The Influence of a Binder in a Composite Electrode: The Case Study of Vanadyl Phosphate in Aqueous Electrolyte
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
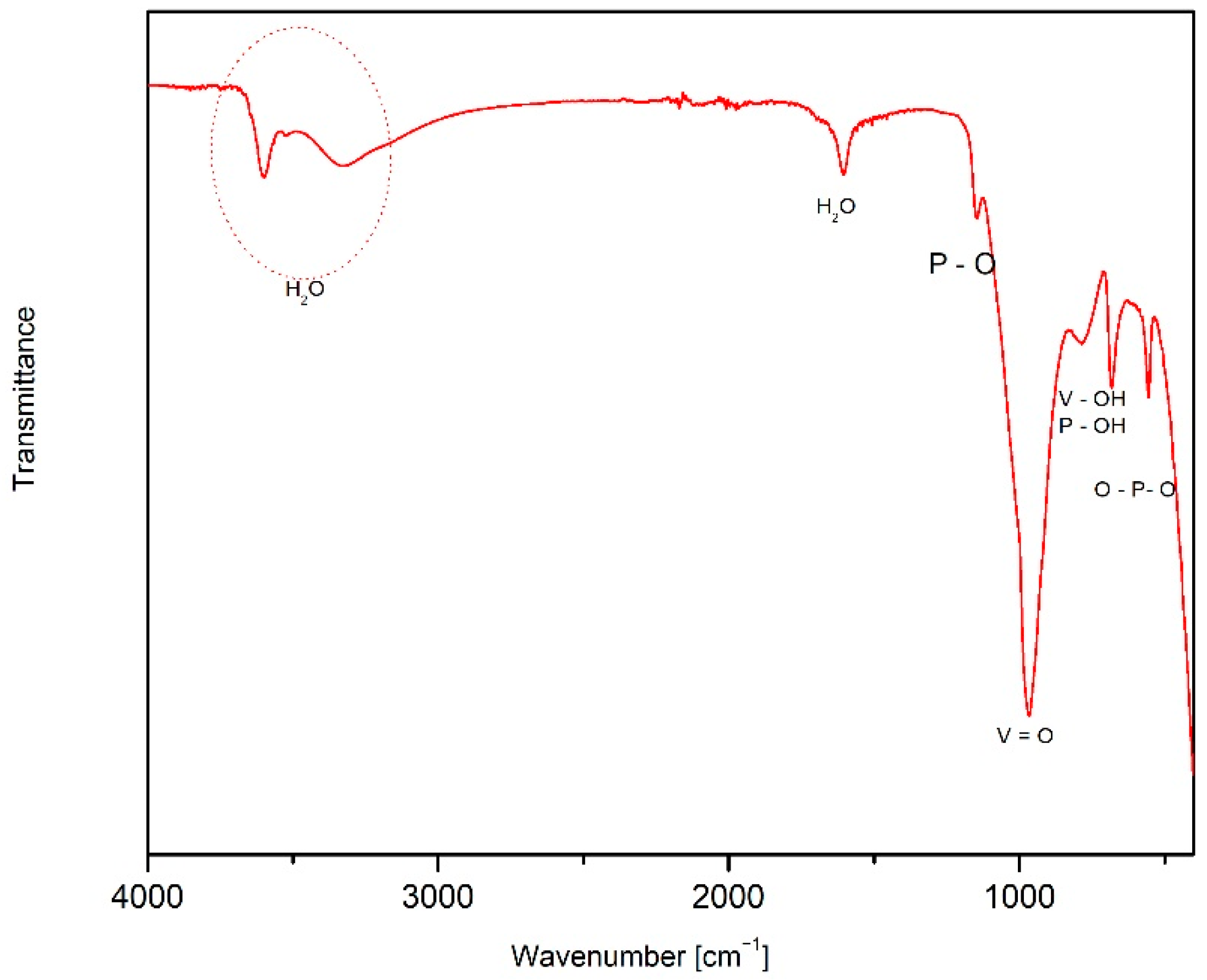
3.1. The Structure and Morphology
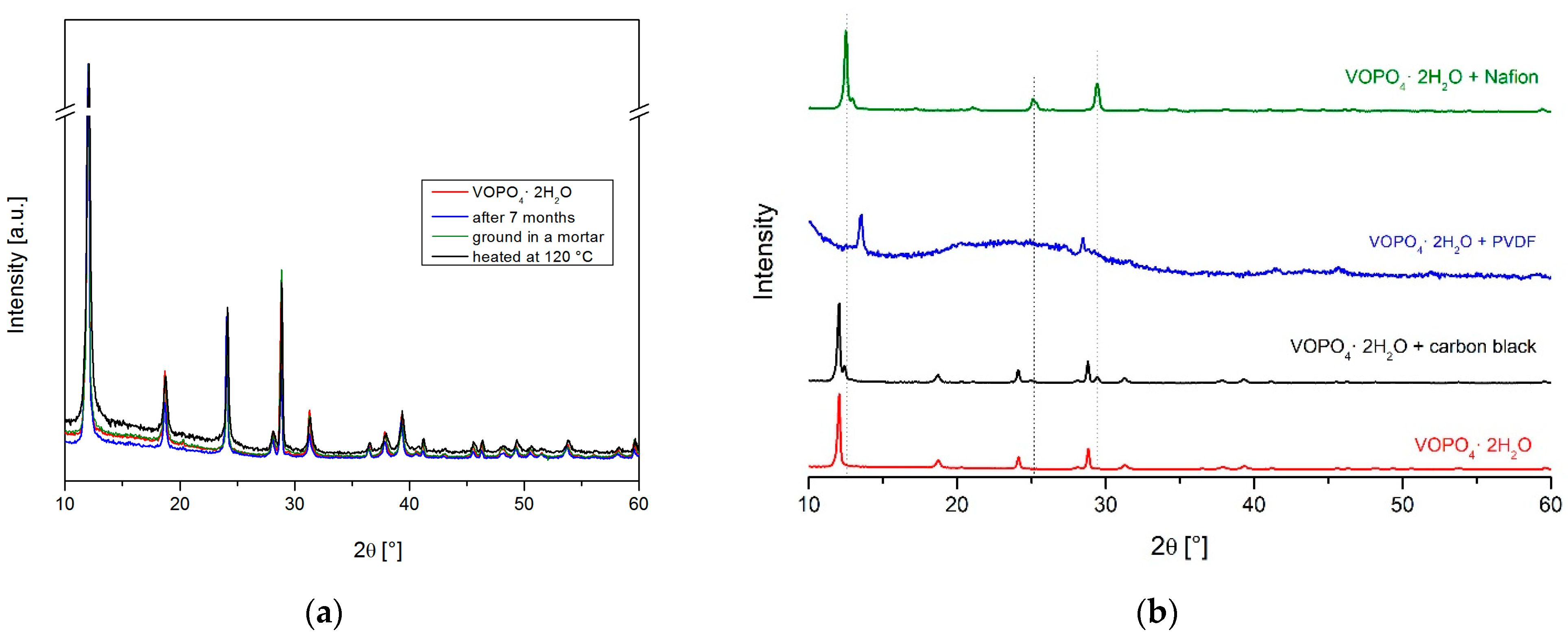
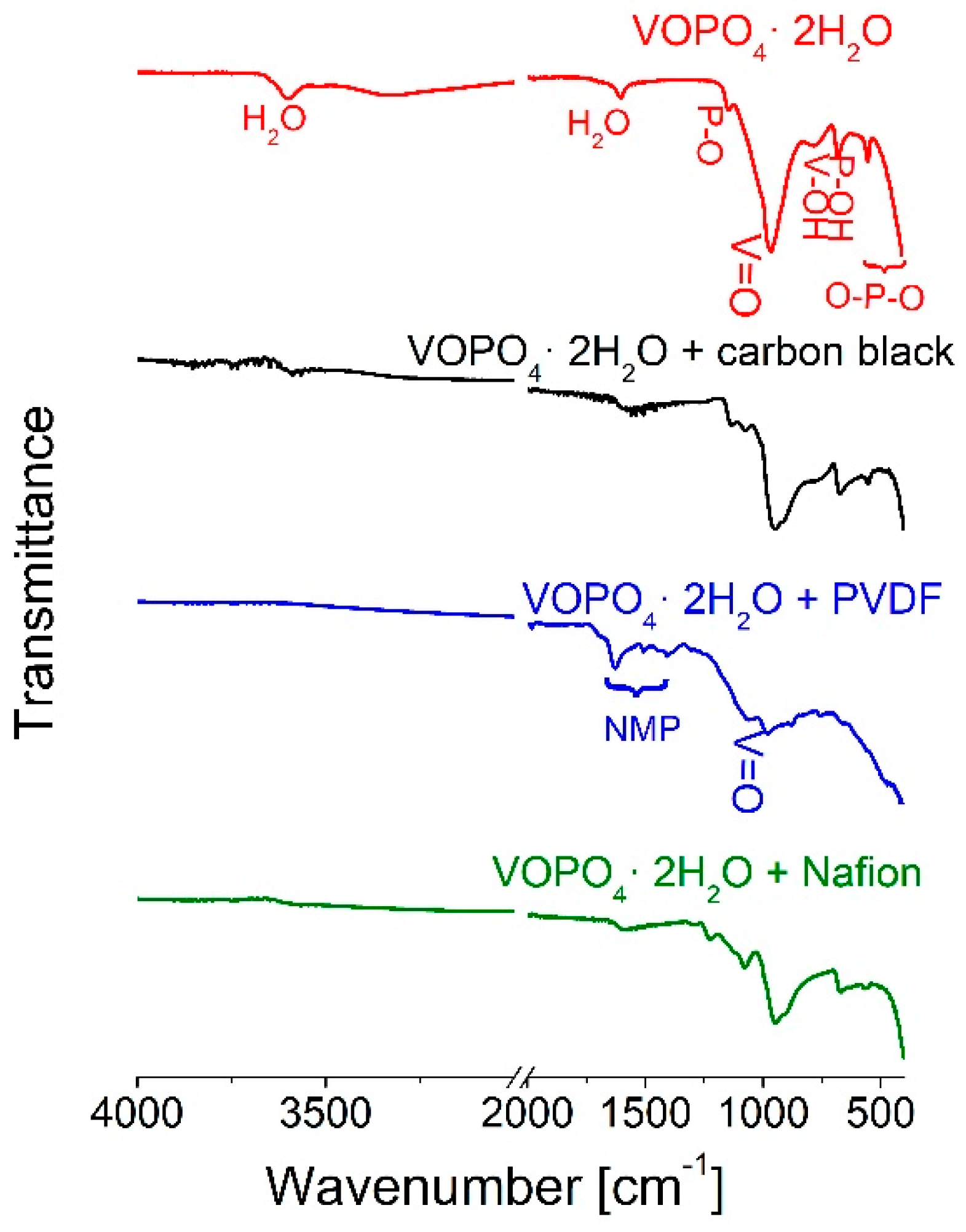
3.2. Behind the Scenes of Electrode Preparation
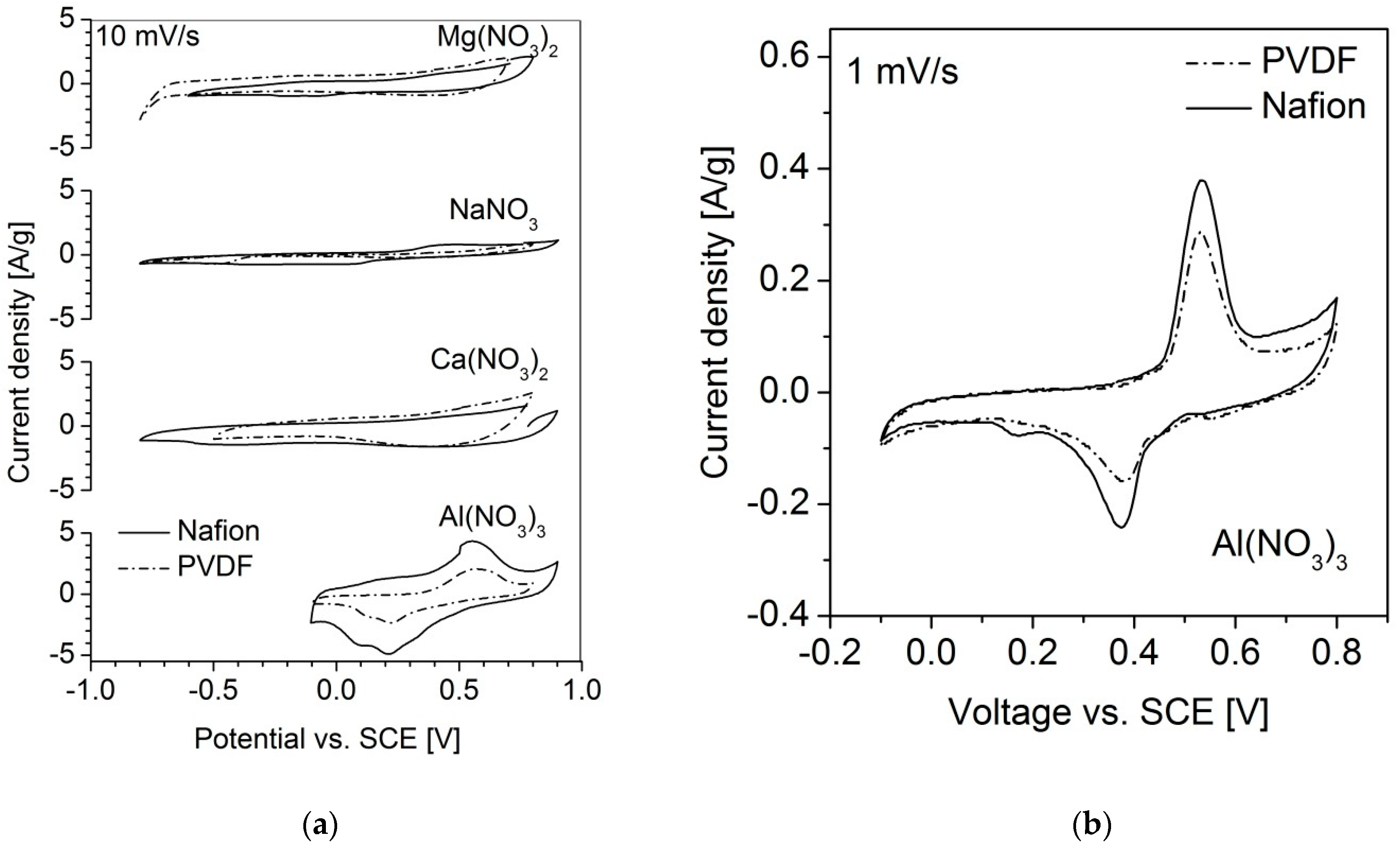
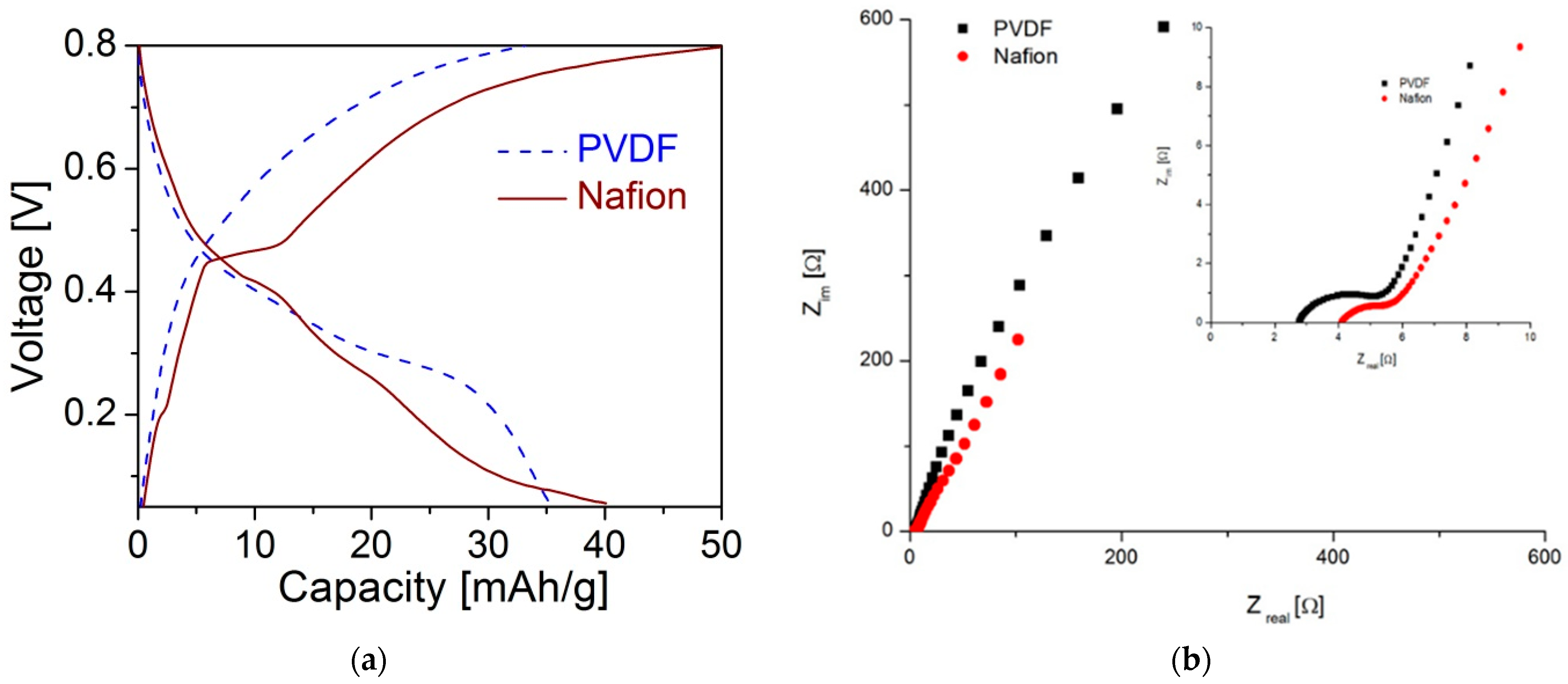
3.3. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Xu, C.; Chen, Z.; Ni, S.; Shen, Z.X. Progress in Aqueous Rechargeable Batteries. Green Energy Environ. 2018, 3, 20–41. [Google Scholar] [CrossRef]
- Yuan, X.; Ma, F.; Zuo, L.; Wang, J.; Yu, N.; Chen, Y.; Zhu, Y.; Huang, Q.; Holze, R.; Wu, Y.; et al. Latest Advances in High-Voltage and High-Energy-Density Aqueous Rechargeable Batteries; Springer: Singapore, 2021; Volume 4, ISBN 0123456789. [Google Scholar]
- Wu, Z.; Lu, C.; Ye, F.; Zhang, L.; Jiang, L.; Liu, Q.; Dong, H.; Sun, Z.; Hu, L. Bilayered VOPO4⋅2H2O Nanosheets with High-Concentration Oxygen Vacancies for High-Performance Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2106816. [Google Scholar] [CrossRef]
- Dupré, N.; Gaubicher, J.; Le Mercier, T.; Wallez, G.; Angenault, J.; Quarton, M. Positive Electrode Materials for Lithium Batteries Based on VOPO4. Solid State Ion. 2001, 140, 209–221. [Google Scholar] [CrossRef]
