Re-Examination of the Microstructural Evolution in Undercooled Co-18.5at.%B Eutectic Alloy
Abstract
:1. Introduction
2. Materials and Methods
3. Results
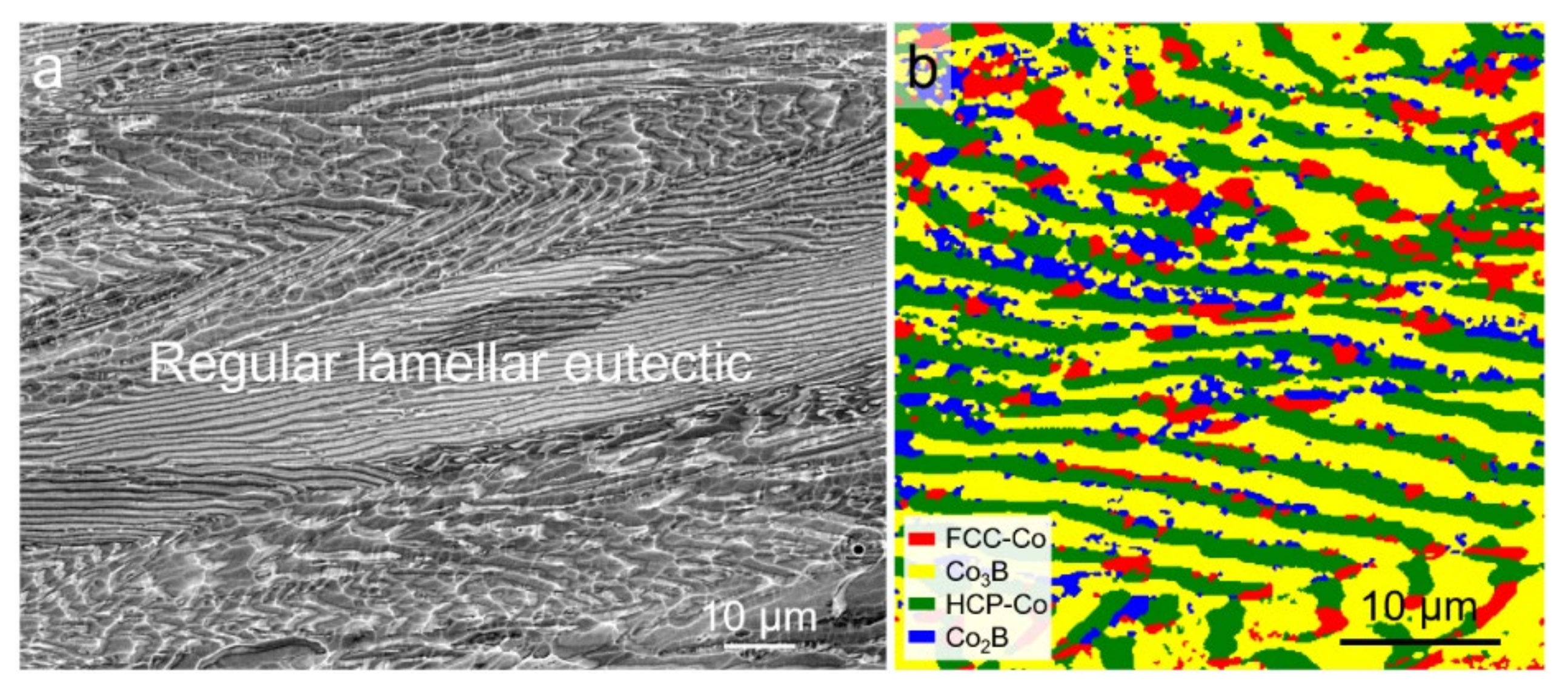
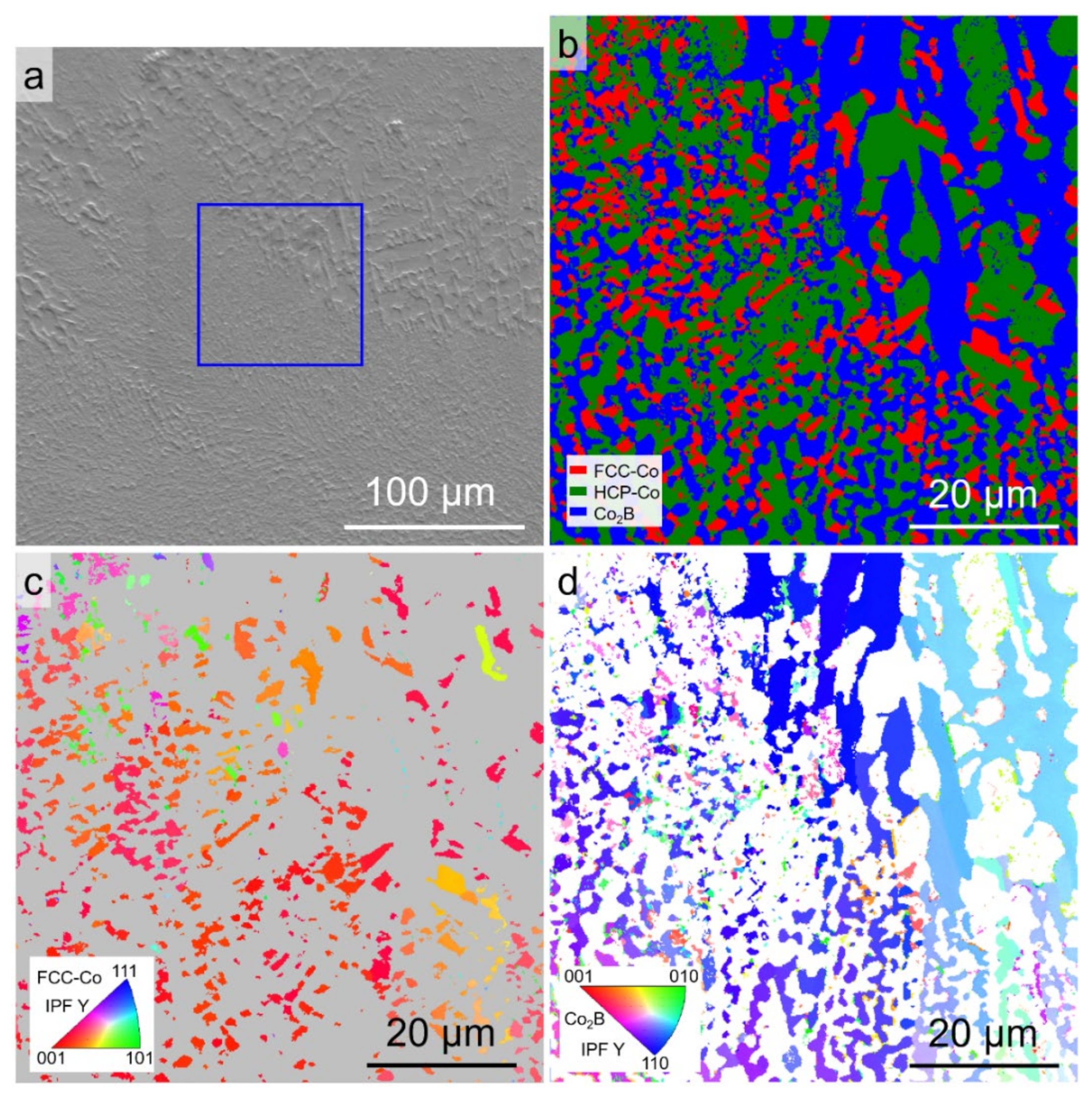
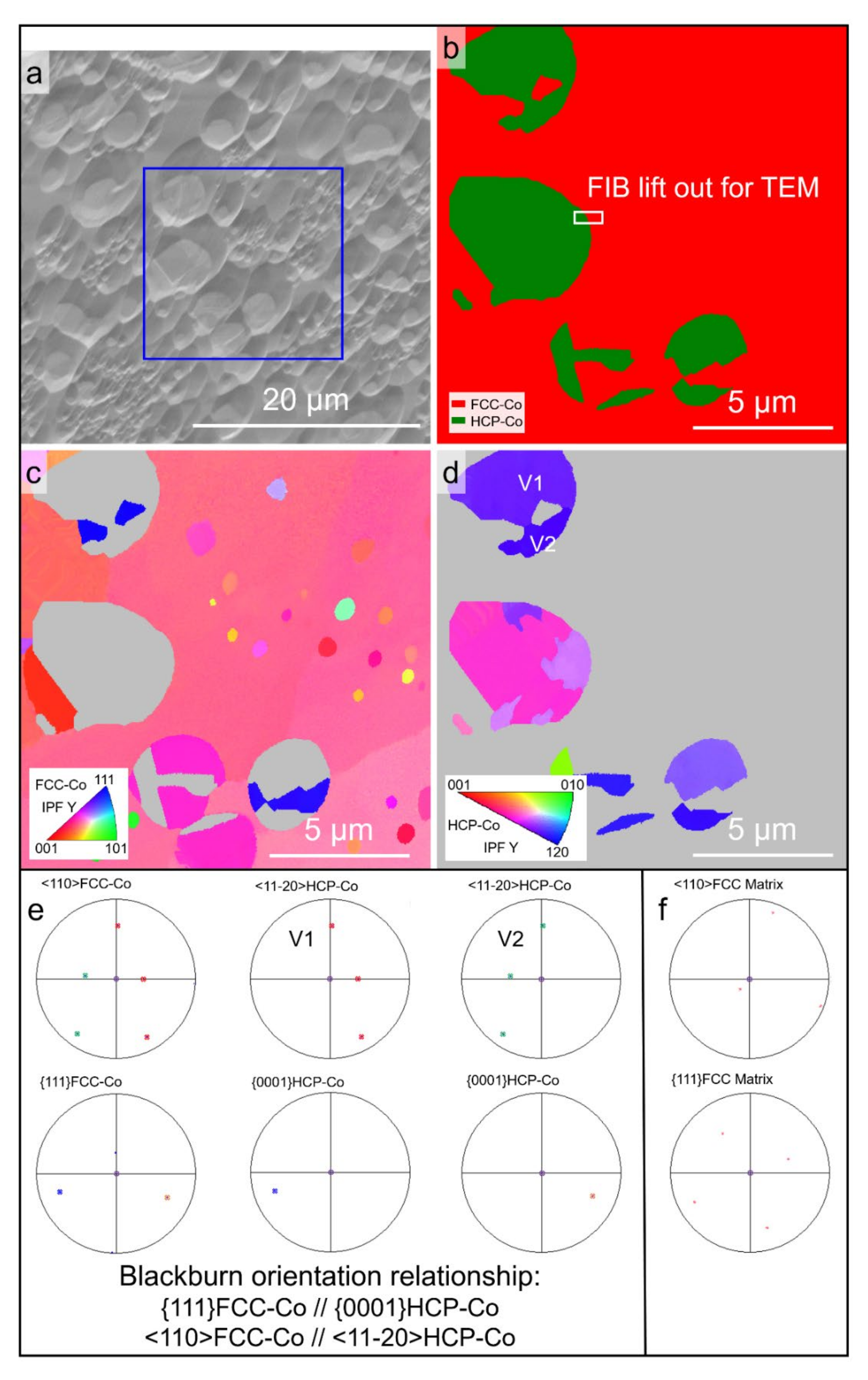
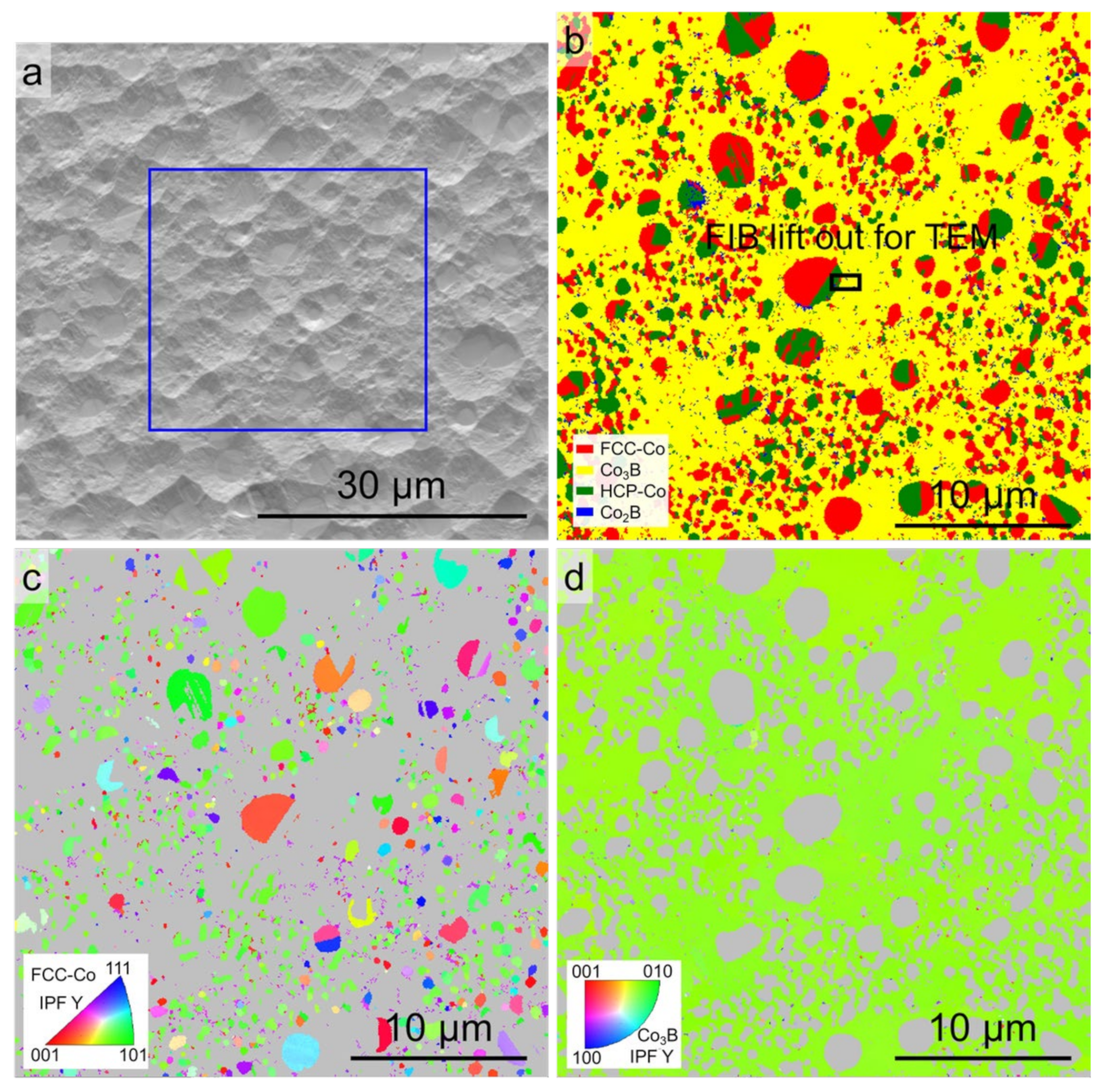
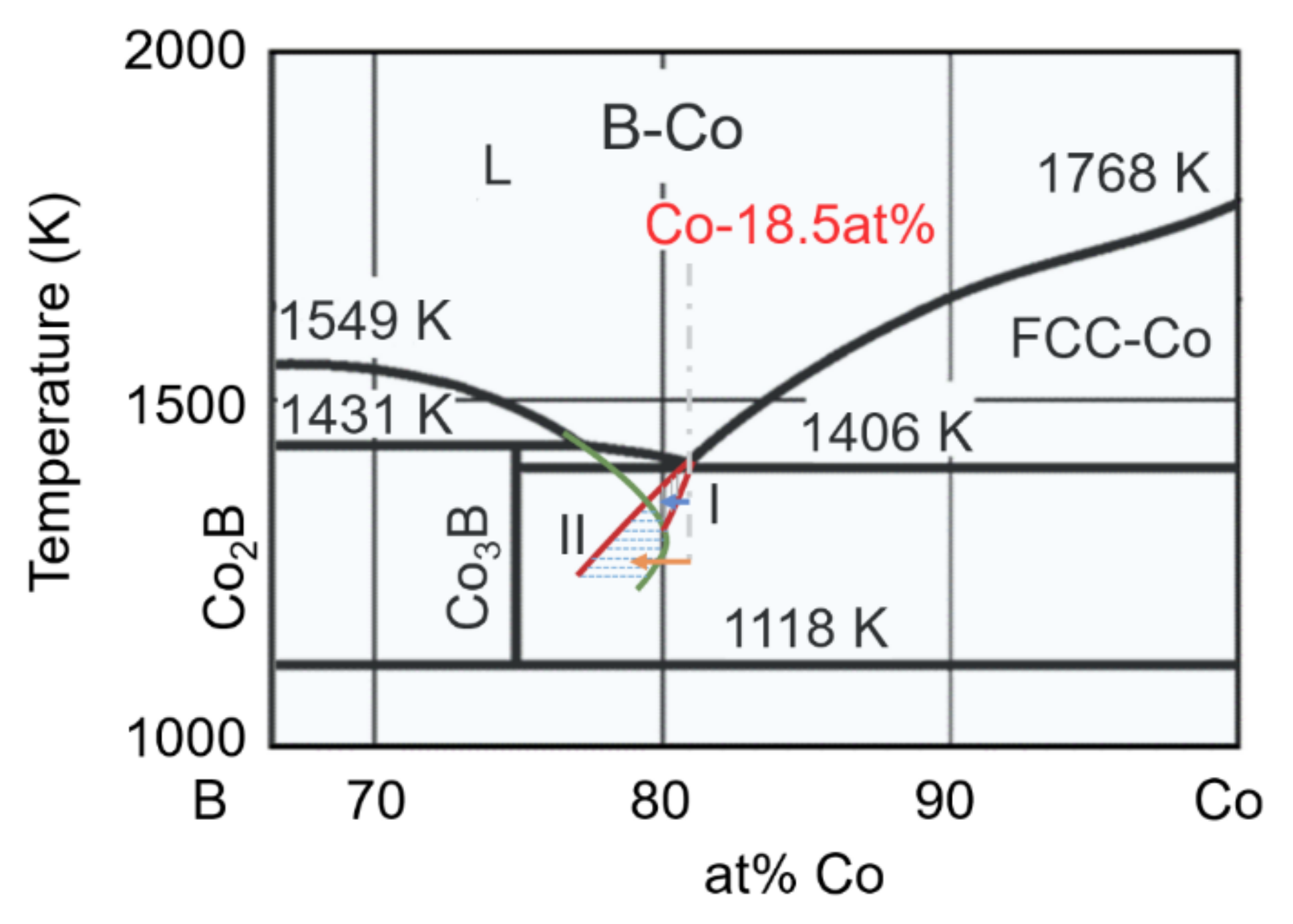
3.1. Original Microstructure and Phase Constitution
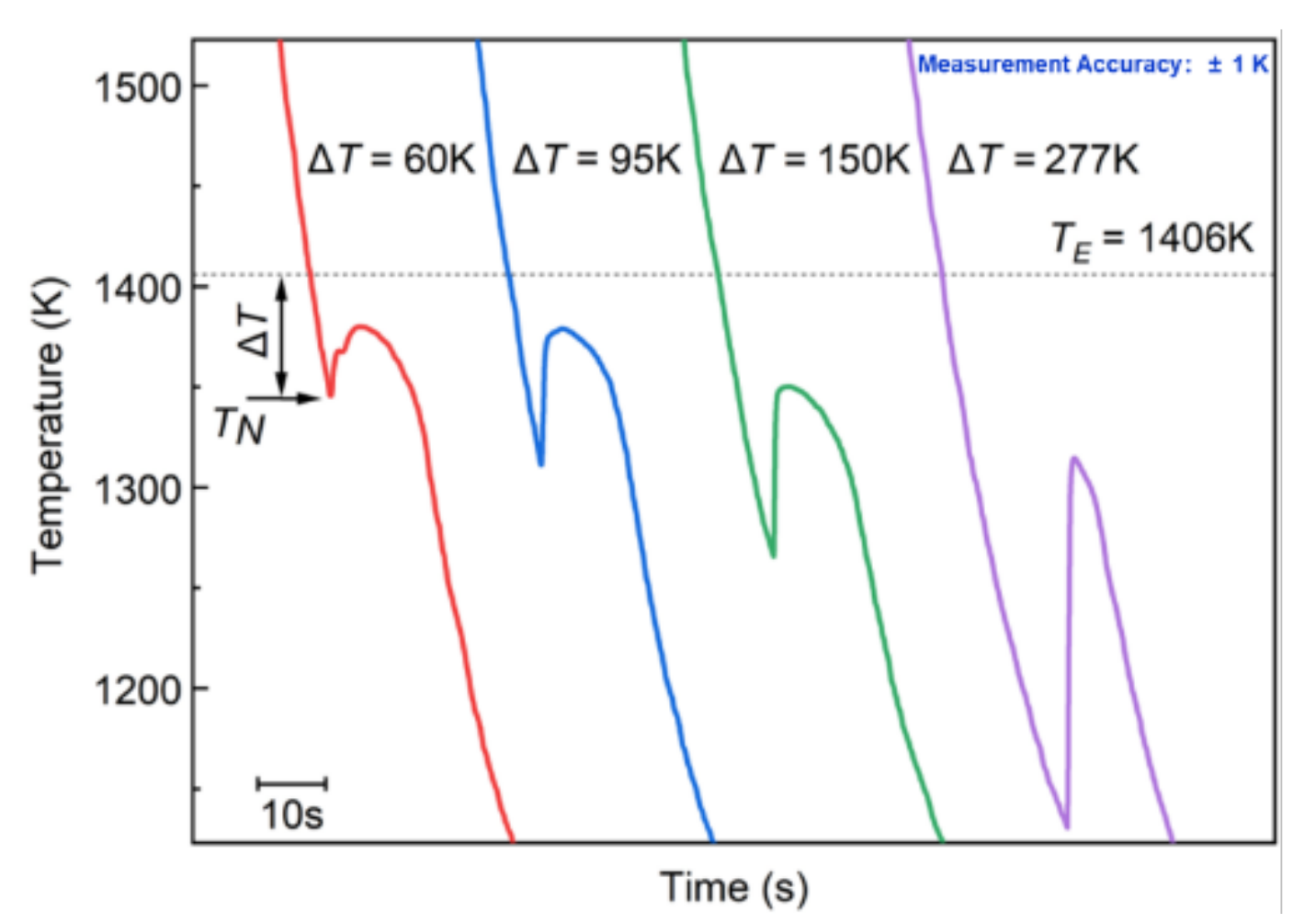
3.2. Cooling Histories
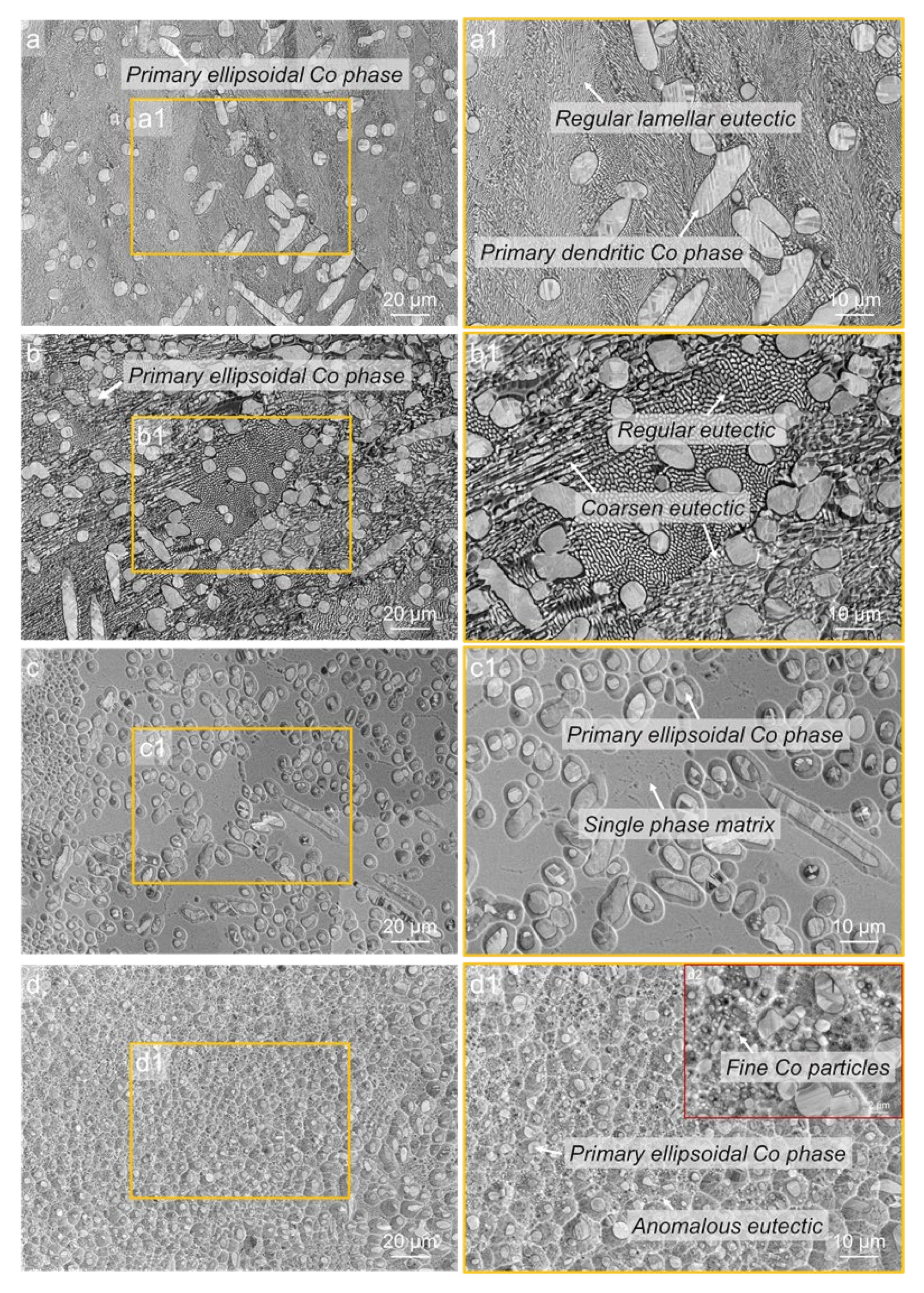
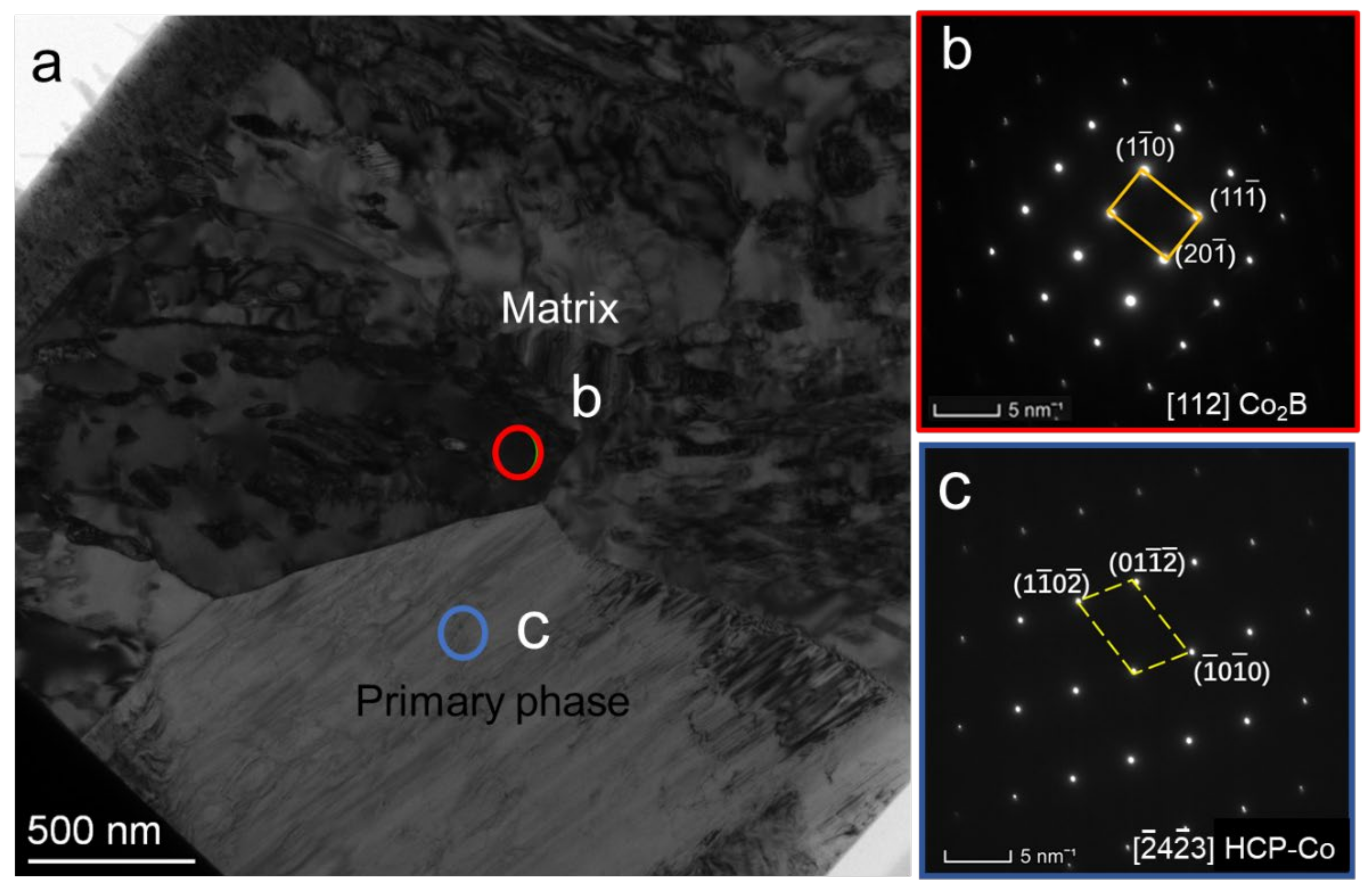
3.3. Microstructures
4. Discussion
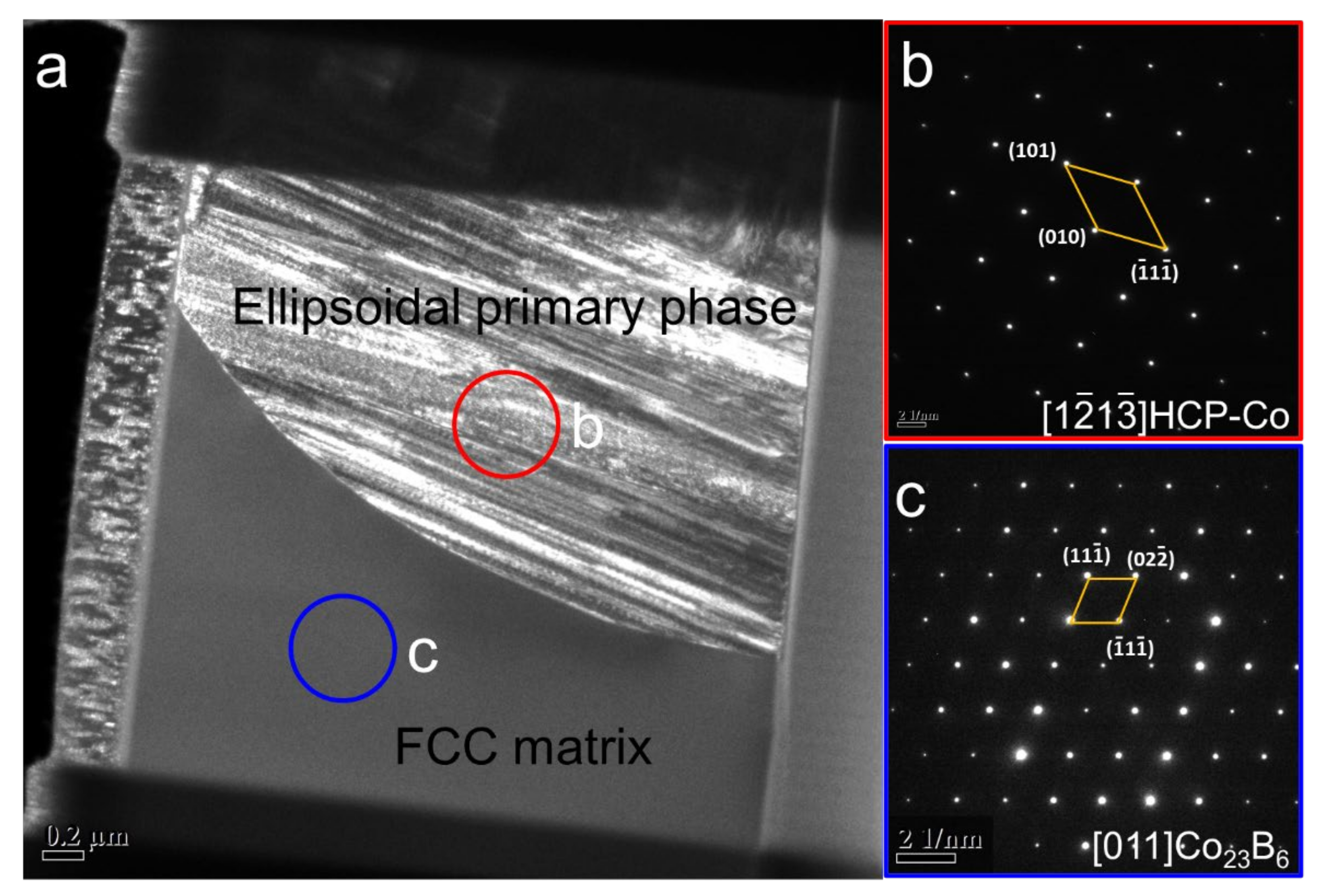
4.1. Formation of FCC–Co/Co2B Eutectic with ΔT Less Than 100 K
4.2. Formation of FCC–Co/Co3B Anomalous Eutectic with High ΔT
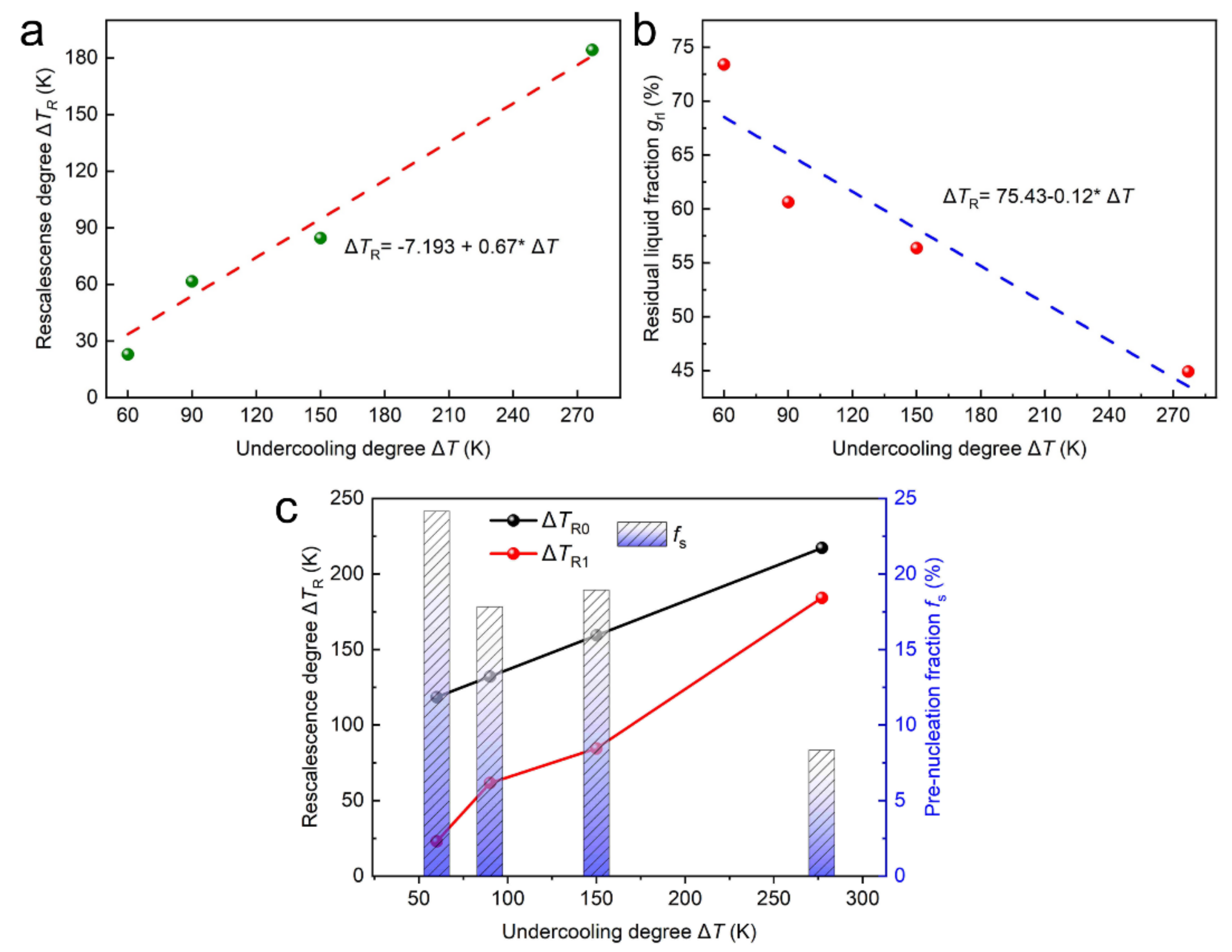
4.3. Prenucleation of Primary FCC–Co Phase
5. Conclusions
- (1)
- The solidification paths are as follows: (1) The regular lamellar eutectic colonies consisting of the FCC–Co and Co3B phase nucleate at a near-equilibrium solidification with extremely low ΔT; (2) As the ΔT increased slightly, the Co3B phase was substituted by the Co2B phase, with a mixture of the lamellar and anomalous eutectic for ΔT < 100 K. (3) With the ΔT increasing further, the Co2B phase changed to the metastable Co23B6 phase for ΔT < 200 K, with the ripened primary α-Co ellipsoid due to the remelting effect; (4) When the ΔT is extremely high, the Co3B phase takes place again, with refined α-Co particles for ΔT > 200 K, where the composition is similar to that of the microstructure formed with low ΔT;
- (2)
