Bioactive Glasses as Carriers of Cancer-Targeted Drugs: Challenges and Opportunities in Bone Cancer Treatment
Abstract
:1. Introduction
2. Methodology
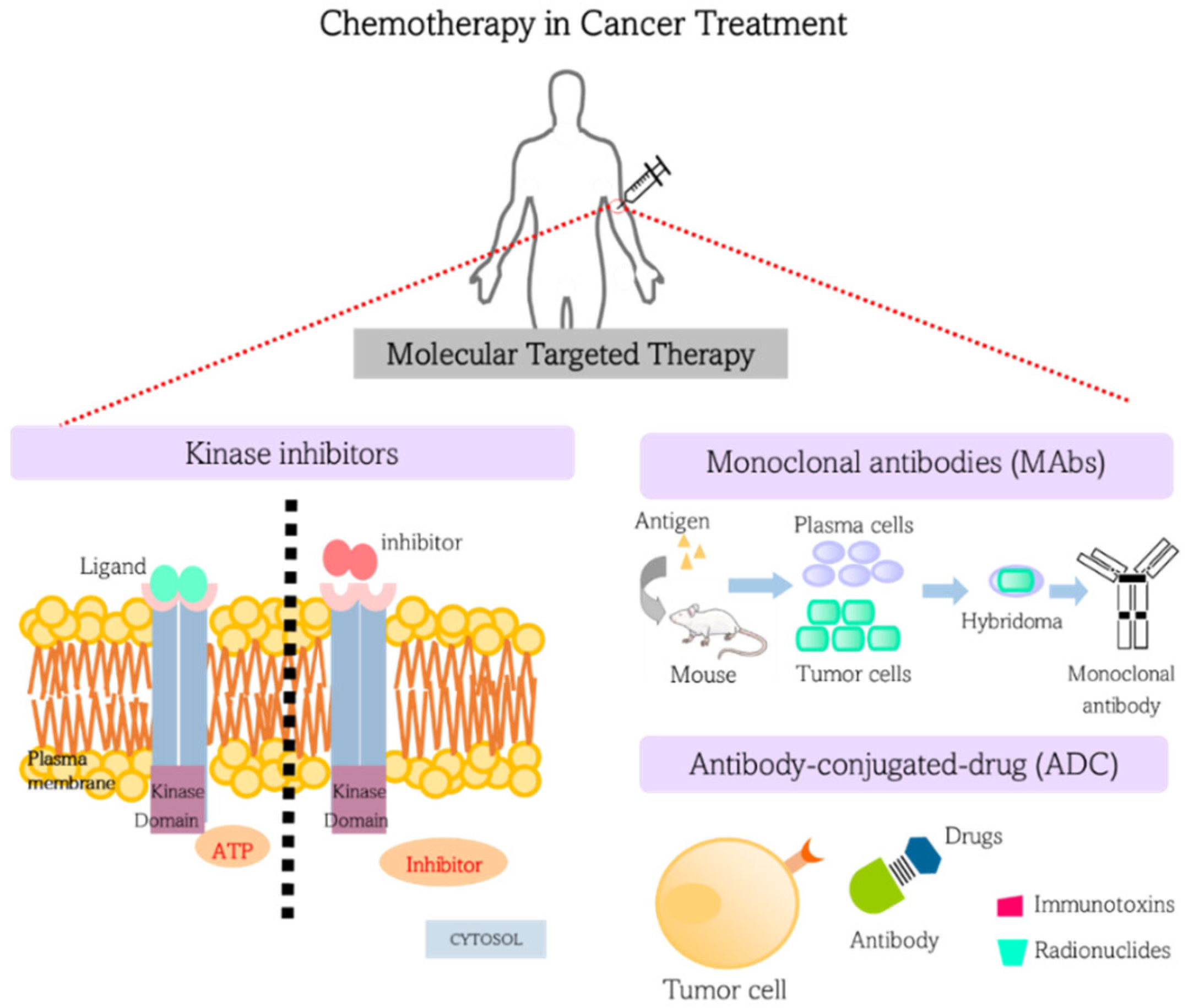
3. Chemotherapy in Cancer Treatment: Targeted Therapies
3.1. Kinase Inhibitors
3.2. Monoclonal Antibodies (MAbs)
3.3. Antibody-Drug Conjugates (ADC)
4. Barriers Affecting Drug Delivery in Cancer Treatment
4.1. Enhanced Permeability Retention (EPR) Effect
4.2. Protein Corona Effect
4.3. High Interstitial Fluid Pressure (IFP)
4.4. Tumor Stroma
5. Bioactive Glasses in Drug Delivery Applied in Cancer Treatment
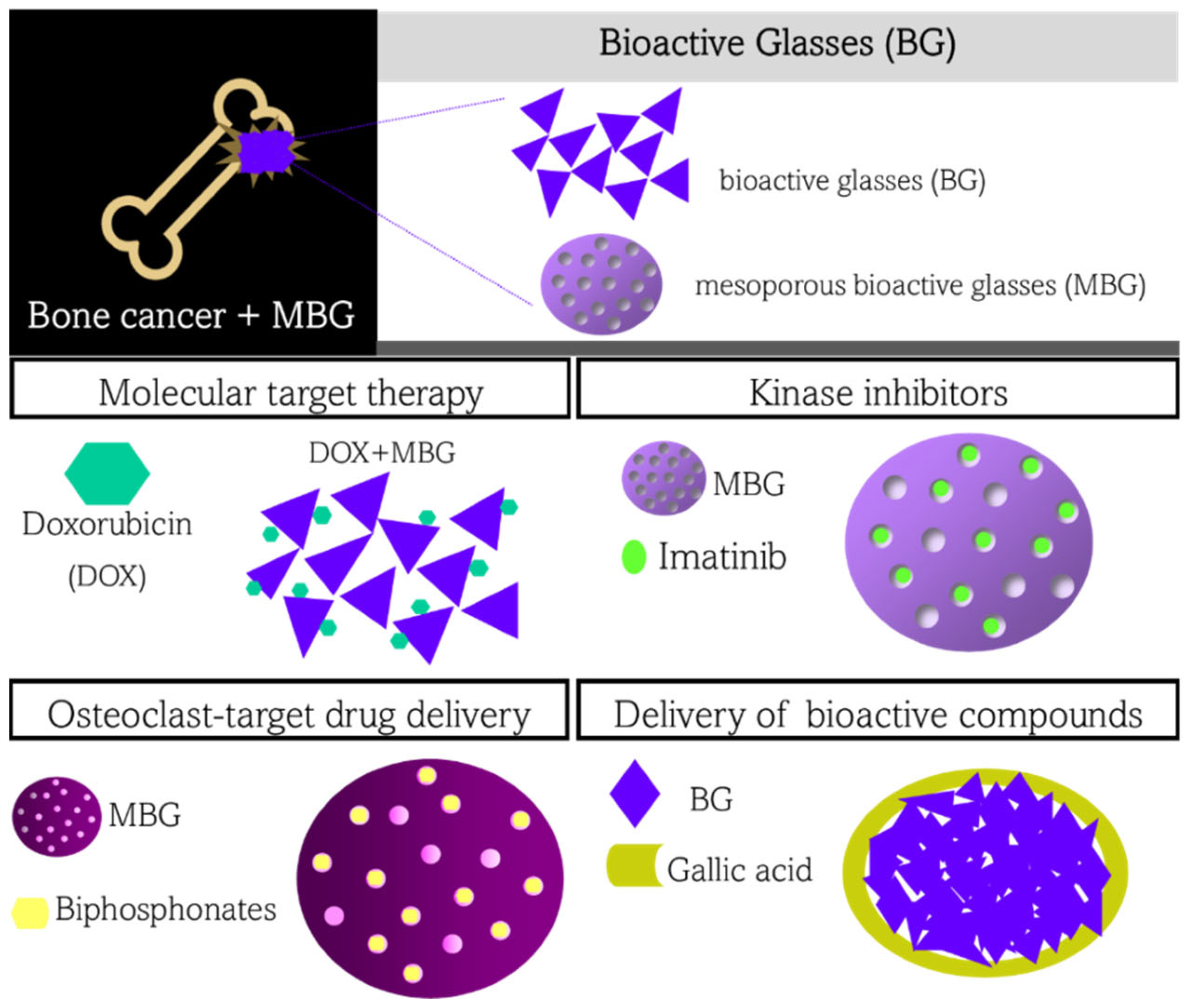
5.1. Bioactive Glasses for Delivery of Molecular Target Therapy
5.2. Bioactive Glasses for Delivery of Kinase Inhibitor
5.3. Bioactive Glasses for Delivery of Osteoclast-Target Drugs
5.4. Bioactive Glasses for Delivery of Other Bioactive Compounds and Therapeutic Ions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tortora, G.J.; Derrickson, B. Principles of Anatomy and Physiology, 13th ed.; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Al-Bari, A.A.; Al Mamun, A. Current Advances in Regulation of Bone Homeostasis. FASEB Bioadv. 2020, 2, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Le Pape, F.; Vargas, G.; Clézardin, P. The Role of Osteoclasts in Breast Cancer Bone Metastasis. J. Bone Oncol. 2016, 5, 93–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Tsuchiya, H. The Role of Surgery in the Treatment of Metastatic Bone Tumor. Int. J. Clin. Oncol. 2022, 27, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M.J.; Livingston, J.A.; Patel, S.R.; Benjamin, R.S. Chemotherapy for Bone Sarcoma in Adults. J. Oncol. Pract. 2016, 12, 208–216. [Google Scholar] [CrossRef]
- Li, X.; Liang, Y.; Lian, C.; Peng, F.; Xiao, Y.; He, Y.; Ma, C.; Wang, Y.; Zhang, P.; Deng, Y.; et al. CST6 Protein and Peptides Inhibit Breast Cancer Bone Metastasis by Suppressing CTSB Activity and Osteoclastogenesis. Theranostics 2021, 11, 9821–9832. [Google Scholar] [CrossRef] [PubMed]
- Zambanini, T.; Borges, R.; de Souza, A.C.S.; Justo, G.Z.; Machado, J.; de Araujo, D.R.; Marchi, J. Holmium-Containing Bioactive Glasses Dispersed in Poloxamer 407 Hydrogel as a Theragenerative Composite for Bone Cancer Treatment. Materials 2021, 14, 1459. [Google Scholar] [CrossRef]
- Chew, S.A.; Danti, S. Biomaterial-Based Implantable Devices for Cancer Therapy. Adv. Healthc. Mater. 2017, 6, 1600766. [Google Scholar] [CrossRef]
- Aspasio, R.D.; Borges, R.; Marchi, J. Biocompatible Glasses for Cancer Treatment. In Biocompatible Glasses: From Bone Regeneration to Cancer Treatment; Marchi, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 249–265. ISBN 978-3-319-44249-5. [Google Scholar]
- Borges, R.; Kai, K.C.; Marchi, J. Biocompatible Glasses for Controlled Release Technology. In Biocompatible Glasses: From Bone Regeneration to Cancer Treatment; Marchi, J., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 285–315. ISBN 978-3-319-44249-5. [Google Scholar]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, T.M.; Berti, F.C.B.; Gasoto, S.C.; Schneider, B.; Stimamiglio, M.A.; Berti, L.F. Calcium Phosphate-Based Bioceramics in the Treatment of Osteosarcoma: Drug Delivery Composites and Magnetic Hyperthermia Agents. Front. Med. Technol. 2021, 3. [Google Scholar] [CrossRef]
- Khalifehzadeh, R.; Arami, H. Biodegradable Calcium Phosphate Nanoparticles for Cancer Therapy. Adv. Colloid Interface Sci. 2020, 279, 102157. [Google Scholar] [CrossRef]
- Moeini, A.; Hassanzadeh Chinijani, T.; Malek Khachatourian, A.; Vinicius Lia Fook, M.; Baino, F.; Montazerian, M. A Critical Review of Bioactive Glasses and Glass–Ceramics in Cancer Therapy. Int. J. Appl. Glass Sci. 2022, 14, 69–87. [Google Scholar] [CrossRef]
- Kargozar, S.; Mozafari, M.; Hamzehlou, S.; Kim, H.-W.; Baino, F. Mesoporous Bioactive Glasses (MBGs) in Cancer Therapy: Full of Hope and Promise. Mater. Lett. 2019, 251, 241–246. [Google Scholar] [CrossRef]
- Kayki-Mutlu, G.; Aksoyalp, Z.S.; Wojnowski, L.; Michel, M.C. A Year in Pharmacology: New Drugs Approved by the US Food and Drug Administration in 2021. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 867–885. [Google Scholar] [CrossRef] [PubMed]
