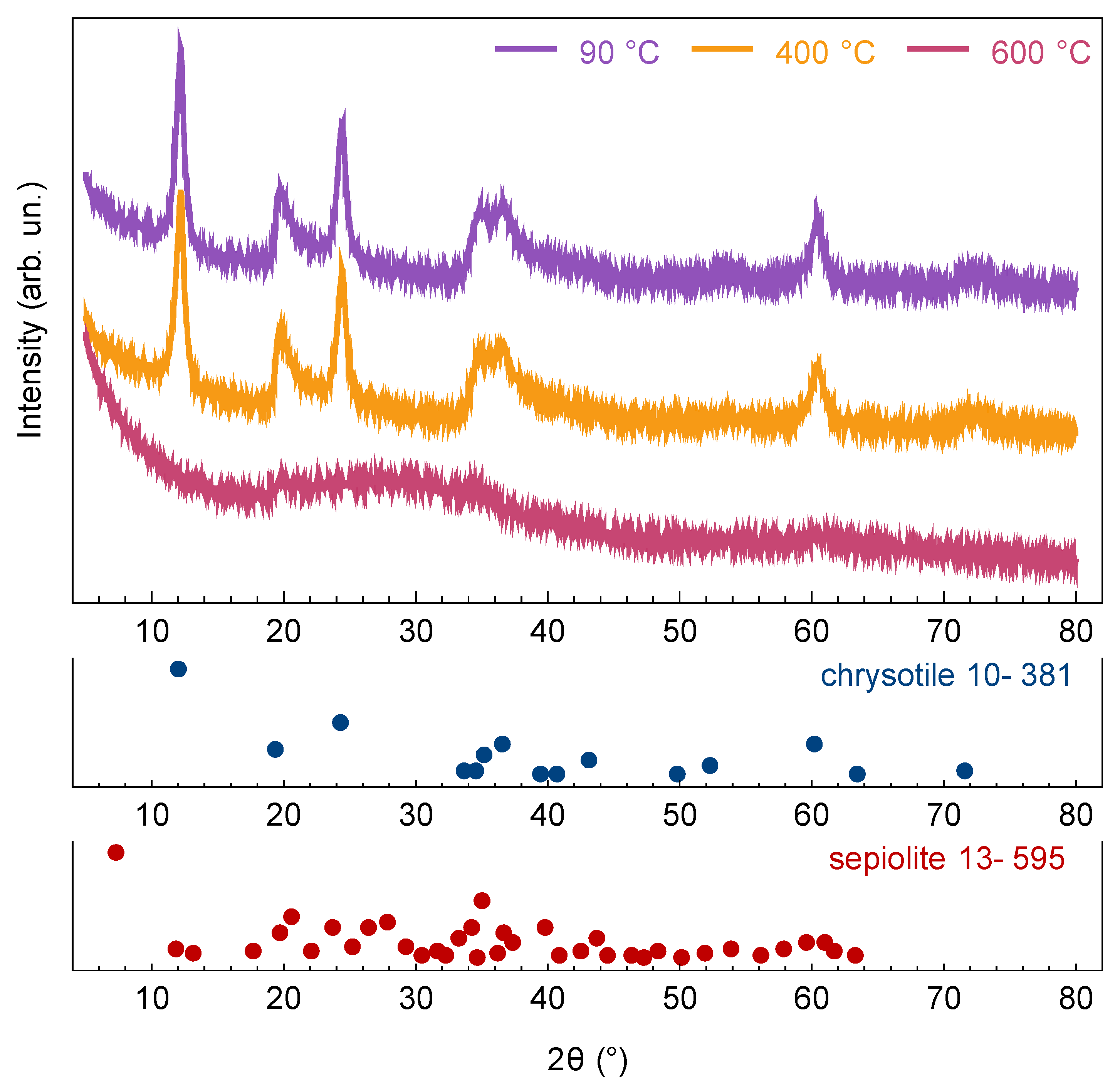

| Structure | Lattice Constants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | β (°) | [100] | [010] | [001] | [100] | [010] | [001] | |
| chrysotile | 5.33 | 9.23 | 14.47 | 91.1 | 222 | 196 | – | 19 | 4 | – |
| sepiolite | 12.75 | 27.43 | 5.32 | 90 | 48 | 153 | 164 | 13 | 54 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasilin, A.; Khalisov, M.; Khrapova, E.; Ugolkov, V.; Enyashin, A.; Ankudinov, A. Thermal Treatment Impact on the Mechanical Properties of Mg3Si2O5(OH)4 Nanoscrolls. Materials 2022, 15, 9023. https://doi.org/10.3390/ma15249023
Krasilin A, Khalisov M, Khrapova E, Ugolkov V, Enyashin A, Ankudinov A. Thermal Treatment Impact on the Mechanical Properties of Mg3Si2O5(OH)4 Nanoscrolls. Materials. 2022; 15(24):9023. https://doi.org/10.3390/ma15249023
Chicago/Turabian StyleKrasilin, Andrei, Maksim Khalisov, Ekaterina Khrapova, Valery Ugolkov, Andrey Enyashin, and Alexander Ankudinov. 2022. "Thermal Treatment Impact on the Mechanical Properties of Mg3Si2O5(OH)4 Nanoscrolls" Materials 15, no. 24: 9023. https://doi.org/10.3390/ma15249023
APA StyleKrasilin, A., Khalisov, M., Khrapova, E., Ugolkov, V., Enyashin, A., & Ankudinov, A. (2022). Thermal Treatment Impact on the Mechanical Properties of Mg3Si2O5(OH)4 Nanoscrolls. Materials, 15(24), 9023. https://doi.org/10.3390/ma15249023






