Effect of Ti3SiC2 and Ti3AlC2 Particles on Microstructure and Wear Resistance of Microarc Oxidation Layers on TC4 Alloy
Abstract
1. Introduction
2. Experiments and Characterization
2.1. Preparation and Experiments
2.2. Analysis and Characterization
3. Results and Discussion
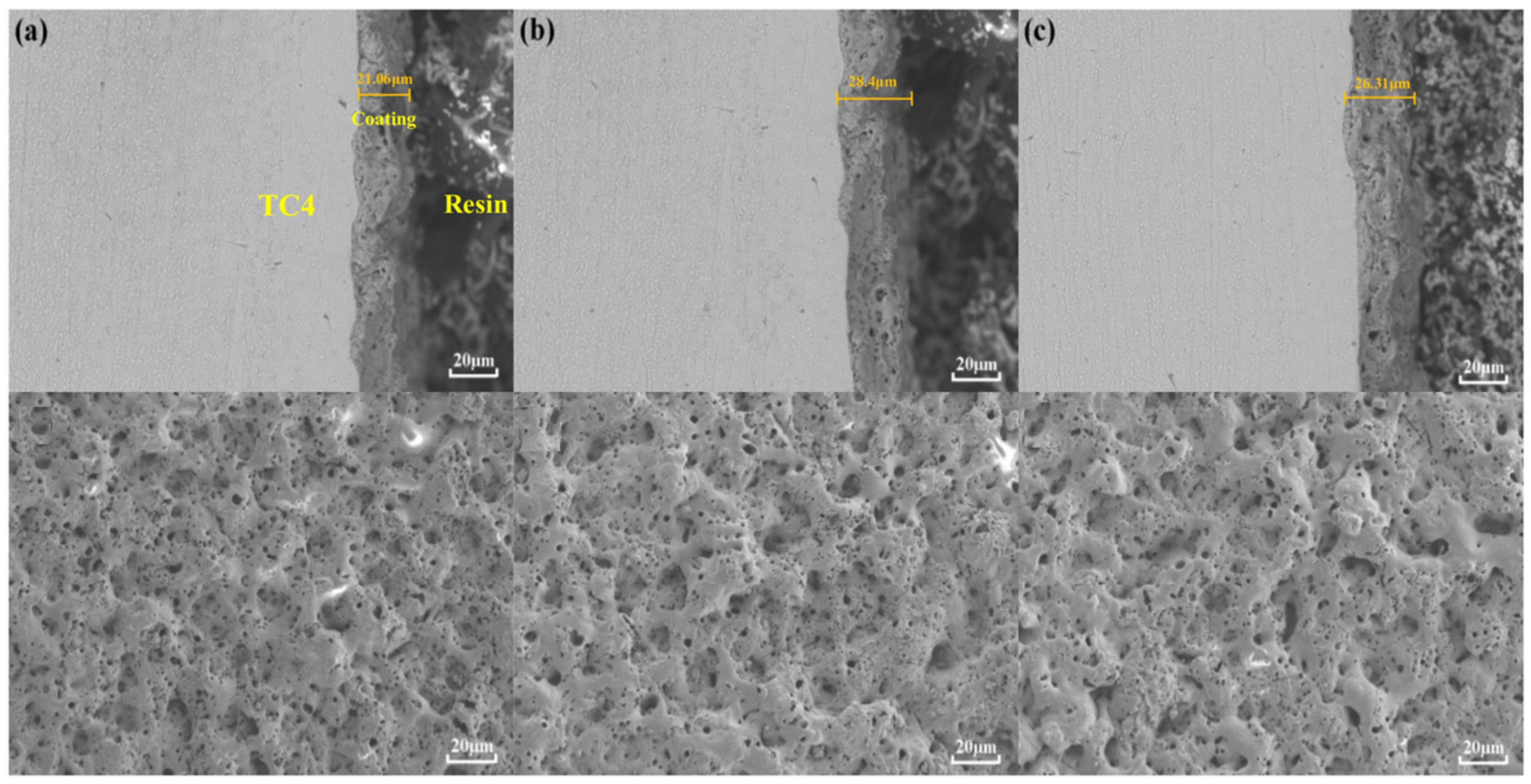
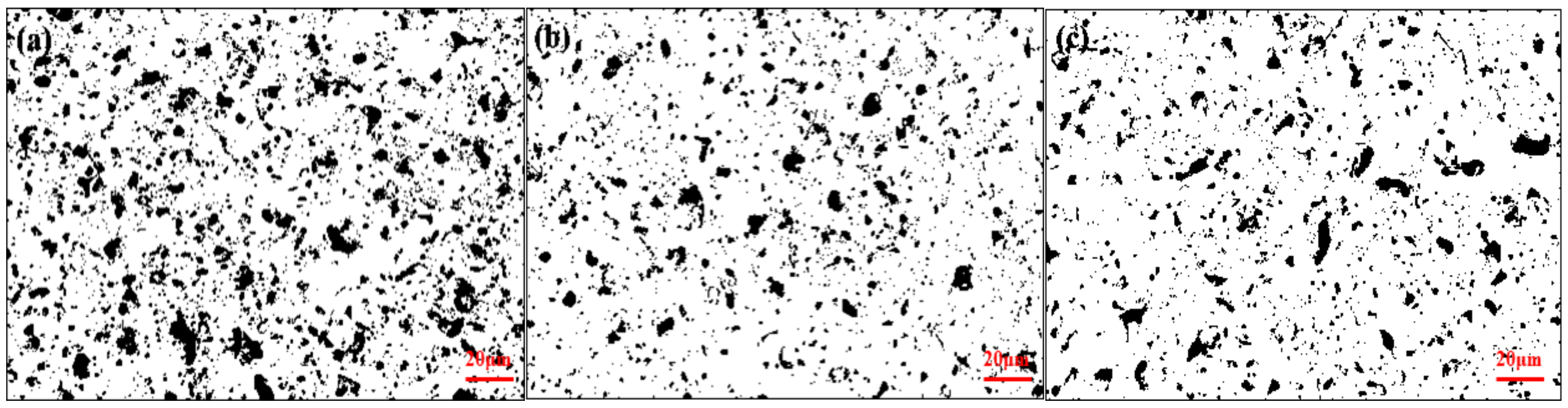
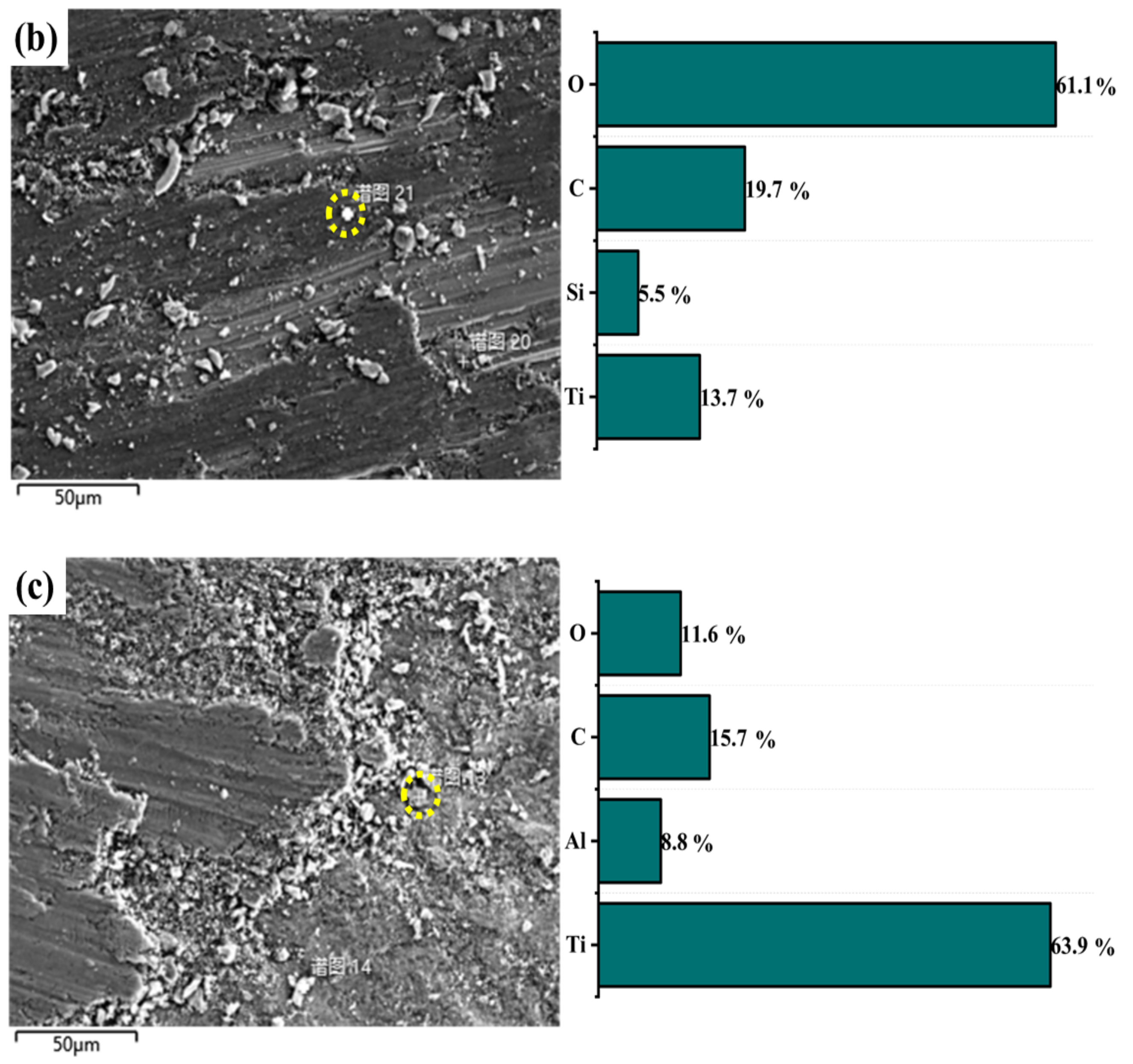
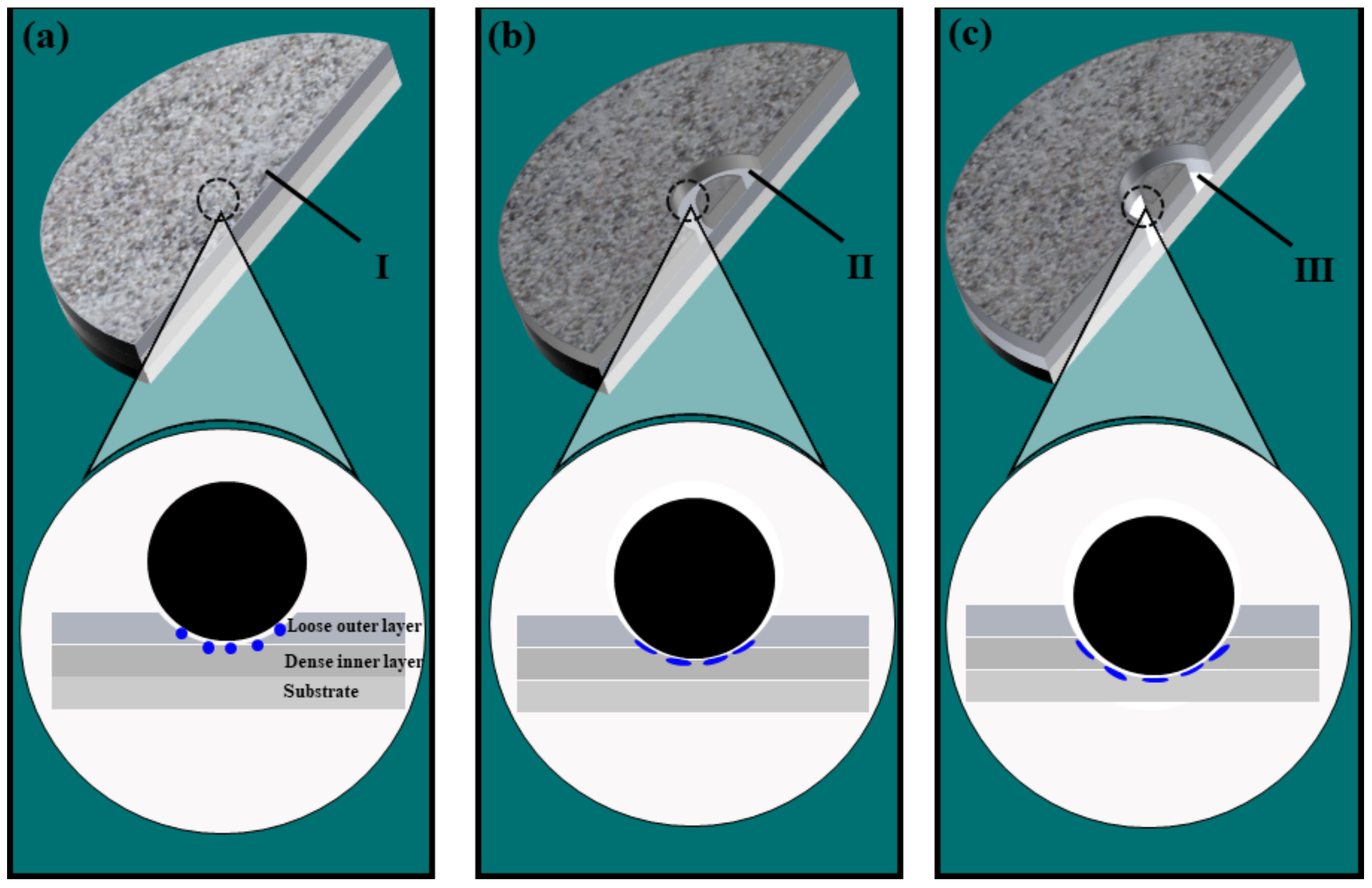
3.1. Cross Section and Surface Topography
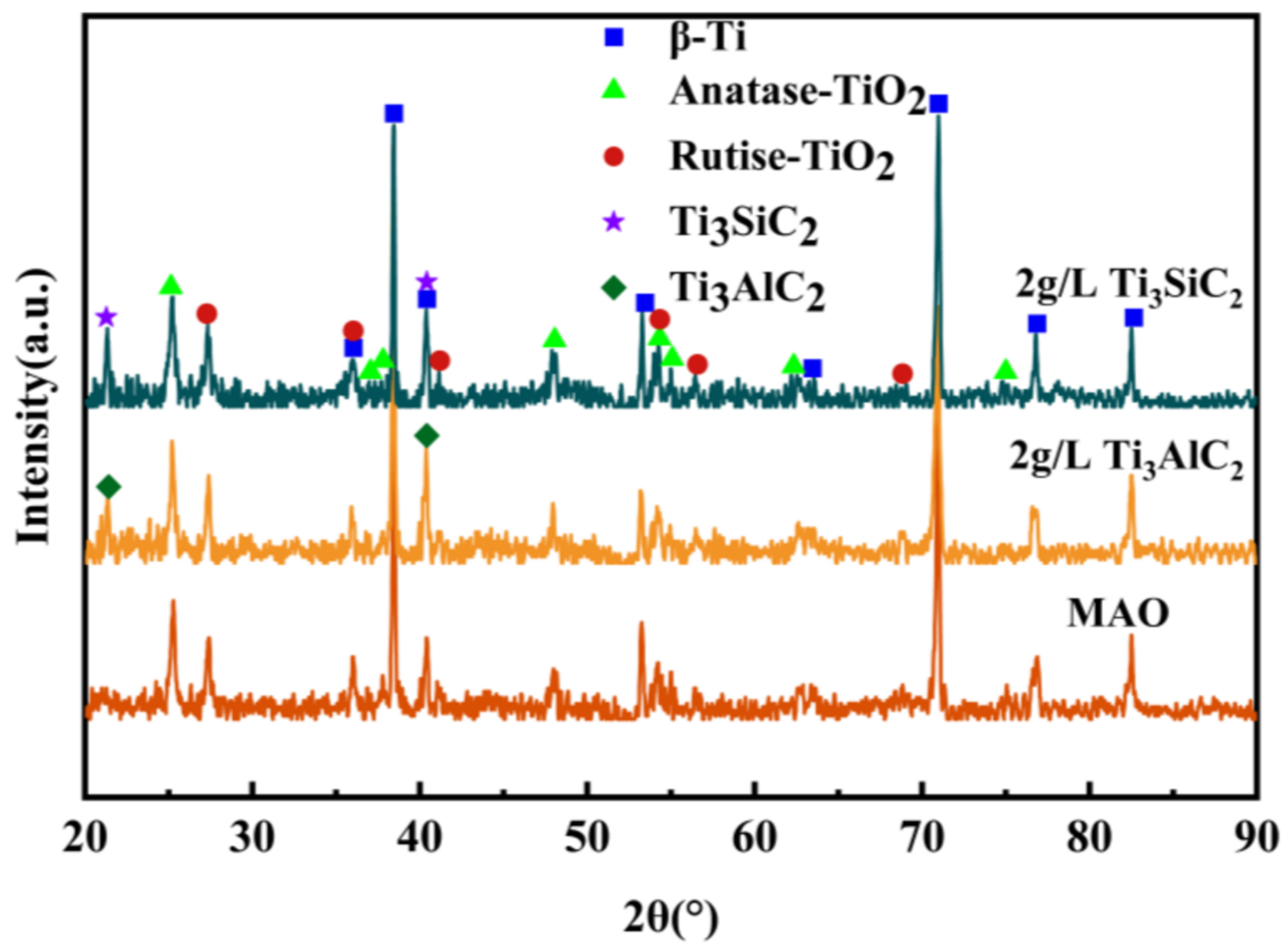
3.2. X-ray Analysis
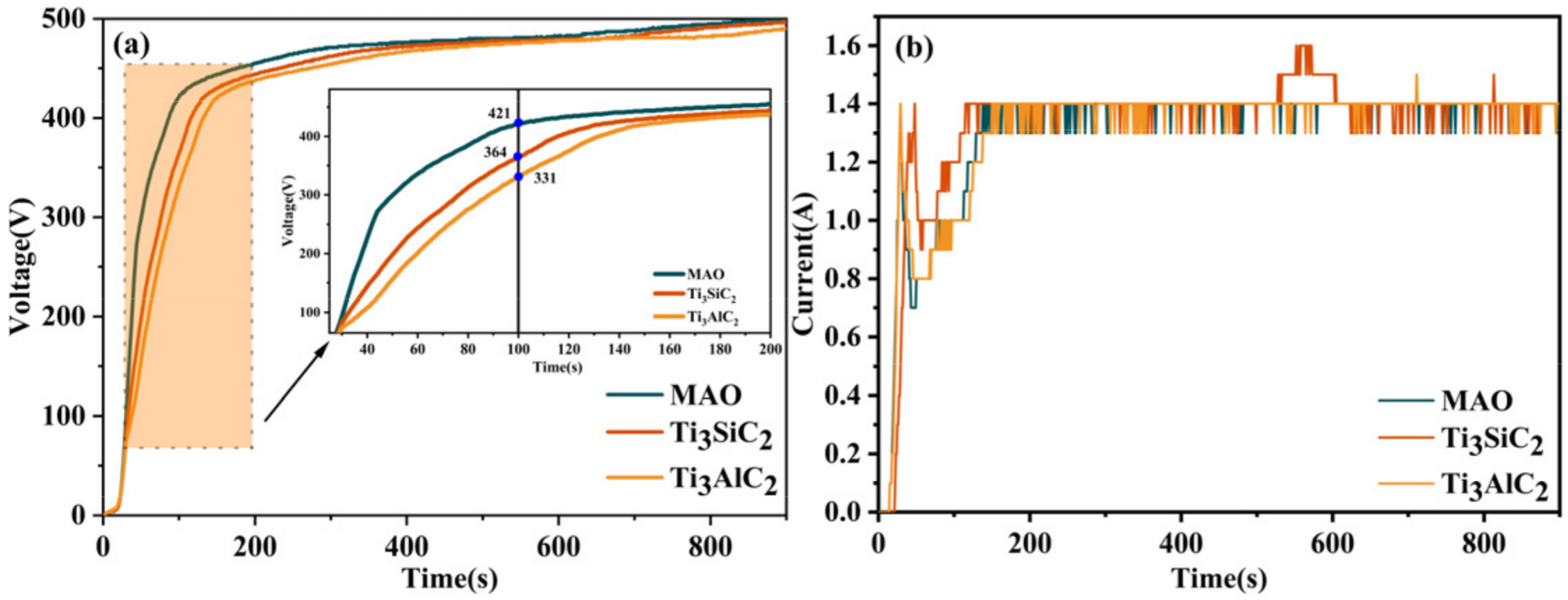
3.3. Friction and Wear Experiment
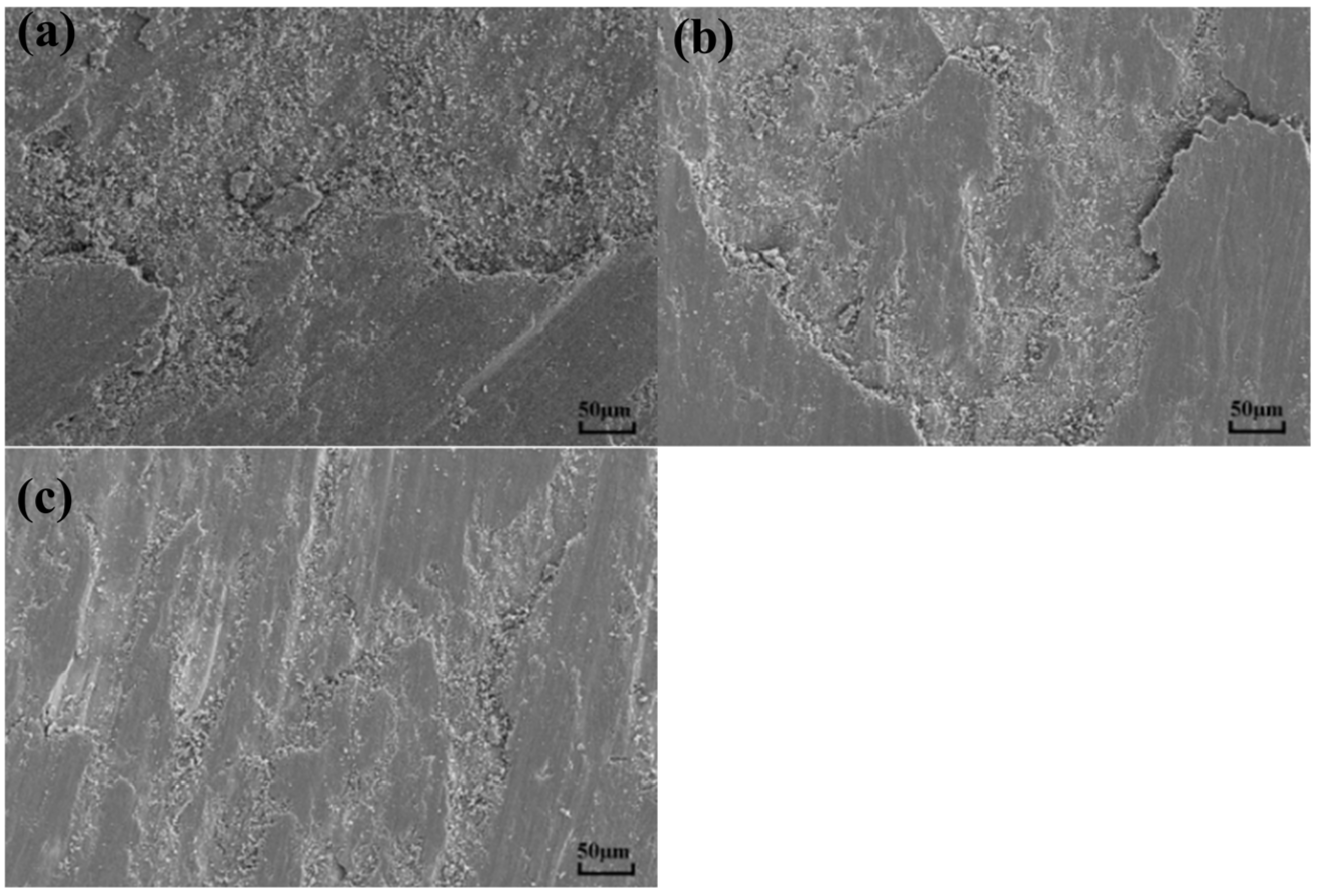
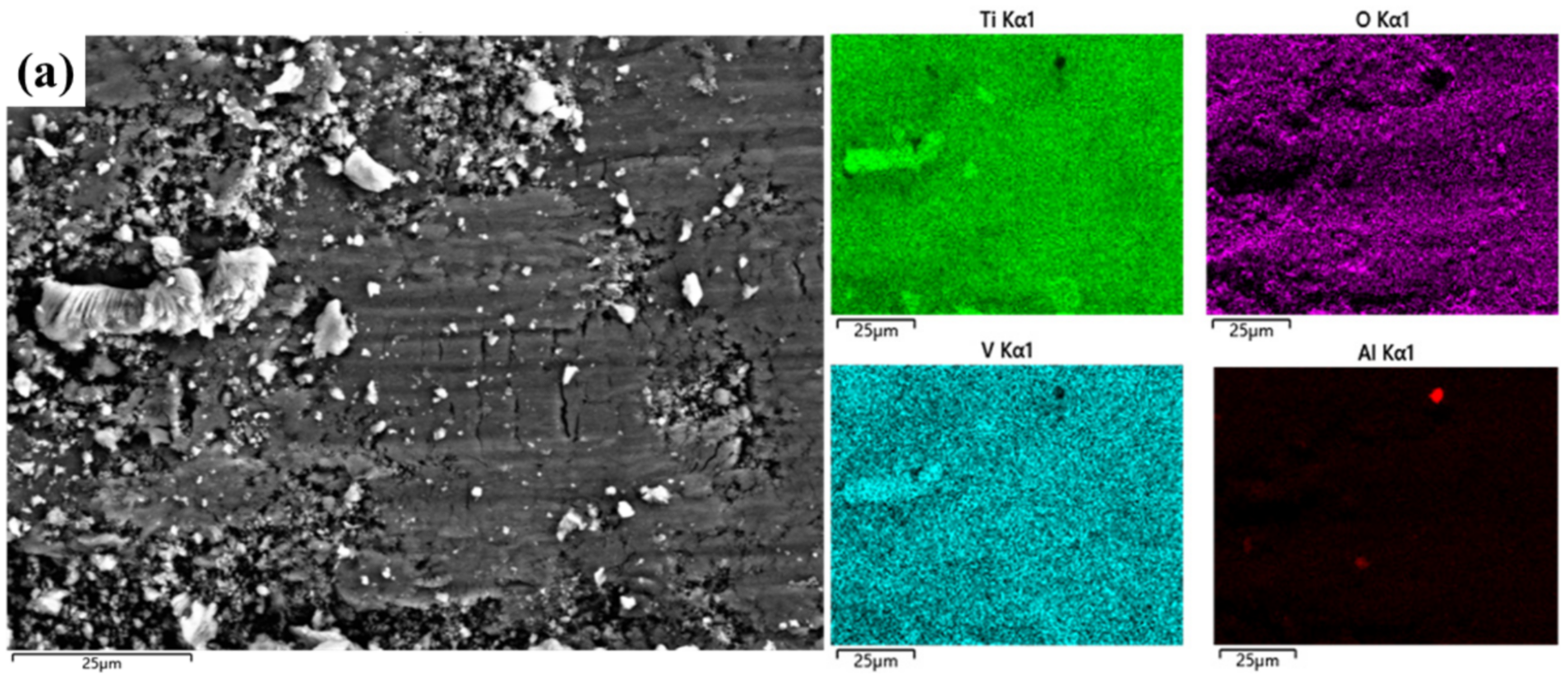
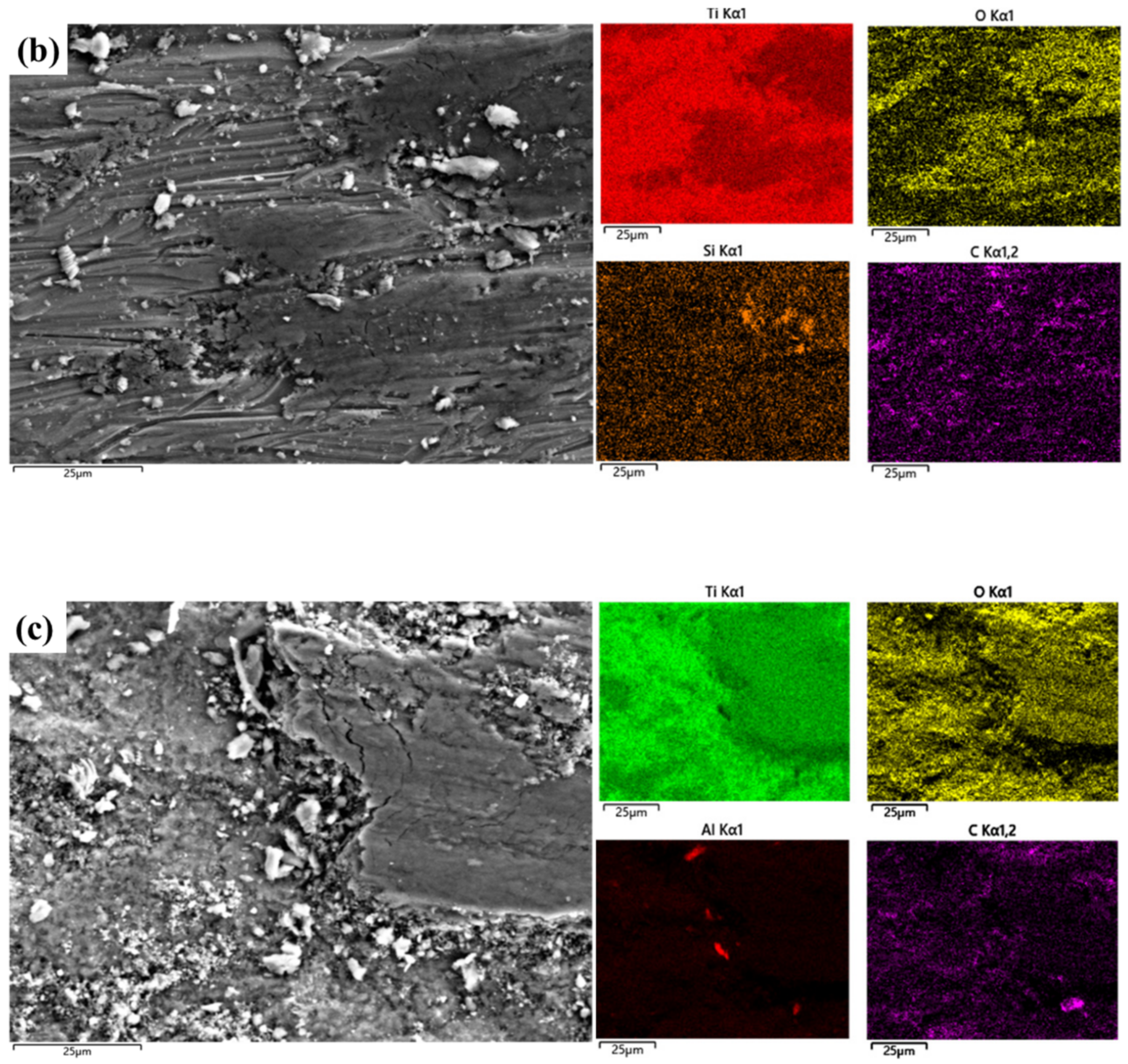
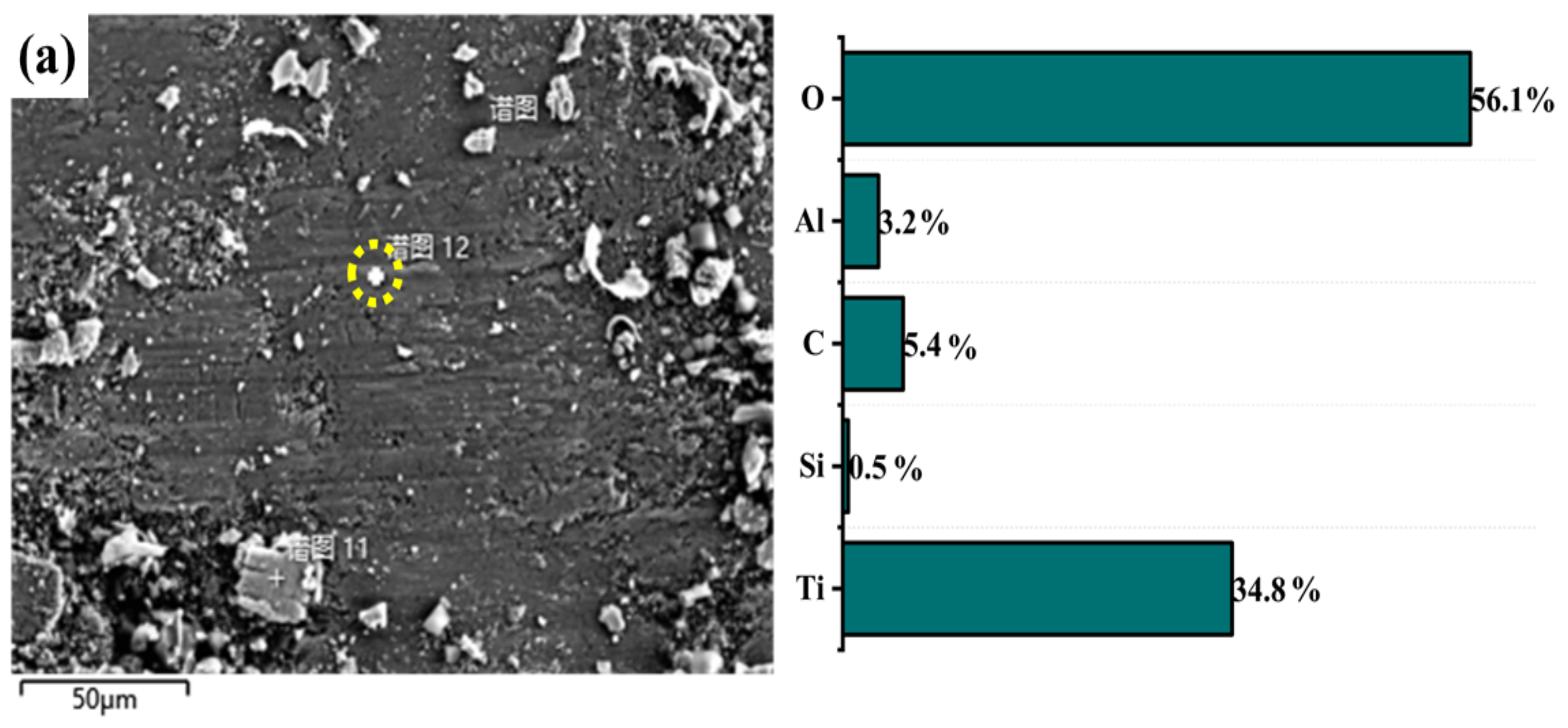
3.4. Wear Mechanisms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korkmaz, K. The effect of Micro-arc Oxidation treatment on the microstructure and properties of open cell Ti–6Al–4V alloy foams. Surf. Coat. Technol. 2015, 272, 72–78. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Zou, Y.-C.; Wang, S.-Q. Fabrication and Performance of Multifuctional Ceramic Coatings Formed by Microarc Oxidation on Metals: A Critial Review. China Surf. Eng. 2018, 31, 20–45. [Google Scholar]