- Ebied, A.M.; Elmariah, H.; Cooper-DeHoff, R.M. New Drugs Approved in 2021. Am. J. Med. 2022, 135, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.-M. Targeted Therapy in Cancer. Cancer Chemother. Pharm. 2015, 76, 1113–1132. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular Targeted Therapy: Treating Cancer with Specificity. Eur. J. Pharm. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Bhullar, K.S.; Lagarón, N.O.; McGowan, E.M.; Parmar, I.; Jha, A.; Hubbard, B.P.; Rupasinghe, H.P.V. Kinase-Targeted Cancer Therapies: Progress, Challenges and Future Directions. Mol. Cancer 2018, 17, 48. [Google Scholar] [CrossRef] [Green Version]
- Yamaoka, T.; Kusumoto, S.; Ando, K.; Ohba, M.; Ohmori, T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lheureux, S.; Denoyelle, C.; Ohashi, P.S.; de Bono, J.S.; Mottaghy, F.M. Molecularly Targeted Therapies in Cancer: A Guide for the Nuclear Medicine Physician. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 41–54. [Google Scholar] [CrossRef] [Green Version]
- Fujita, K.; Ishida, H.; Kubota, Y.; Sasaki, Y. Toxicities of Receptor Tyrosine Kinase Inhibitors in Cancer Pharmacotherapy: Management with Clinical Pharmacology. Curr. Drug Metab. 2017, 18, 186–198. [Google Scholar] [CrossRef]
- Lodish, M.B. Clinical Review: Kinase Inhibitors: Adverse Effects Related to the Endocrine System. J. Clin. Endocrinol. Metab. 2013, 98, 1333–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, G.; Milstein, C. Continuous Cultures of Fused Cells Secreting Antibody of Predefined Specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal Antibodies: Versatile Platforms for Cancer Immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falzone, L.; Salomone, S.; Libra, M. Evolution of Cancer Pharmacological Treatments at the Turn of the Third Millennium. Front. Pharm. 2018, 9, 1300. [Google Scholar] [CrossRef] [Green Version]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [Green Version]
- Hamid, O.; Puzanov, I.; Dummer, R.; Schachter, J.; Daud, A.; Schadendorf, D.; Blank, C.; Cranmer, L.D.; Robert, C.; Pavlick, A.C.; et al. Final Overall Survival for KEYNOTE-002: Pembrolizumab (Pembro) versus Investigator-Choice Chemotherapy (Chemo) for Ipilimumab (Ipi)-Refractory Melanoma. Ann. Oncol. 2016, 27, vi379. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H.; Lao, C.D.; et al. Nivolumab versus Chemotherapy in Patients with Advanced Melanoma Who Progressed after Anti-CTLA-4 Treatment (CheckMate 037): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Peters, C.; Brown, S. Antibody-Drug Conjugates as Novel Anti-Cancer Chemotherapeutics. Biosci. Rep. 2015, 35, e00225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Younes, A.; Bartlett, N.L.; Leonard, J.P.; Kennedy, D.A.; Lynch, C.M.; Sievers, E.L.; Forero-Torres, A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N. Engl. J. Med. 2010, 363, 1812–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Sun, Q. Novel Targeted Drugs Approved by the NMPA and FDA in 2019. Signal Transduct Target Ther. 2020, 5, 65. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, S. Moxetumomab Pasudotox: First Global Approval. Drugs 2018, 78, 1763–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosio, F.; Brusa, P.; Cattel, L. Immunotoxins and Anticancer Drug Conjugate Assemblies: The Role of the Linkage between Components. Toxins 2011, 3, 848–883. [Google Scholar] [CrossRef] [Green Version]
- Steiner, M.; Neri, D. Antibody-Radionuclide Conjugates for Cancer Therapy: Historical Considerations and New Trends. Clin. Cancer Res. 2011, 17, 6406–6416. [Google Scholar] [CrossRef] [Green Version]
- Tsumoto, K.; Isozaki, Y.; Yagami, H.; Tomita, M. Future Perspectives of Therapeutic Monoclonal Antibodies. Immunotherapy 2019, 11, 119–127. [Google Scholar] [CrossRef]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to Watch in 2020. MAbs 2020, 12, 1703531. [Google Scholar] [CrossRef] [Green Version]
- Pillay, V.; Gan, H.K.; Scott, A.M. Antibodies in Oncology. New Biotechnol. 2011, 28, 518–529. [Google Scholar] [CrossRef]
- Mikhail, A.S.; Eetezadi, S.; Ekdawi, S.N.; Stewart, J.; Allen, C. Image-Based Analysis of the Size- and Time-Dependent Penetration of Polymeric Micelles in Multicellular Tumor Spheroids and Tumor Xenografts. Int. J. Pharm. 2014, 464, 168–177. [Google Scholar] [CrossRef]
- Joyce, J.A.; Pollard, J.W. Microenvironmental Regulation of Metastasis. Nat. Rev. Cancer 2009, 9, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Matsumura, Y. Tumoritropic and Lymphotropic Principles of Macromolecular Drugs. Crit. Rev. Ther. Drug Carr. Syst. 1989, 6, 193–210. [Google Scholar]
- Maeda, H. Toward a Full Understanding of the EPR Effect in Primary and Metastatic Tumors as Well as Issues Related to Its Heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef]