- Zhang, Z.; Ni, Y.; Avdeev, M.; Kan, W.H.; He, G. Dual-Ion Intercalation to Enable High-Capacity VOPO4 Cathodes for Na-Ion Batteries. Electrochim. Acta 2021, 365, 137376. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, D.; Liu, W.; Rui, X. VOPO4⋅2H2O: Large-Scale Synthesis and Zinc-Ion Storage Application. Front. Energy Res. 2020, 8, 211. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, S.; Yu, X.; He, W.; Zhang, S.; Tian, Y.; Xing, M.; Fu, Y.; Luo, X. Realizing Reversible Storage of Trivalent Aluminium Ions Using VOPO4·2H2O nanosheets as cathode material in aqueous aluminum metal batteries. J. Alloys Compd. 2021, 885, 161008. [Google Scholar] [CrossRef]
- Wang, J.; Tan, S.; Xiong, F.; Yu, R.; Wu, P.; Cui, L.; An, Q. VOPO4·2H2O as a New Cathode Material for Rechargeable Ca-Ion Batteries. Chem. Commun. 2020, 56, 3805–3808. [Google Scholar] [CrossRef]
- Verma, V.; Kumar, S.; Manalastas, W.; Zhao, J.; Chua, R.; Meng, S.; Kidkhunthod, P.; Srinivasan, M. Layered VOPO4 as a Cathode Material for Rechargeable Zinc-Ion Battery: Effect of Polypyrrole Intercalation in the Host and Water Concentration in the Electrolyte. ACS Appl. Energy Mater. 2019, 2, 8667–8674. [Google Scholar] [CrossRef]
- Park, N.G.; Kim, K.M.; Chang, S.H. Sonochemical Synthesis of the High Energy Density Cathode Material VOPO4·2H2O. Electrochem. Commun. 2001, 3, 553–556. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Z.; Wang, H.; Ji, Z.; Feng, Y.; Wang, J.; Liu, J.; Hu, M.; Fei, J.; Gan, W.; et al. A High-Performance Flexible Aqueous Al Ion Rechargeable Battery with Long Cycle Life. Energy Storage Mater. 2020, 25, 426–435. [Google Scholar] [CrossRef]