- Jin, H.-X.; Wei, K.-X.; Li, J.-M. Progress in research on titanium alloys for aviation. Chin. J. Nonferrous Met. 2015, 25, 280–292. [Google Scholar]
- Fu, C.; Weng, S.; Fan, J. Boron-based materials modified on the surface of TiO2 nanorods via electroless plating toward high-efficient solar-driven water splitting. Chem. Eng. J. 2022, 430, 132881. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Yang, K. Microstructure and performance of YTaO4 coating deposited by atmospheric plasma spraying on TC4 titanium alloy surface. Surf. Coat. Technol. 2022, 431, 128004. [Google Scholar] [CrossRef]
- Yumusak, G.; Leyland, A.; Matthews, A. A microabrasion wear study of nitrided α-Ti and β-TiNb PVD metallic thin films, pre-deposited onto titanium alloy substrates. Surf. Coat. Technol. 2022, 442, 128423. [Google Scholar] [CrossRef]
- Grabowski, A.; Florian, T.; Wieczorek, J.; Adamiak, M. Structuring of the Ti6Al4V alloy surface by pulsed laser remelting. Appl. Surf. Sci. 2021, 535, 147618. [Google Scholar] [CrossRef]
- Liao, S.-C.; Chang, C.-T.; Chen, C.-Y. Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications. Surf. Coat. Technol. 2020, 394, 125812. [Google Scholar] [CrossRef]