- The mechanism of the phase selection is interpreted on the basis of the composition segregation, the skewed coupled zone, the strain-induced transformation, and the solute trapping. When ΔT < 100 K, the Co2B phase nucleates preferentially within the residual liquid, which is attributed to the existence of the FCC–Co/Co2B pseudo-eutectic region and the accumulated strain during recalescence. Moreover, the Co3B phase forms at an ultrahigh ΔT, which results from the solute trapping during the rapid solidification;
- (3)
- The recalescence behaviors of the primary FCC–Co phase were analyzed, and the prenucleation solid fraction, owing to the existence of solid-like clusters or SROs within the heterogenous melt, decreases as the undercooling increases and, hence, the recalescence degree rises dramatically.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Du, J.; Wang, H.; Li, H.; Zhao, X. Numerical analysis of particulate migration behavior within molten pool during TIG-assisted droplet deposition manufacturing of SiC particle-reinforced aluminum matrix composites. Materials 2021, 14, 2430. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Li, J.; Wang, J.; Beaugnon, E. Liquid-liquid structure transition in metallic melt and its impact on solidification: A review. Trans. Nonferrous Met. Soc. China 2020, 30, 2293–2310. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, X.; Xu, X.; Lin, X.; Liu, L. A review on the effect of external fields on solidification, melting and heat transfer enhancement of phase change materials. J. Energy Storage 2020, 31, 101567. [Google Scholar] [CrossRef]