- Shi, H.; Song, Y.; Qin, Z.; Li, C.; Guo, D.; Liu, X.; Sun, X. Inhibiting VOPO4⋅X H2O Decomposition and Dissolution in Rechargeable Aqueous Zinc Batteries to Promote Voltage and Capacity Stabilities. Angew. Chem. 2019, 131, 16203–16207. [Google Scholar] [CrossRef]
- Hyoung, J.; Heo, J.W.; Chae, M.S.; Hong, S.T. Electrochemical Exchange Reaction Mechanism and the Role of Additive Water to Stabilize the Structure of VOPO4⋅2H2O as a Cathode Material for Potassium-Ion Batteries. ChemSusChem 2019, 12, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lu, X.; Peng, L.; Xu, K.; Peng, X.; Huang, J.; Yu, G.; Xie, Y. Two-Dimensional Vanadyl Phosphate Ultrathin Nanosheets for High Energy Density and Flexible Pseudocapacitors. Nat. Commun. 2013, 4, 2431. [Google Scholar] [CrossRef] [PubMed]
- Sithamparappillai, U.; Nuño, J.L.; Dummer, N.F.; Weng, W.; Kiely, C.J.; Bartley, J.K.; Hutchings, G.J. Effect on the Structure and Morphology of Vanadium Phosphates of the Addition of Alkanes during the Alcohol Reduction of VOPO4·2H2O. J. Mater. Chem. 2010, 20, 5310–5318. [Google Scholar] [CrossRef]
- De Farias, R.F.; Airoldi, C. Synthesis and Characterization of an VOPO4-Polyaniline Lamellar Hybrid Compound. Solid State Sci. 2003, 5, 611–613. [Google Scholar] [CrossRef]
- Borah, P.; Datta, A. Exfoliated VOPO4·2H2O Dispersed on Alumina as a Novel Catalyst for the Selective Oxidation of Cyclohexane. Appl. Catal. A Gen. 2010, 376, 19–24. [Google Scholar] [CrossRef]