- Maeda, H.; Takeshita, J.; Kanamaru, R. A lipophilic derivative of neocarzinostatin a polymer conjugation of an antitumor protein antibiotic. Int. J. Pept. Protein Res. 1979, 14, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Maeda, H. Polymer Therapeutics and the EPR Effect. J. Drug Target 2017, 25, 781–785. [Google Scholar] [CrossRef]
- Taurin, S.; Nehoff, H.; Greish, K. Anticancer Nanomedicine and Tumor Vascular Permeability; Where Is the Missing Link? J. Control. Release 2012, 164, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Greish, K. Enhanced Permeability and Retention of Macromolecular Drugs in Solid Tumors: A Royal Gate for Targeted Anticancer Nanomedicines. J. Drug Target 2007, 15, 457–464. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR Effect: Unique Features of Tumor Blood Vessels for Drug Delivery, Factors Involved, and Limitations and Augmentation of the Effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Arnida; Janát-Amsbury, M.M.; Ray, A.; Peterson, C.M.; Ghandehari, H. Geometry and Surface Characteristics of Gold Nanoparticles Influence Their Biodistribution and Uptake by Macrophages. Eur. J. Pharm. Biopharm. 2011, 77, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagamitsu, A.; Greish, K.; Maeda, H. Elevating Blood Pressure as a Strategy to Increase Tumor-Targeted Delivery of Macromolecular Drug SMANCS: Cases of Advanced Solid Tumors. Jpn. J. Clin. Oncol. 2009, 39, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Chen, C. The Crown and the Scepter: Roles of the Protein Corona in Nanomedicine. Adv. Mater. 2018, 31, 1805740. [Google Scholar] [CrossRef] [PubMed]
- Tonigold, M.; Simon, J.; Estupiñán, D.; Kokkinopoulou, M.; Reinholz, J.; Kintzel, U.; Kaltbeitzel, A.; Renz, P.; Domogalla, M.P.; Steinbrink, K.; et al. Pre-Adsorption of Antibodies Enables Targeting of Nanocarriers despite a Biomolecular Corona. Nat. Nanotechnol. 2018, 13, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, C.; Lim, M.; Marquis, C.P.; Amal, R. Nanoparticle–Protein Corona Complexes Govern the Biological Fates and Functions of Nanoparticles. J. Mater. Chem. B 2014, 2, 2060. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Shi, B.; Pei, Y.-Y.; Hong, M.-H.; Wu, J.; Chen, H.-Z. In Vivo Tumor Targeting of Tumor Necrosis Factor-α-Loaded Stealth Nanoparticles: Effect of MePEG Molecular Weight and Particle Size. Eur. J. Pharm. Sci. 2006, 27, 27–36. [Google Scholar] [CrossRef]
- Johnstone, S.A.; Masin, D.; Mayer, L.; Bally, M.B. Surface-Associated Serum Proteins Inhibit the Uptake of Phosphatidylserine and Poly(Ethylene Glycol) Liposomes by Mouse Macrophages. Biochim. Et Biophys. Acta BBA Biomembr. 2001, 1513, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Owensiii, D.; Peppas, N. Opsonization, Biodistribution, and Pharmacokinetics of Polymeric Nanoparticles. Int. J. Pharm 2006, 307, 93–102. [Google Scholar] [CrossRef]
- Pozzi, D.; Colapicchioni, V.; Caracciolo, G.; Piovesana, S.; Capriotti, A.L.; Palchetti, S.; de Grossi, S.; Riccioli, A.; Amenitsch, H.; Laganà, A. Effect of Polyethyleneglycol (PEG) Chain Length on the Bio-Nano-Interactions between PEGylated Lipid Nanoparticles and Biological Fluids: From Nanostructure to Uptake in Cancer Cells. Nanoscale 2014, 6, 2782. [Google Scholar] [CrossRef]
- Vonarbourg, A.; Passirani, C.; Saulnier, P.; Simard, P.; Leroux, J.C.; Benoit, J.P. Evaluation of Pegylated Lipid Nanocapsules versus Complement System Activation and Macrophage Uptake. J. Biomed. Mater. Res. A 2006, 78A, 620–628. [Google Scholar] [CrossRef]
- Gaucher, G.; Asahina, K.; Wang, J.; Leroux, J.-C. Effect of Poly(N-vinyl-pyrrolidone)-block-poly(D,L-lactide) as Coating Agent on the Opsonization, Phagocytosis, and Pharmacokinetics of Biodegradable Nanoparticles. Biomacromolecules 2009, 10, 408–416. [Google Scholar] [CrossRef]
- Bros, M.; Nuhn, L.; Simon, J.; Moll, L.; Mailänder, V.; Landfester, K.; Grabbe, S. The Protein Corona as a Confounding Variable of Nanoparticle-Mediated Targeted Vaccine Delivery. Front. Immunol. 2018, 9, 1760. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Sakurai, Y.; Harashima, H.; Bae, Y.H. Nano-Sized Drug Carriers: Extravasation, Intratumoral Distribution, and Their Modeling. J. Control. Release 2017, 267, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2010, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sun, F.; Liu, S.; Jiang, S. Anti-PEG Antibodies in the Clinic: Current Issues and beyond PEGylation. J. Control. Release 2016, 244, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Brault, N.D.; Sundaram, H.S.; Huang, C.-J.; Li, Y.; Yu, Q.; Jiang, S. Two-Layer Architecture Using Atom Transfer Radical Polymerization for Enhanced Sensing and Detection in Complex Media. Biomacromolecules 2012, 13, 4049–4056. [Google Scholar] [CrossRef]