- Bisztyga-Szklarz, M.; Rząd, E.; Boroń, Ł.; Klimczyk, P.; Polczyk, T.; Łętocha, A.; Rajska, M.; Hebda, M.; Długosz, P. Properties of Microplasma Coating on AZ91 Magnesium Alloy Prepared from Electrolyte with and without the Borax Addition. Materials 2022, 15, 1354. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Gofer, Y.; Borodianskiy, K.; Sobolev, A. Hydroxyapatite Coating on Ti-6Al-7Nb Alloy by Plasma Electrolytic Oxidation in Salt-Based Electrolyte. Materials 2022, 15, 7374. [Google Scholar] [CrossRef]
- Yao, Z.; Jiang, Y.; Jia, F. Growth Characteristics of Plasma Electrolytic Oxidation Ceramic coatings On Ti–6Al–4V Alloy. Appl. Surf. Sci. 2008, 254, 4084–4091. [Google Scholar] [CrossRef]
- Gao, G.-R.; Li, Z.-X.; Du, J.-H. Study of the corrosion resistance and friction properties of TC4 alloy surface micro-arc oxide film. Rare Met. Mater. Eng. 2008, 37, 602–605. [Google Scholar]
- Sun, C.; Hui, R.; Qu, W. Effects of processing parameters on microstructures of TiO2 coatings formed on titanium by plasma electrolytic oxidation. J. Mater. Sci. 2010, 45, 6235–6241. [Google Scholar] [CrossRef]