- Šišková, R.; Beneš, L.; Zima, V.; Vlček, M.; Votinský, J.; Kalousová, J. Redox Intercalation Reaction of Crystalline VOPO4·2H2O with NaI Solution in Acetone. Polyhedron 1993, 12, 181–185. [Google Scholar] [CrossRef]
- Chauvel, A.; de Roy, M.E.; Besse, J.P.; Benarbia, A.; Legrouri, A.; Barroug, A. Redox Intercalation of Alkali Metals into Vanadyl Phosphate Dihydrate. Mater. Chem. Phys. 1995, 40, 207–211. [Google Scholar] [CrossRef]
- Jacobson, A.J.; Johnson, J.W.; Brody, J.F.; Scanlon, J.C.; Lewandowski, J.T. Redox Intercalation Reactions of VOPO4·2H2O with Mono—and Divalent Cations. Inorg. Chem. 1985, 24, 1782–1787. [Google Scholar] [CrossRef]
- Shi, H.Y.; Wu, W.; Yang, X.; Jia, Z.; Lin, Z.; Qin, Z.; Song, Y.; Guo, D.; Sun, X. Accessing the 2 V VV/VIV Redox Process of Vanadyl Phosphate Cathode for Aqueous Batteries. J. Power Sources 2021, 507, 230270. [Google Scholar] [CrossRef]
- Ma, L.; Li, N.; Long, C.; Dong, B.; Fang, D.; Liu, Z.; Zhao, Y.; Li, X.; Fan, J.; Chen, S.; et al. Achieving Both High Voltage and High Capacity in Aqueous Zinc-Ion Battery for Record High Energy Density. Adv. Funct. Mater. 2019, 29, 1906142. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Ying, Y.; Ma, F.; Chen, S.; Li, Y.; Huang, H.; Zhi, C. Toward Practical High-Areal-Capacity Aqueous Zinc-Metal Batteries: Quantifying Hydrogen Evolution and a Solid-Ion Conductor for Stable Zinc Anodes. Adv. Mater. 2021, 33, 2007406. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, S.; Li, H.; Ruan, Z.; Tang, Z.; Liu, Z.; Wang, Z.; Huang, Y.; Pei, Z.; Zapien, J.A.; et al. Initiating a Mild Aqueous Electrolyte Co3O4/Zn Battery with 2.2 V-High Voltage and 5000-Cycle Lifespan by a Co(iii) Rich-Electrode. Energy Environ. Sci. 2018, 11, 2521–2530. [Google Scholar] [CrossRef]
- Dell, R.; Rand, D.A.J. Understanding Batteries; Royal Society of Chemistry: Cambridge, UK, 2001. [Google Scholar]
- Morales, D.M.; Villalobos, J.; Kazakova, M.A.; Xiao, J.; Risch, M. Nafion-Induced Reduction of Manganese and Its Impact on the Electrocatalytic Properties of a Highly Active MnFeNi Oxide for Bifunctional Oxygen Conversion. ChemElectroChem 2021, 8, 2979–2983. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.E.; Zhenova, A.; Roberts, S.; Petchey, T.; Zhu, P.; Dancer, C.E.J.; McElroy, C.R.; Kendrick, E.; Goodship, V. On the Solubility and Stability of Polyvinylidene Fluoride. Polymers 2021, 13, 1354. [Google Scholar] [CrossRef]
- Cholewinski, A.; Si, P.; Uceda, M.; Pope, M.; Zhao, B. Polymer Binders: Characterization and Development toward Aqueous Electrode Fabrication for Sustainability. Polymers 2021, 13, 631. [Google Scholar] [CrossRef]
- Rietveld, H.M. A Profile Refinement Method for Nuclear and Magnetic Structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Cheary, R.W.; Coelho, A. A Fundamental Parameters Approach to X-ray Line-Profile Fitting. J. Appl. Crystallogr. 1992, 25, 109–121. [Google Scholar] [CrossRef]
- R’Kha, C.; Vandenborre, M.T.; Livage, J.; Prost, R.; Huard, E. Spectroscopic Study of Colloidal VOPO4·2H2O. J. Solid State Chem. 1986, 63, 202–215. [Google Scholar] [CrossRef]