- Kane, R.S.; Deschatelets, P.; Whitesides, G.M. Kosmotropes Form the Basis of Protein-Resistant Surfaces. Langmuir 2003, 19, 2388–2391. [Google Scholar] [CrossRef]
- Shao, Q.; Jiang, S. Molecular Understanding and Design of Zwitterionic Materials. Adv. Mater. 2014, 27, 15–26. [Google Scholar] [CrossRef]
- Tsai, W.-B.; Shi, Q.; Grunkemeier, J.M.; McFarland, C.; Horbett, T.A. Platelet Adhesion to Radiofrequency Glow-Discharge-Deposited Fluorocarbon Polymers Preadsorbed with Selectively Depleted Plasmas Show the Primary Role of Fibrinogen. J. Biomater. Sci. Polym. Ed. 2004, 15, 817–840. [Google Scholar] [CrossRef]
- Huo, D.; Jiang, X.; Hu, Y. Recent Advances in Nanostrategies Capable of Overcoming Biological Barriers for Tumor Management. Adv. Mater. 2019, 32, 1904337. [Google Scholar] [CrossRef]
- Bentley, A.A.; Adams, J.C. The Evolution of Thrombospondins and Their Ligand-Binding Activities. Mol. Biol. Evol. 2010, 27, 2187–2197. [Google Scholar] [CrossRef] [Green Version]
- Brown, E. Integrin-Associated Protein (CD47) and Its Ligands. Trends. Cell Biol. 2001, 11, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.-A.; Zheleznyak, A.; Fang, Y.-F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.L.; Harada, T.; Christian, D.A.; Pantano, D.A.; Tsai, R.K.; Discher, D.E. Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles. Science 2013, 339, 971–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torosean, S.; Flynn, B.; Axelsson, J.; Gunn, J.; Samkoe, K.S.; Hasan, T.; Doyley, M.M.; Pogue, B.W. Nanoparticle Uptake in Tumors Is Mediated by the Interplay of Vascular and Collagen Density with Interstitial Pressure. Nanomedicine 2013, 9, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High Interstitial Fluid Pressure—An Obstacle in Cancer Therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Ernsting, M.J.; Murakami, M.; Roy, A.; Li, S.-D. Factors Controlling the Pharmacokinetics, Biodistribution and Intratumoral Penetration of Nanoparticles. J. Control. Release 2013, 172, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Overchuk, M.; Zheng, G. Overcoming Obstacles in the Tumor Microenvironment: Recent Advancements in Nanoparticle Delivery for Cancer Theranostics. Biomaterials 2018, 156, 217–237. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the Ligand-Coated Nanoparticle Delivery to Cancer Cells in Solid Tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef]
- Netti, P.; Baxter, L.; Coucher, Y.; Skalak, R.; Jain, R. A Poroelastic Model for Interstitial Pressure in Tumors. Biorheology 1995, 32, 346. [Google Scholar] [CrossRef]
- Kalluri, R. Basement Membranes: Structure, Assembly and Role in Tumour Angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Tong, R.T.; Boucher, Y.; Kozin, S.V.; Winkler, F.; Hicklin, D.J.; Jain, R.K. Vascular Normalization by Vascular Endothelial Growth Factor Receptor 2 Blockade Induces a Pressure Gradient Across the Vasculature and Improves Drug Penetration in Tumors. Cancer Res. 2004, 64, 3731–3736. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Miller, R.T. Mechanisms of Mechanical Signaling in Development and Disease. J. Cell Sci. 2011, 124, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahoney, L.; Csima, A. Clinical Screening for Breast Cancer. N. Engl. J. Med. 1982, 306, 546. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadi, H.; Sahai, E. Mechanisms and Impact of Altered Tumour Mechanics. Nat. Cell Biol. 2018, 20, 766–774. [Google Scholar] [CrossRef]
- Skalak, R.; Zargaryan, S.; Jain, R.K.; Netti, P.A.; Hoger, A. Compatibility and the Genesis of Residual Stress by Volumetric Growth. J. Math. Biol. 1996, 34, 889–914. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Martin, J.D.; Chauhan, V.P.; Jain, S.R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, Consequences, and Remedies for Growth-Induced Solid Stress in Murine and Human Tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 15101–15108. [Google Scholar] [CrossRef] [Green Version]
- Baish, J.W.; Stylianopoulos, T.; Lanning, R.M.; Kamoun, W.S.; Fukumura, D.; Munn, L.L.; Jain, R.K. Scaling Rules for Diffusive Drug Delivery in Tumor and Normal Tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 1799–1803. [Google Scholar] [CrossRef] [Green Version]