- Li, Z.; Xie, Y.; Yuan, Y.; Ji, Y.; Zhou, S. Phase selection in Mn-Si alloys by fast solid-state reaction with enhanced skyrmion stability. Adv. Funct. Mater. 2021, 31, 2009723. [Google Scholar] [CrossRef]
- Zhang, A.; Peng, P.; Zheng, W.; Yang, J.; Xu, Y. Phase selection and nano-mechanical properties of intermetallic compounds in directionally solidified Cu-68at. %Sn peritectic alloy. J. Alloys Compd. 2020, 859, 157866. [Google Scholar] [CrossRef]
- Qiu, X.; Li, J.; Wang, J.; Guo, T.; Kou, H.; Beaugnon, E. Effect of liquid-liquid structure transition on the nucleation in undercooled Co-Sn eutectic alloy. Mater. Chem. Phys. 2016, 170, 261–265. [Google Scholar] [CrossRef]
- Welk, B.A.; Fraser, H.L.; Dixit, V.; Williams, T.; Gibson, M.A. Phase selection in a laser surface melted Zr-Cu-Ni-Al-Nb alloy. Metall. Mater. Trans. B 2014, 45, 547–554. [Google Scholar] [CrossRef]
- Karagadde, S.; Leung, C.L.A.; Lee, P.D. Progress on in situ and operando X-ray imaging of solidification processes. Materials 2021, 14, 2374. [Google Scholar] [CrossRef]
- Mebed, A.M.; Abd-Elnaiem, A.M. Microstructural study and numerical simulation of phase decomposition of heat-treated Co–Cu alloys. Prog. Nat. Sci. Mater. 2014, 24, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Xu, P.; Xiang, S.; Liang, Y.; Hu, X.; Jing, L. Kinetic behavior and microstructure of pearlite isothermal transformation under high undercooling. Metall. Mater. Trans. A 2018, 49, 4785–4797. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, Z.; Cojocaru-Miredin, O.; Zhu, B.; Wang, X.; Gao, N.; Huang, Z.; Zu, F.Q. Dependence of solidification for Bi2Te3-xSex alloys on their liquid states. Sci. Rep. 2017, 7, 2463. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sierra, A.M.; Pons, J.; Santamarta, R.; Vermaut, P.; Ochin, P. Solidification process and effect of thermal treatments on Ni–Co–Mn–Sn metamagnetic shape memory alloys. Acta Mater. 2015, 93, 164–174. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Wang, J.; Beaugnon, E. Transition from hypereutectic to hypoeutectic for rapid solidification in an undercooled Co-B alloy. J. Cryst. Growth 2018, 499, 98–105. [Google Scholar] [CrossRef]
- Liu, L.; Yang, L.; Li, J. Solidification pathways in highly undercooled Co79.3B20.7 alloy. Metall. Mater. Trans. A 2021, 52, 4324–4330. [Google Scholar] [CrossRef]
- Vizureanu, P.; Nabiaek, M.; Sandu, A.V.; Je, B.J.M. Investigation into the effect of thermal treatment on the obtaining of magnetic phases: Fe5Y, Fe23B6, Y2Fe14B and αFe within the amorphous matrix of rapidly-quenched Fe61+xCo10-xW1Y8B20 alloys (where x = 0, 1 or 2). Materials 2020, 13, 835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alma, M.H.; Alia, M.A.; Earving, A.D.; Flores, J.G.; Pérez-Bueno, J.J.; Yunny, M.; Gabriel, T. Effect of heat treatment on the hardness and wear resistance of electrodeposited Co-B alloy coatings. J. Mater. Res. Technol. 2019, 8, 960–968. [Google Scholar]
- Mizuno, A.; Tamura, J.; Kohara, S.; Watanabe, M. Comparative study on structural variations during containerless solidification processes of Fe-B and Fe-C eutectic alloys. ISIJ Int. 2012, 52, 770–773. [Google Scholar] [CrossRef] [Green Version]
- Quirinale, D.G.; Rustan, G.E.; Kreyssig, A.; Goldman, A.I. Synergistic stabilization of metastable Fe23B6 and γ-Fe in undercooled Fe83B17. Appl. Phys. Lett. 2015, 106, 241906. [Google Scholar] [CrossRef]