- Tietze, H.R. The Crystal and Molecular Structure of Oxovanadium(v) Orthophosphate Dihydrate, VOPO4·2H2O. Aust. J. Chem. 1981, 34, 2035–2038. [Google Scholar] [CrossRef]
- Antonio, M.R.; Barbour, R.L.; Blum, P.R. Interlayer Coordination Environments of Iron, Cobalt, and Nickel in Vanadyl Phosphate Dihydrate, VOPO4·2H2O, Intercalation Compounds. Inorg. Chem. 1987, 26, 1235–1243. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Moghadam, M.H.M.; Sharif, F. Spontaneous Exfoliation of Graphite Oxide in Polar Aprotic Solvents as the Route to Produce Graphene Oxide—Organic Solvents Liquid Crystals. Carbon 2013, 64, 403–415. [Google Scholar] [CrossRef]
- Shpeizer, B.G.; Ouyang, X.; Heising, J.M.; Clearfield, A. Synthesis and Crystal Structure of a New Vanadyl Phosphate [H0.6(VO)3(PO4)3(H2O)3]·4H2O and Its Conversion to Porous Products. Chem. Mater. 2001, 13, 2288–2296. [Google Scholar] [CrossRef]
- Le Bail, A. Whole Powder Pattern Decomposition Methods and Applications: A Retrospection. Powder Diffr. 2005, 20, 316. [Google Scholar] [CrossRef]
- Jugović, D.; Milović, M.; Ivanovski, V.N.; Škapin, S.; Barudžija, T.; Mitrić, M. Microsized Fayalite Fe2SiO4 as Anode Material: The Structure, Electrochemical Properties and Working Mechanism. J. Electroceramics 2021. [CrossRef]
- Zhao, C.; Zhang, X.; He, Z.; Guan, Q.; Li, W. Demystifying the Mechanism of NMP Ligands in Promoting Cu-Catalyzed Acetylene Hydrochlorination: Insights from a Density Functional Theory Study. Inorg. Chem. Front. 2020, 7, 3204–3216. [Google Scholar] [CrossRef]