- Helmlinger, G.; Yuan, F.; Dellian, M.; Jain, R.K. Interstitial PH and PO2 Gradients in Solid Tumors In Vivo: High-Resolution Measurements Reveal a Lack of Correlation. Nat. Med. 1997, 3, 177–182. [Google Scholar] [CrossRef]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef]
- Hagendoorn, J.; Tong, R.; Fukumura, D.; Lin, Q.; Lobo, J.; Padera, T.P.; Xu, L.; Kucherlapati, R.; Jain, R.K. Onset of Abnormal Blood and Lymphatic Vessel Function and Interstitial Hypertension in Early Stages of Carcinogenesis. Cancer Res. 2006, 66, 3360–3364. [Google Scholar] [CrossRef] [PubMed]
- Dimou, A.; Syrigos, K.N.; Saif, M.W. Overcoming the Stromal Barrier: Technologies to Optimize Drug Delivery in Pancreatic Cancer. Ther. Adv. Med. Oncol. 2012, 4, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, R.K. Normalizing Tumor Microenvironment to Treat Cancer: Bench to Bedside to Biomarkers. J. Clin. Oncol. 2013, 31, 2205–2218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMillin, D.W.; Delmore, J.; Weisberg, E.; Negri, J.M.; Geer, D.C.; Klippel, S.; Mitsiades, N.; Schlossman, R.L.; Munshi, N.C.; Kung, A.L.; et al. Tumor Cell-Specific Bioluminescence Platform to Identify Stroma-Induced Changes to Anticancer Drug Activity. Nat. Med. 2010, 16, 483–489. [Google Scholar] [CrossRef]
- McMillin, D.W.; Negri, J.M.; Mitsiades, C.S. The Role of Tumour–Stromal Interactions in Modifying Drug Response: Challenges and Opportunities. Nat. Rev. Drug Discov. 2013, 12, 217–228. [Google Scholar] [CrossRef]
- Ni Chonghaile, T.; Sarosiek, K.A.; Vo, T.-T.; Ryan, J.A.; Tammareddi, A.; Moore, V.D.G.; Deng, J.; Anderson, K.C.; Richardson, P.; Tai, Y.-T.; et al. Pretreatment Mitochondrial Priming Correlates with Clinical Response to Cytotoxic Chemotherapy. Science 2011, 334, 1129–1133. [Google Scholar] [CrossRef] [Green Version]
- Kalluri, R. The Biology and Function of Fibroblasts in Cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Sherman, M.H.; Yu, R.T.; Engle, D.D.; Ding, N.; Atkins, A.R.; Tiriac, H.; Collisson, E.A.; Connor, F.; van Dyke, T.; Kozlov, S.; et al. Vitamin D Receptor-Mediated Stromal Reprogramming Suppresses Pancreatitis and Enhances Pancreatic Cancer Therapy. Cell 2014, 159, 80–93. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Miao, L.; Goodwin, T.J.; Li, J.; Liu, Q.; Huang, L. Quercetin Remodels the Tumor Microenvironment To Improve the Permeation, Retention, and Antitumor Effects of Nanoparticles. ACS Nano 2017, 11, 4916–4925. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Zhao, Y.; Dong, J.; Xue, M.; Lin, Y.-S.; Ji, Z.; Mai, W.X.; Zhang, H.; Chang, C.H.; Brinker, C.J.; et al. Two-Wave Nanotherapy to Target the Stroma and Optimize Gemcitabine Delivery to a Human Pancreatic Cancer Model in Mice. ACS Nano 2013, 7, 10048–10065. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, S.J.; Finch, S.K.; Hallahan, D.E.; Giorgio, T.D. Proteolytic Surface Functionalization Enhances In Vitro Magnetic Nanoparticle Mobility through Extracellular Matrix. Nano Lett. 2006, 6, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the Extracellular Matrix in Development and Disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Gore, J.; Korc, M. Pancreatic Cancer Stroma: Friend or Foe? Cancer Cell 2014, 25, 711–712. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-Activatable Polymer–Drug Conjugate Augments Tumour Penetration and Treatment Efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Hench, L.L. The Story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Lepry, W.C.; Nazhat, S.N. A Review of Pho.osphate and Borate Sol–Gel Glasses for Biomedical Applications. Adv. Nanobiomed. Res. 2021, 1, 2000055. [Google Scholar] [CrossRef]
- Ege, D.; Zheng, K.; Boccaccini, A.R. Borate Bioactive Glasses (BBG): Bone Regeneration, Wound Healing Applications, and Future Directions. ACS Appl. Biol. Mater. 2022, 5, 3608–3622. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting In Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Borges, R.; Souza, A.C.S.; Genova, L.A.; Machado, J., Jr.; Justo, G.Z.; Marchi, J. Biocompatible Glasses Applied in Cancer Treatment: Magnetic Hyperthermia and Brachytherapy. Bioactive Glasses and Glass-Ceramics. 2022, pp. 537–579. Available online: https://onlinelibrary.wiley.com/action/showCitFormats?doi=10.1002%2F9781119724193.ch22&mobileUi=0 (accessed on 12 December 2022).