- Xu, J.; Di, Z.; Feng, L.; Jian, Z. Multi-transformations in rapid solidification of highly undercooled hypoeutectic Ni–Ni3B alloy melt. J. Mater. Res. 2015, 30, 3307–3315. [Google Scholar] [CrossRef]
- Xu, J.; Feng, L.; Bo, D. Phase Selection in Undercooled Ni-3.3 Wt Pct B Alloy Melt. Metall. Mater. Trans. A 2013, 44, 1401–1408. [Google Scholar] [CrossRef]
- Feng, L.; Xu, J.; Di, Z.; Jian, Z. Solidification of highly undercooled hypereutectic Ni-Ni3B alloy melt. Metall. Mater. Trans. A 2014, 45, 4810–4819. [Google Scholar]
- Wei, X.; Xu, W.; Kang, J.; Ferry, M.; Li, J. Phase selection in solidification of undercooled Co–B alloys. J. Mater. Sci. Technol. 2017, 33, 352–358. [Google Scholar] [CrossRef]
- Wei, X.; Xu, W.; Kang, J.; Ferry, M.; Li, J. Metastable Co23B6 phase solidified from deeply undercooled Co79.3B20.7 alloy melt. J. Mater. Sci. 2016, 51, 6436–6443. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Li, X.; Li, J. Solidification of undercooled Co75B25 alloy. Acta Metall. 2018, 54, 1165–1170. [Google Scholar]
- Lizárraga, R.; Pan, F.; Bergqvist, L.; Holmström, E.; Gercsi, Z.; Vitos, L. First principles theory of the hcp-fcc phase transition in cobalt. Sci. Rep. 2017, 7, 3778. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H. B-Co (Boron-Cobalt). J. Phase Equilib. 2003, 24, 376. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Xu, X.; Chen, Y. Determination of solid fraction from cooling curve. Metall. Mater. Trans. A 2012, 43, 1268–1276. [Google Scholar] [CrossRef]
- Liao, P.K.; Spear, K.E. The B-Co (Boron-Cobalt) system. Bullet. Alloy Phase Diag. 1988, 9, 452–457. [Google Scholar] [CrossRef]