- Milović, M.; Vujković, M.; Jugović, D.; Mitrić, M. Electrochemical and Structural Study on Cycling Performance of Γ-LiV2O5 Cathode. Ceram. Int. 2021, 47, 17077–17083. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective Ionic Radii in Oxides and Fluorides. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1969, 25, 925–946. [Google Scholar] [CrossRef]
- Arun, T.; Mohanty, A.; Rosenkranz, A.; Wang, B.; Yu, J.; Morel, M.J.; Udayabhaskar, R.; Hevia, S.A.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; et al. Role of Electrolytes on the Electrochemical Characteristics of Fe3O4/MXene/RGO Composites for Supercapacitor Applications. Electrochim. Acta 2021, 367, 137473. [Google Scholar] [CrossRef]
- Iqbal, M.Z.; Zakar, S.; Haider, S.S. Role of Aqueous Electrolytes on the Performance of Electrochemical Energy Storage Device. J. Electroanal. Chem. 2020, 858, 113793. [Google Scholar] [CrossRef]
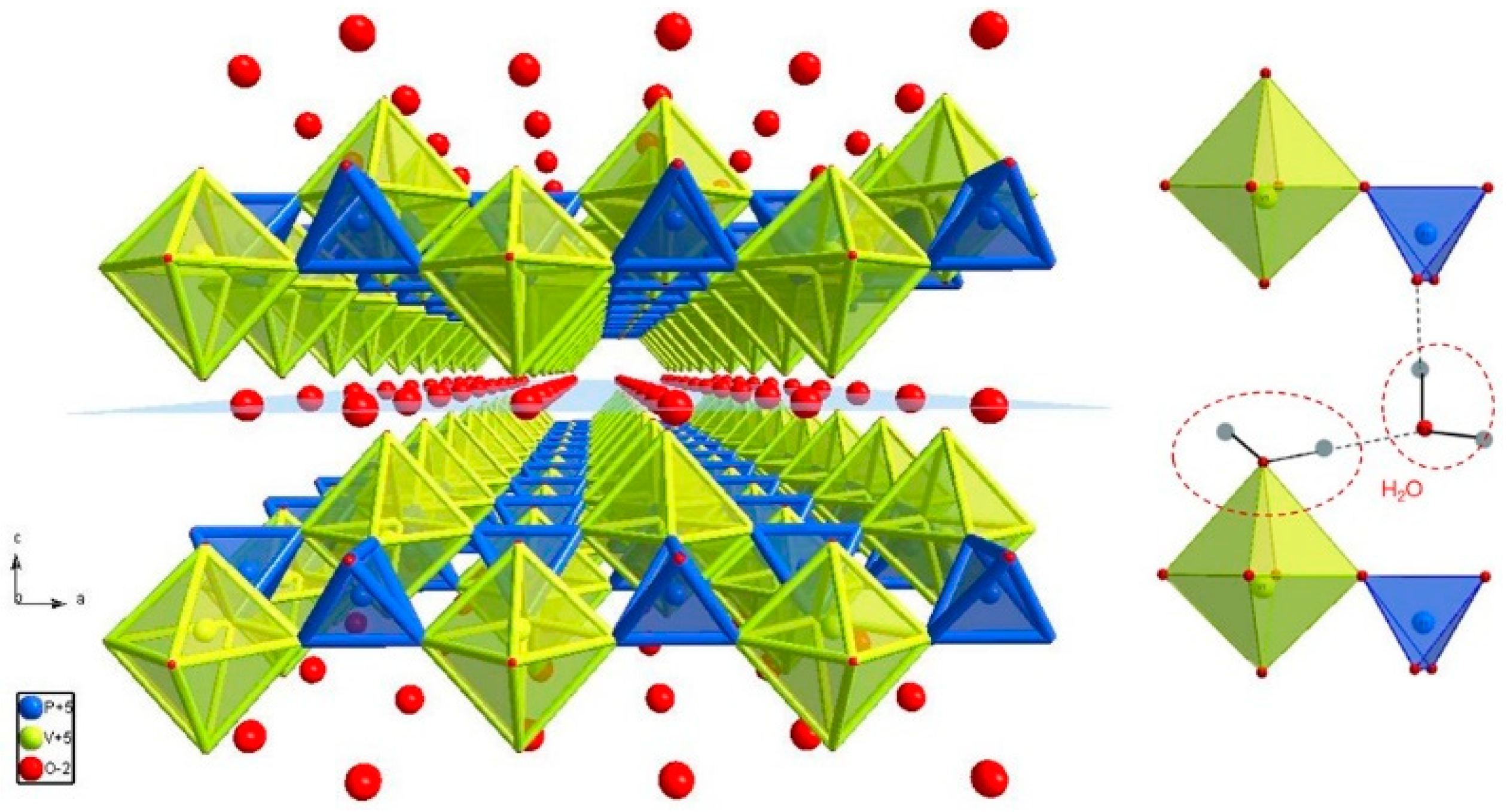
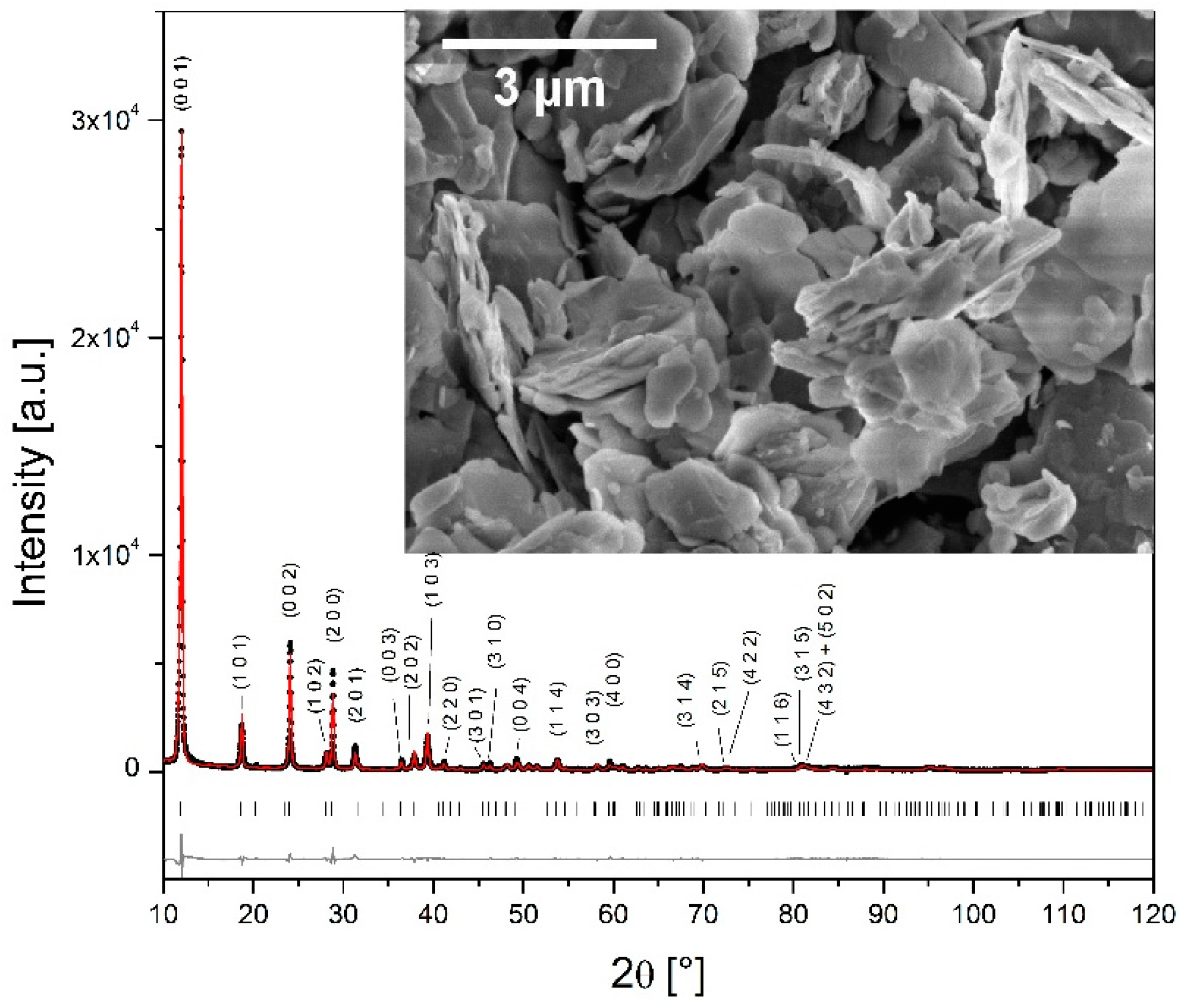
| Lattice parameters (Å) | a = 6.2138 (9) b = 6.2138 (9) c = 7.4140 (10) |
| Primitive cell volume (Å3) | V = 286.26 (9) |
| Mean crystallite size (nm) | 280 (20) |
| Microstrain (%) | 0.38 (2) |
| Fractional Coordinates | x | y | z | B [Å2] |
|---|---|---|---|---|
| P1 (2b) | 0.75 | 0.25 | 0.5 | 2.5 |
| V (2c) | 0.25 | 0.25 | 0.4092 (5) | 0.5 |
| O1 (8i) | 0.25 | 0.941 (2) | 0.3809 (8) | 0.4 |
| O2 (2c) | 0.25 | 0.25 | 0.091 (2) | 2.1 |