- Baino, F.; Fiume, E.; Ciavattini, S.; Kargozar, S.; Borges, R.; Genova, L.A.; Marchi, J.; Verné, E. Biomedical Radioactive Glasses for Brachytherapy. Materials 2021, 14, 1131. [Google Scholar] [CrossRef]
- Danewalia, S.S.; Singh, K. Bioactive Glasses and Glass–Ceramics for Hyperthermia Treatment of Cancer: State-of-Art, Challenges, and Future Perspectives. Mater. Today Biol. 2021, 10, 100100. [Google Scholar] [CrossRef] [PubMed]
- Vernè, E.; Miola, M.; Ferraris, S.; Bianchi, C.L.; Naldoni, A.; Maina, G.; Bretcanu, O. Surface Activation of a Ferrimagnetic Glass–Ceramic for Antineoplastic Drugs Grafting. Adv. Eng. Mater. 2010, 12, B309–B319. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-Printed Magnetic Fe3O4/MBG/PCL Composite Scaffolds with Multifunctionality of Bone Regeneration, Local Anticancer Drug Delivery and Hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595. [Google Scholar] [CrossRef] [PubMed]
- Czarnobaj, K.; Prokopowicz, M.; Sawicki, W. Formulation and In Vitro Characterization of Bioactive Mesoporous Silica with Doxorubicin and Metronidazole Intended for Bone Treatment and Regeneration. AAPS Pharm. Sci. Tech. 2017, 18, 3163–3171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Hu, M.; Wang, X.; Zhou, Z.; Liu, Y. Design and Evaluation of Europium Containing Mesoporous Bioactive Glass Nanospheres: Doxorubicin Release Kinetics and Inhibitory Effect on Osteosarcoma MG 63 Cells. Nanomaterials 2018, 8, 961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Zhang, Y.; Lin, C.; Zhong, W. Sol-Gel Derived Terbium-Containing Mesoporous Bioactive Glasses Nanospheres: In Vitro Hydroxyapatite Formation and Drug Delivery. Colloids. Surf. B Biointerfaces 2017, 160, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Polo, L.; Gómez-Cerezo, N.; Aznar, E.; Vivancos, J.-L.; Sancenón, F.; Arcos, D.; Vallet-Regí, M.; Martínez-Máñez, R. Molecular Gates in Mesoporous Bioactive Glasses for the Treatment of Bone Tumors and Infection. Acta Biomater. 2017, 50, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Aina, V.; Malavasi, G.; Magistris, C.; Cerrato, G.; Martra, G.; Viscardi, G.; Menabue, L.; Lusvardi, G. Conjugation of Amino-Bioactive Glasses with 5-Aminofluorescein as Probe Molecule for the Development of PH Sensitive Stimuli-Responsive Biomaterials. J. Mater. Sci. Mater. Med. 2014, 25, 2243–2253. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Chen, X.; Yang, Z.; Tan, X.; Wang, J.; Chen, Y. Preparation and Characterization of Folic Acid Functionalized Bioactive Glass for Targeted Delivery and Sustained Release of Methotrexate. J. Biomed. Mater. Res. A 2019, 107, 319–329. [Google Scholar] [CrossRef]
- Ur Rahman, M.S.; Tahir, M.A.; Noreen, S.; Yasir, M.; Khan, M.B.; Mahmood, T.; Bahadur, A.; Shoaib, M. Osteogenic Silver Oxide Doped Mesoporous Bioactive Glass for Controlled Release of Doxorubicin against Bone Cancer Cell Line (MG-63): In Vitro and In Vivo Cytotoxicity Evaluation. Ceram. Int. 2020, 46, 10765–10770. [Google Scholar] [CrossRef]
- Shoaib, M.; Saeed, A.; Rahman, M.S.U.; Naseer, M.M. Mesoporous Nano-Bioglass Designed for the Release of Imatinib and In Vitro Inhibitory Effects on Cancer Cells. Mater. Sci. Eng. C 2017, 77, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, E.; Righi, A.; Staals, E.L. Rare Primary Malignant Bone Sarcomas. Cancers 2020, 12, 3092. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebert, R.; Meissner-Weigl, J.; Zeck, S.; Määttä, J.; Auriola, S.; Coimbra de Sousa, S.; Mentrup, B.; Graser, S.; Rachner, T.D.; Hofbauer, L.C.; et al. Probenecid as a Sensitizer of Bisphosphonate-Mediated Effects in Breast Cancer Cells. Mol. Cancer 2014, 13, 265. [Google Scholar] [CrossRef] [Green Version]
- Boanini, E.; Panseri, S.; Arroyo, F.; Montesi, M.; Rubini, K.; Tampieri, A.; Covarrubias, C.; Bigi, A. Alendronate Functionalized Mesoporous Bioactive Glass Nanospheres. Materials 2016, 9, 135. [Google Scholar] [CrossRef] [Green Version]
- Välimäki, V.-V.; Moritz, N.; Yrjans, J.J.; Vuorio, E.; Aro, H.T. Effect of Zoledronic Acid on Incorporation of a Bioceramic Bone Graft Substitute. Bone 2006, 38, 432–443. [Google Scholar] [CrossRef]
- Srisubut, S.; Teerakapong, A.; Vattraphodes, T.; Taweechaisupapong, S. Effect of Local Delivery of Alendronate on Bone Formation in Bioactive Glass Grafting in Rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2007, 104, e11–e16. [Google Scholar] [CrossRef]
- Mosbahi, S.; Oudadesse, H.; Lefeuvre, B.; Barroug, A.; Elfeki, H.; Elfeki, A.; Roiland, C.; Keskes, H. Risedronate Adsorption on Bioactive Glass Surface for Applications as Bone Biomaterial. Appl. Surf. Sci. 2016, 367, 205–213. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, J.; He, Q.; Zhang, L.; Chen, F.; Chen, Y. An Emulsification–Solvent Evaporation Route to Mesoporous Bioactive Glass Microspheres for Bisphosphonate Drug Delivery. J. Mater. Sci. 2012, 47, 2256–2263. [Google Scholar] [CrossRef]
- Borges, R.; Zambanini, T.; Pelosine, A.M.; Justo, G.Z.; Souza, A.C.S.; Machado, J., Jr.; Araujo, D.R.; Marchi, J. A Colloidal Hydrogel-Based Drug Delivery System Overcomes the Limitation of Combining Bisphosphonates with Bioactive Glasses: A Selective Bone Cancer Treatment Allied with Bone Regeneration. Biomater. Adv. 2022. submitted. [Google Scholar]
- Corazzari, I.; Tomatis, M.; Turci, F.; Ferraris, S.; Bertone, E.; Prenesti, E.; Vernè, E. Gallic Acid Grafting Modulates the Oxidative Potential of Ferrimagnetic Bioactive Glass-Ceramic SC-45. Colloids Surf. B Biointerfaces 2016, 148, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xue, Y.; Ma, P.X.; Mao, C.; Lei, B. Intrinsic Ultrahigh Drug/MiRNA Loading Capacity of Biodegradable Bioactive Glass Nanoparticles toward Highly Efficient Pharmaceutical Delivery. ACS Appl. Mater. Interfaces 2017, 9, 8460–8470. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liang, Q.; Zhang, W.; Li, Y.; Ye, J.; Zhao, F.; Chen, X.; Wang, S. Bio-Inspired Bioactive Glasses for Efficient MicroRNA and Drug Delivery. J. Mater. Chem. B 2017, 5, 6376–6384. [Google Scholar] [CrossRef] [PubMed]