- Li, Q.-B.; Yang, W.-B.; Liu, C.-C.; Wang, D.-A.; Liang, J. Correlations between the growth mechanism and properties of micro-arc oxidation coatings on titanium alloy: Effects of electrolytes. Surf. Coat. Technol. 2017, 316, 162–170. [Google Scholar] [CrossRef]
- Liu, H.-H.; Wang, S.-X.; Liu, X.-H.; Zhao, Q.; Yu, L.-X.; Gao, A.; Zhong, H. Optimisation of Ti2AlNb alloy micro-arc oxidation electrolyte and study of wear resistance. Surf. Technol. 2018, 47, 85–91. [Google Scholar]
- Chen, X.-X.; Liao, D.-D.; Chen, W.-X.; Cai, L.-P.; Zhao, P.-C.; Jiang, H.; Shi, T.-H. Effect of sodium tungstate addition on the wear resistance of micro-arc oxide layer of titanium alloy drill pipe. Mater. Prot. 2019, 52, 90–94+107. [Google Scholar]
- Guo, Y.-F.; Xu, L.-Y.; Luan, J.-J.; Wan, Y.; Li, R.-C. Effect of carbon nanotubes additive on tribocorrosion performance of micro-arc oxidized coatings on Ti6Al4V alloy. Surf. Interfaces 2022, 28, 101626. [Google Scholar] [CrossRef]
- Di, S.-C.; Guo, Y.-P.; Lv, H.-W.; Yu, J.; Li, Z.-W. Microstructure and properties of rare earth CeO2-doped TiO2 nanostructured composite coatings through micro-arc oxidation. Ceram. Int. 2015, 41, 6178–6186. [Google Scholar] [CrossRef]
- Terleeva, O.-P.; Sharkeev, Y.-P.; Slonova, A.-I. Effect of microplasma modes and electrolyte composition on micro-arc oxidation coatings on titanium for medical applications. Surf. Coat. Technol. 2010, 205, 1723–1729. [Google Scholar] [CrossRef]