| O3 (2a) | 0.75 | 0.25 | 0 | 2.1 |
| O4 (2c) | 0.25 | 0.25 | 0.631 (1) | 2.1 |
| M–O Bond | Bond Length [Å] |
|---|---|
| P–O1 × 4 | 1.4804 (97) |
| P–O3 × 2 | 3.7070 (5) |
| (P–O1) aver. | 1.4804 (97) |
| PO4 distortion | 0 |
| V–O1 × 4 | 1.930 (11) |
| V–O2 | 2.357 (13) |
| V–O4 | 1.644 (11) |
| (V–O) aver. | 1.954 |
| VO6 distortion | 1.1 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jugović, D.; Milović, M.D.; Barudžija, T.; Kuzmanović, M.; Vujković, M.; Mitrić, M. The Influence of a Binder in a Composite Electrode: The Case Study of Vanadyl Phosphate in Aqueous Electrolyte. Materials 2022, 15, 9041. https://doi.org/10.3390/ma15249041
Jugović D, Milović MD, Barudžija T, Kuzmanović M, Vujković M, Mitrić M. The Influence of a Binder in a Composite Electrode: The Case Study of Vanadyl Phosphate in Aqueous Electrolyte. Materials. 2022; 15(24):9041. https://doi.org/10.3390/ma15249041
Chicago/Turabian StyleJugović, Dragana, Miloš D. Milović, Tanja Barudžija, Maja Kuzmanović, Milica Vujković, and Miodrag Mitrić. 2022. "The Influence of a Binder in a Composite Electrode: The Case Study of Vanadyl Phosphate in Aqueous Electrolyte" Materials 15, no. 24: 9041. https://doi.org/10.3390/ma15249041
APA StyleJugović, D., Milović, M. D., Barudžija, T., Kuzmanović, M., Vujković, M., & Mitrić, M. (2022). The Influence of a Binder in a Composite Electrode: The Case Study of Vanadyl Phosphate in Aqueous Electrolyte. Materials, 15(24), 9041. https://doi.org/10.3390/ma15249041