- Kearns, L.; Chapman, C.R.; Moch, K.I.; Caplan, A.L.; Watson, T.; McFadyen, A.; Furlong, P.; Bateman-House, A. Gene Therapy Companies Have an Ethical Obligation to Develop Expanded Access Policies. Mol. Ther. 2021, 29, 1367–1369. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A Review of the Biological Response to Ionic Dissolution Products from Bioactive Glasses and Glass-Ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Westhauser, F.; Arango-Ospina, M.; Losch, S.; Wilkesmann, S.; Lehner, B.; Ali, M.S.; Peukert, W.; Boccaccini, A.R.; Fellenberg, J. Selective and Caspase-Independent Cytotoxicity of Bioactive Glasses towards Giant Cell Tumor of Bone Derived Neoplastic Stromal Cells but Not to Bone Marrow Derived Stromal Cells. Biomaterials 2021, 275, 120977. [Google Scholar] [CrossRef]
- Sui, B.; Liu, X.; Sun, J. Dual-Functional Dendritic Mesoporous Bioactive Glass Nanospheres for Calcium Influx-Mediated Specific Tumor Suppression and Controlled Drug Delivery In Vivo. ACS Appl. Mater. Interfaces 2018, 10, 23548–23559. [Google Scholar] [CrossRef]
- Chen, S.; Greasley, S.L.; Ong, Z.Y.; Naruphontjirakul, P.; Page, S.J.; Hanna, J.V.; Redpath, A.N.; Tsigkou, O.; Rankin, S.; Ryan, M.P.; et al. Biodegradable Zinc-Containing Mesoporous Silica Nanoparticles for Cancer Therapy. Mater. Today Adv. 2020, 6, 100066. [Google Scholar] [CrossRef]
- Kilcup, N.; Gaynard, S.; Werner-Zwanziger, U.; Tonkopi, E.; Hayes, J.; Boyd, D. Stimulation of Apoptotic Pathways in Liver Cancer Cells: An Alternative Perspective on the Biocompatibility and the Utility of Biomedical Glasses. J. Biomater. Appl. 2015, 30, 1445–1459. [Google Scholar] [CrossRef]
- Delpino, G.P.; Borges, R.; Zambanini, T.; Joca, J.F.S.; Gaubeur, I.; de Souza, A.C.S.; Marchi, J. Sol-Gel-Derived 58S Bioactive Glass Containing Holmium Aiming Brachytherapy Applications: A Dissolution, Bioactivity, and Cytotoxicity Study. Mater. Sci. Eng. C 2021, 119, 111595. [Google Scholar] [CrossRef]
- Zambanini, T.; Borges, R.; Faria, P.C.; Delpino, G.P.; Pereira, I.S.; Marques, M.M.; Marchi, J. Dissolution, Bioactivity Behavior, and Cytotoxicity of Rare Earth-Containing Bioactive Glasses (RE = Gd, Yb). Int. J. Appl. Ceram. Technol. 2019, 16, 2028–2039. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive Glasses Incorporating Less-Common Ions to Improve Biological and Physical Properties. J. Mater. Sci. Mater. Med. 2021, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Kang, X.; Cheng, Z.; Ma, P.; Jia, Y.; Lin, J. Electrospinning Preparation and Drug Delivery Properties of Eu3+/Tb3+ Doped Mesoporous Bioactive Glass Nanofibers. J. Colloid Interface Sci. 2012, 387, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, M.; Chang, L.; Ma, L.; Chen, Z.; Qiu, J.; Yu, J.; Chang, J. Upconversion Luminescence Ca–Mg–Si Bioactive Glasses Synthesized Using the Containerless Processing Technique. Front. Mater. Sci. 2019, 13, 399–409. [Google Scholar] [CrossRef]
- Morais, D.S.; Coelho, J.; Ferraz, M.P.; Gomes, P.S.; Fernandes, M.H.; Hussain, N.S.; Santos, J.D.; Lopes, M.A. Samarium Doped Glass-Reinforced Hydroxyapatite with Enhanced Osteoblastic Performance and Antibacterial Properties for Bone Tissue Regeneration. J. Mater. Chem. B 2014, 2, 5872–5881. [Google Scholar] [CrossRef]
- Zhu, D.; Lu, B.; Yang, Q.; Yu, H.; Liu, P.; Yin, J.; Chen, Y.; Huang, Y.; Ke, Q.; Zhang, C.; et al. Lanthanum-Doped Mesoporous Bioglasses/Chitosan Composite Scaffolds Enhance Synchronous Osteogenesis and Angiogenesis for Augmented Osseous Regeneration. Chem. Eng. J. 2021, 405, 127077. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Su, Y.; Chen, D.; Zhong, W. A Doxorubicin Delivery System: Samarium/Mesoporous Bioactive Glass/Alginate Composite Microspheres. Mater. Sci. Eng. C 2016, 67, 205–213. [Google Scholar] [CrossRef]
- Shoaib, M.; Bahadur, A.; Iqbal, S.; AL-Anazy, M.M.; Laref, A.; Tahir, M.A.; Channar, P.A.; Noreen, S.; Yasir, M.; Iqbal, A.; et al. Magnesium Doped Mesoporous Bioactive Glass Nanoparticles: A Promising Material for Apatite Formation and Mitomycin c Delivery to the MG-63 Cancer Cells. J. Alloys Compd 2021, 866, 159013. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Ma, Y.; Chen, D.; Yang, H.; Li, M. Selenium—Containing Mesoporous Bioactive Glass Particles: Physicochemical and Drug Delivery Properties. Ceram. Int. 2016, 42, 3609–3617. [Google Scholar] [CrossRef]
- Hu, M.; Fang, J.; Zhang, Y.; Wang, X.; Zhong, W.; Zhou, Z. Design and Evaluation a Kind of Functional Biomaterial for Bone Tissue Engineering: Selenium/Mesoporous Bioactive Glass Nanospheres. J. Colloid Interface Sci. 2020, 579, 654–666. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, M.; Zhang, W.; Zhang, X. Construction of Tellurium-Doped Mesoporous Bioactive Glass Nanoparticles for Bone Cancer Therapy by Promoting ROS-Mediated Apoptosis and Antibacterial Activity. J. Colloid Interface Sci. 2022, 610, 719–730. [Google Scholar] [CrossRef] [PubMed]
| Material | Glass System | Cancer Therapy | Main Finds | Ref. | |
|---|---|---|---|---|---|
| Molecular Target Therapy | Melt-derived magnetic bioactive glass | 24.7SiO2-13.5Na2O-13.5CaO-3.3P2O5-14FeO-31Fe2O3 (wt.%) | DOX + cisplatin + magnetic hyperthermia | Pre-treatment of the glass surface with an aqueous solution led to an increase in the hydroxyl group that, in turn, interacted with DOX and cisplatin increasing drug loading, but causing random drug release kinetics. | [116] |
| Fe3O3/MBG 1/PCL 2 | 80SiO2-15CaO-5PO5 (mol%) | DOX + magnetic hyperthermia | DOX was loaded into the mesopores of the MBG glass agitation of the glass particles in solution, yielding an 84.8% loading efficiency. Later, the DOX-loaded MBG was 3D-printed together with Fe3O4 and PCL. The resulting scaffold showed controlled release properties. | [117] | |
| MCM-41 | SiO2 | DOX + metronidazole | DOX and an anti-inflammatory drug were loaded into the mesopores. The competitive release between DOX and metronidazole was modulated by the number of polar moieties in the drug molecular structure. As DOX has more polar groups, it showed slower release. | [118] | |
| Tb/MBG nanospheres | 80SiO2-15CaO-5P2O5 doped with 0.5 or 5 mol% Tb2O3 | DOX + Tb3+ | DOX release was dominated by the quantity of doping tellurium, and pH of the solution. | [120] | |