- Yue, Y.-J.; Wu, H. Effects of Current Density on Microstructure of Titania Coatings by Micro-arc Oxidation. J. Mater. Sci. Technol. 2012, 28, 321–324. [Google Scholar]
- Sobolev, A.; Kossenko, A.; Borodianskiy, K. Study of the effect of current pulse frequency on Ti6Al4V alloy coating formation by micro arc oxidation. Materials 2019, 12, 3983. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Huang, T.; Wang, D. Effects of duty ratio on properties of micro-arc film on Ti3Zr2Sn3Mo25Nb. Trans. IMF 2018, 96, 269–274. [Google Scholar] [CrossRef]
- Li, H.; Sun, Y.; Zhang, J. Effect of ZrO2 particle on the performance of micro-arc oxidation coatings on Ti6Al4V. Appl. Surf. Sci. 2015, 324, 183–190. [Google Scholar] [CrossRef]
- Mu, M.; Liang, J.; Zhou, X. One-step preparation of TiO2/MoS2 composite coating on Ti6Al4V alloy by plasma electrolytic oxidation and its tribological properties. Surf. Coat. Technol. 2013, 214, 124–130. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, X.; Zhang, C. Effect of the graphene oxide additive on the corrosion resistance of the plasma electrolytic oxidation coating of the AZ31 magnesium alloy. Corros. Sci. 2017, 114, 146–155. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Jiang, Z.-H.; Yao, Z.-P. Electrochemical study of growth behaviour of plasma electrolytic oxidation coating on Ti6Al4V: Effects of the additive. Corros. Sci. 2010, 52, 3465–3473. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zheng, M.-Y.; Wu, K. Microarc oxidation coating formed on SiCw/AZ91 magnesium matrix composite and its corrosion resistance. Mater. Lett. 2005, 59, 1727–1731. [Google Scholar] [CrossRef]
- Li, H.-Z.; Ma, Y.-P.; Li, Z.-X. Effect of graphite content on the micro-arc oxide film of TC4 titanium alloy. Titan. Ind. Prog. 2017, 34, 26–29. [Google Scholar]
- Zhang, Q.; Li, Y.-H.; Liu, X. TC4 Properties of ZrO2/TiO2 composite ceramic films on the surface of titanium alloys. Trans. Mater. Heat Treat. 2014, 9, 199–204. [Google Scholar]
- Zhai, W.; Lu, W.; Zhang, P. Wear-triggered self-healing behavior on the surface of nanocrystalline nickel aluminum bronze/Ti3SiC2 composites. Appl. Surf. Sci. 2018, 436, 1038–1049. [Google Scholar] [CrossRef]
- Dong, H.-R.; Ma, Y.; Guo, H.-X.; Zhang, Y.-F.; Hao, Y. Denseness of AZ91D magnesium alloy micro-arc oxide films and its effect on corrosion resistance. Trans. Nonferrous Met. Soc. China 2015, 25, 844–851. [Google Scholar]