- Witusiewicz, V.T. Thermodynamics of binary and ternary melts of the 3d transition metals (Cr, Mn, Fe, Co and Ni) with boron. Thermochim. Acta 1995, 264, 41–58. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Zhang, F.; Lü, X.; Zhang, Y.; Zhou, Q. Growth kinetics and grain refinement mechanisms in an undercooled melt of a CoSi intermetallic compound. J. Alloys Compd. 2019, 781, 13–25. [Google Scholar] [CrossRef]
- He, Y.; Wu, Y.; Bu, F.; Zou, C.; Bian, Z.; Huang, Q.; Liu, T.; Wang, Q.; Wang, J.; Li, J.; et al. Effects of an ultra-high magnetic field up to 25 T on the phase transformations of undercooled Co-B eutectic alloy. J. Mater. Sci. Technol. 2021, 93, 79–88. [Google Scholar] [CrossRef]
- Li, Y. Bulk metallic glasses: Eutectic coupled zone and amorphous formation. JOM 2005, 57, 60–63. [Google Scholar] [CrossRef]
- Wang, W.; He, S.; Iwasaki, H.; Syono, Y.; Goto, T. Phase stability of an amorphous Co80B20 alloy under high-temperature and high-pressure. Acta Phys. Sin. Chin. 1984, 33, 914–920. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, F.; Wang, H.; Yang, G. Grain refinement in highly undercooled solidification of Ni85Cu15 alloy melt: Direct evidence for recrystallization mechanism. Scr. Mater. 2010, 63, 43–46. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, F.; Yang, G.; Liu, N.; Yang, C.; Zhou, Y. Discussion on nucleation mechanism of undercooled Ni80.3B19.7 melts. J. Alloys Compd. 2007, 439, 166–170. [Google Scholar] [CrossRef]
- Hunt, J.D.; Jackson, K.A. Nucleation of solid in an undercooled liquid by cavitation. J. Appl. Phys. 1966, 37, 254–257. [Google Scholar] [CrossRef]