| Sm/MBG/alginate | 60SiO2-36CaO-4P2O5 doped with 5 or 15 mol% Sm2O3 | DOX + Sm3+ | The composite showed a sustained drug release. Besides, DOX release was modulated by the samarium quantity and pH environment of the dilution solution. | [151] | |
| Eu/MBG nanospheres | 60%SiO2-(36–x)%CaO-x%Eu2O3–4%P2O5 (x = 0, 0.5, 1, 2 mol%) | DOX + Eu3+ | The addition of Eu3+ in the synthesis led to changes in pore sizes and surface area, allowing different DOX loading in the MBG. Also, Eu3+ increased bioactivity, and the system was cytotoxic against MG-63 osteosarcoma cells. | [119] | |
| MBG functionalized with amine or isocyanate groups and capped with ATP 3 or ε-poly-L-lysine | 85%SiO2–10%CaO–5%P2O5 (% mol) | DOX | MBG functionalized with triamine and capped with ATP showed a gate-opening mechanism in a solution containing ALP 4, while MBG functionalized with isocyanate and capped with ε-poly-L-lysine was sensitive to pronase. Those MBG capped with ATP were bioactive only after the gate-opening mechanism. | [121] | |
| Sol-gel-derived bioactive glass nanoparticles functionalized with NH3 and grafted with folic acid (FA). | 80SiO2-16CaO-4P2O5 (mol%) | Methotrexate (MTX) | MTX was grafted on FA and sustained release in an aqueous solution. Due to FA grafting, the systems could enter HeLa cells by receptor-mediated endocytosis, but only the system BG-FA-MTX was cytotoxic. | [123] | |
| Fe2O3/MBG nanocomposite | 80SiO-15CaO-5P2O5 (mol%) | Mitomycin C + magnetic hyperthermia | Mitomycin C release kinetics was dependent on the pH of the release media, being faster delivered at lower pH. Mitomycin C showed toxicity in MG-63 cells. | [124] | |
| Mg-MBG | 51SiO2-18CaO-20Na2O-4P2O5-7MgO (mol%) | Mitomycin C | Mitomycin C release kinetics was dependent on the pH of the release media, being faster delivered at lower pH. Mitomycin C showed toxicity in MG-63 cells. | [152] | |
| Kinase inhibitor | MBG | 51SiO2·20CaO·20Na2O·5K2O·4P2O5 | Imatinib | Imatinib release kinetics was dependent on the pH of the release media, being faster delivered at lower pH. Imatinib showed toxicity in MG-63 cells. | [125] |
| Bisphosphonates | MBG nanospheres | 80SiO2-15CaO-5P2O5 (mol%) | Alendronate | The drug-delivery system showed cytotoxicity to MG-63 cells, besides promoting in vitro anti-bone absorption response by killing the osteoclast model (RAW 264.7). | [129] |
| Melt-derived 13-93 bioactive glass | 53SiO2-6Na2O-20CaO-12K2O-5MgO-4P2O5 (wt.%) | Zoledronic Acid | The system led to the favorable remodeling of the tubular bone structure in bone fracture regeneration in in vivo models using Sprague-Dawley rats. | [130] | |
| Melt-derived 45S5 Bioactive glass | 45SiO2-24.5Na2O-24.5CaO-6P2O5 (wt.%) | Alendronate | In vivo tests in Sprague-Dawley rats showed bone regeneration, but no anti-osteoclast activity. | [131] | |
| Pluronic F127 hydrogel/Ho-doped 58S Bioactive glass | 58SiO2-33CaO-P2O5 (wt.%) | Zoledronic Acid | Part of the zoledronic acid was encapsulated in the hydrogel (free-ZA), and another part was bonded to the glass (bonded-ZA). The free-ZA was responsible for promoting selective cytotoxicity in MG-63 osteosarcoma cells, but not in MC3T3 pre-osteoblast cells. | [134] | |
| Bioactive compounds and therapeutic ions | Ferrimagnetic bioactive glass grafted with gallic acid | 24.7SiO2-13.5CaO-13.5Na2O-3.3P2O5-31Fe2O3-14FeO (wt.%) | Gallic acid + magnetic hyperthermia | Gallic acid showed promoted the formation of ROS species that could be used to cause oxidative stress in cancer cells. | [135] |
| Sol-gel-derived Bioactive glass nanoparticle | 80SiO2-16CaO-4P2O5 (mol%) | miRNA | The bioactive glass nanoparticles showed higher gene transfection than commercial transfection reagents, such as polyethyleneimine (PEI 25KD) and lipofectamine 3000. | [136] | |
| MBG nanoparticle | 70SiO2-30CaO (mol%) | miRNA + DOX | MBG nanoparticles were uptaken by HeLa cells and showed cytotoxicity in transfected cells. | [137] | |
| 45S5 Bioactive glass | 45SiO2-24.5Na2O-24.5CaO-6P2O5 (wt.%) | n/a | Glasses were able to disrupt the cell membrane of giant cell tumors of the bone and cause necrotic death. | [140] | |
| MBG nanospheres | 80SiO2-15CaO-5P2O5 and 70SiO2-25CaO-5P2O5 (mol%) | Ca2+ | Ca2+ ions activate transient receptor potential channels and calcium-sensing receptors on hepatocellular carcinoma cells (HepG2), signaling cell death by the calpain-1Bcl-2caspase-3 signaling pathway without affecting healthy cells. | [141] | |
| Zn-MBG | 86SiO2-14ZnO | Zn2+ | Zn2+ release was enhanced in the acid microenvironment and caused cytotoxicity in breast cancer cells (MDA-MB-231 and MCF-7 (ER+). | [142] | |
| Melt—derived bioactive glass | 0.51SiO2–0.29Na2O–(0.20-X)ZnO–XV2O5, 0 ≤ X ≤ 0.09 | Zn2+ + V5+ | The glass showed selective apoptosis of liver cancer cells, which was addressed to vanadium rather than zinc. | [143] | |
| Sol-gel-derived 58S bioactive glass | 58SIO2-33CaO-9P2O5 doped with 1-5 wt.% of Ho2O3 | Ho3+ + brachytherapy | Ho-containing bioactive glasses display selective cytotoxicity on human osteosarcoma MG63 cell lineages while favoring high biocompatibility in pre-osteoblast-like cells (MC3T3-E1). | [7,144] | |
| (Se, Te)-MBG | 80SiO2-15CaO-5P2O5 doped with 5 mol% of Se2O4 or Te2O4 replacing SiO2. | Se4+ or Te4+ | Caused oxidative stress, which was more pronounced in MG-63 cells than in MC3T3. | [153,154,155] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, R.; Pelosine, A.M.; de Souza, A.C.S.; Machado, J., Jr.; Justo, G.Z.; Gamarra, L.F.; Marchi, J. Bioactive Glasses as Carriers of Cancer-Targeted Drugs: Challenges and Opportunities in Bone Cancer Treatment. Materials 2022, 15, 9082. https://doi.org/10.3390/ma15249082
Borges R, Pelosine AM, de Souza ACS, Machado J Jr., Justo GZ, Gamarra LF, Marchi J. Bioactive Glasses as Carriers of Cancer-Targeted Drugs: Challenges and Opportunities in Bone Cancer Treatment. Materials. 2022; 15(24):9082. https://doi.org/10.3390/ma15249082
Chicago/Turabian StyleBorges, Roger, Agatha Maria Pelosine, Ana Carolina Santos de Souza, Joel Machado, Jr., Giselle Zenker Justo, Lionel Fernel Gamarra, and Juliana Marchi. 2022. "Bioactive Glasses as Carriers of Cancer-Targeted Drugs: Challenges and Opportunities in Bone Cancer Treatment" Materials 15, no. 24: 9082. https://doi.org/10.3390/ma15249082