- Nie, H.-C. Study on The Influence of Solution Components and Sealing Process on The Insulation Properties of Aluminium Alloy Micro Lone Oxide Layer; Xi’an University of Technology: Xi’an, China, 2017. [Google Scholar]
- Hu, Z.-C.; Xie, F.-Q.; Wu, X.-Q. Effects of Voltage on the Characteristics and Corrosion Resistance of Microarc Oxidation Coatings on Ti6Al4V Alloy. Adv. Mat. Res. 2010, 97, 1336–1339. [Google Scholar] [CrossRef]
- Li, B.-J.; Zhao, H.; Wang, X.-H.; Zhu, Q.-Z. Effect of electrical parameters on the micro-arc oxidation ceramic film of TC4 titanium alloy. J. Shenyang Univ. Technol. 2011, 30, 59–61. [Google Scholar]
- Qi, Y.-M.; Peng, Z.-J.; Liu, B.-X. Preparation of high hardness micro-arc oxide films on alloy surfaces and study of wear resistance. Surf. Technol. 2019, 48, 81–88. [Google Scholar]
- Pezzato, L.; Brunelli, K.; Dabala, M. Corrosion properties of plasma electrolytic oxidation coated AA7075 treated using an electrolyte containing lanthanum-salts. Surf. Interface. Anal. 2016, 48, 729–738. [Google Scholar] [CrossRef]



| Name | Formula | Purity Manufacturer | Country |
|---|---|---|---|