- Kavousi, S.; Zaeem, M.A. Quantitative phase-field modeling of solute trapping in rapid solidification. Acta Mater. 2021, 205, 116562. [Google Scholar] [CrossRef]
- Baker, E.B.; Jeon, S.; Shuleshova, O.; Kaban, I.; Wang, Y.; Gao, J.; Kolbe, M.; SanSoucie, M.P.; Matson, D.M. Dendrite remelting during rapid solidification of undercooled CoSi-CoSi2 eutectic alloys quantified by in situ synchrotron X-ray diffraction. Scr. Mater. 2021, 194, 113645. [Google Scholar] [CrossRef]
- Bondorf, J.P.; Donangelo, R.; Mishustin, I.N.; Pethick, C.J.; Schulz, H.; Sneppen, K. Statistical multifragmentation of nuclei: (I). Formulation of the model. Nucl. Phys. A 1985, 443, 321–347. [Google Scholar] [CrossRef]
- Liu, F.; Yang, G. Stress-induced recrystallization mechanism for grain refinement in highly undercooled superalloy. J. Cryst. Growth 2001, 231, 295–305. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Yang, J.; Shen, J. Thermodynamic optimization of the boron–cobalt–iron system. J. Alloys Compd. 2011, 509, 4805–4810. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Yu, W.; Wang, X.; Zheng, H.; Tian, X. Pre-nucleation clusters mediated crystallization in Al–Si melts. Scr. Mater. 2016, 110, 87–91. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Wang, J.; Kou, H.; Beaugnon, E. Liquid–liquid structure transition and nucleation in undercooled Co-B eutectic alloys. Appl. Phys. A 2017, 123, 391. [Google Scholar] [CrossRef]