| TC4 | - | HuiJing Metal Materials Limited | China |
| Na2SiO3 | AR | Sinopharm Chemical Reagent Co. | China |
| Na3PO4 | AR | Sinopharm Chemical Reagent Co. | China |
| Na2WO4 | AR | Sinopharm Chemical Reagent Co. | China |
| Ti3SiC2 | 98% | Forsman | China |
| Ti3AlC2 | 98% | Forsman | China |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, G.; Shang, J.; Lin, D. Effect of Ti3SiC2 and Ti3AlC2 Particles on Microstructure and Wear Resistance of Microarc Oxidation Layers on TC4 Alloy. Materials 2022, 15, 9078. https://doi.org/10.3390/ma15249078
Gu G, Shang J, Lin D. Effect of Ti3SiC2 and Ti3AlC2 Particles on Microstructure and Wear Resistance of Microarc Oxidation Layers on TC4 Alloy. Materials. 2022; 15(24):9078. https://doi.org/10.3390/ma15249078
Chicago/Turabian StyleGu, Gaoyang, Jian Shang, and Dan Lin. 2022. "Effect of Ti3SiC2 and Ti3AlC2 Particles on Microstructure and Wear Resistance of Microarc Oxidation Layers on TC4 Alloy" Materials 15, no. 24: 9078. https://doi.org/10.3390/ma15249078
APA StyleGu, G., Shang, J., & Lin, D. (2022). Effect of Ti3SiC2 and Ti3AlC2 Particles on Microstructure and Wear Resistance of Microarc Oxidation Layers on TC4 Alloy. Materials, 15(24), 9078. https://doi.org/10.3390/ma15249078