- Tournier, R.F. First-order transitions in glasses and melts induced by solid superclusters nucleated and melted by homogeneous nucleation instead of surface melting. Chem. Phys. 2019, 524, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Tournier, R.F.; Ojovan, M.I. Building and breaking bonds by homogenous nucleation in glass-forming melts leading to transitions in three liquid states. Materials 2021, 14, 2287. [Google Scholar] [CrossRef]
- Tournier, R.F.; Ojovan, M.I. Prediction of second melting temperatures already observed in pure elements by molecular dynamics simulations. Materials 2021, 14, 6509. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Wang, J.; Yildiz, E.; Pairis, S.; Beaugnon, E. Temperature-induced structure transition in a liquid Co-B eutectic alloy. Mater. Lett. 2019, 234, 351–353. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Wu, Y.; Bu, F.; Zhang, Y.; Zhang, Y.; Hei, B.; Zhang, J.; Wang, H. Re-Examination of the Microstructural Evolution in Undercooled Co-18.5at.%B Eutectic Alloy. Materials 2022, 15, 1315. https://doi.org/10.3390/ma15041315
He Y, Wu Y, Bu F, Zhang Y, Zhang Y, Hei B, Zhang J, Wang H. Re-Examination of the Microstructural Evolution in Undercooled Co-18.5at.%B Eutectic Alloy. Materials. 2022; 15(4):1315. https://doi.org/10.3390/ma15041315
Chicago/Turabian StyleHe, Yixuan, Yuhao Wu, Fan Bu, Yiyuan Zhang, Yifan Zhang, Bo Hei, Jianbao Zhang, and Haifeng Wang. 2022. "Re-Examination of the Microstructural Evolution in Undercooled Co-18.5at.%B Eutectic Alloy" Materials 15, no. 4: 1315. https://doi.org/10.3390/ma15041315
APA StyleHe, Y., Wu, Y., Bu, F., Zhang, Y., Zhang, Y., Hei, B., Zhang, J., & Wang, H. (2022). Re-Examination of the Microstructural Evolution in Undercooled Co-18.5at.%B Eutectic Alloy. Materials, 15(4), 1315. https://doi.org/10.3390/ma15041315






