Preparation and Characterization of Glass-Ceramic Foam from Clay-Rich Waste Diatomaceous Earth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
3. Results and Discussion
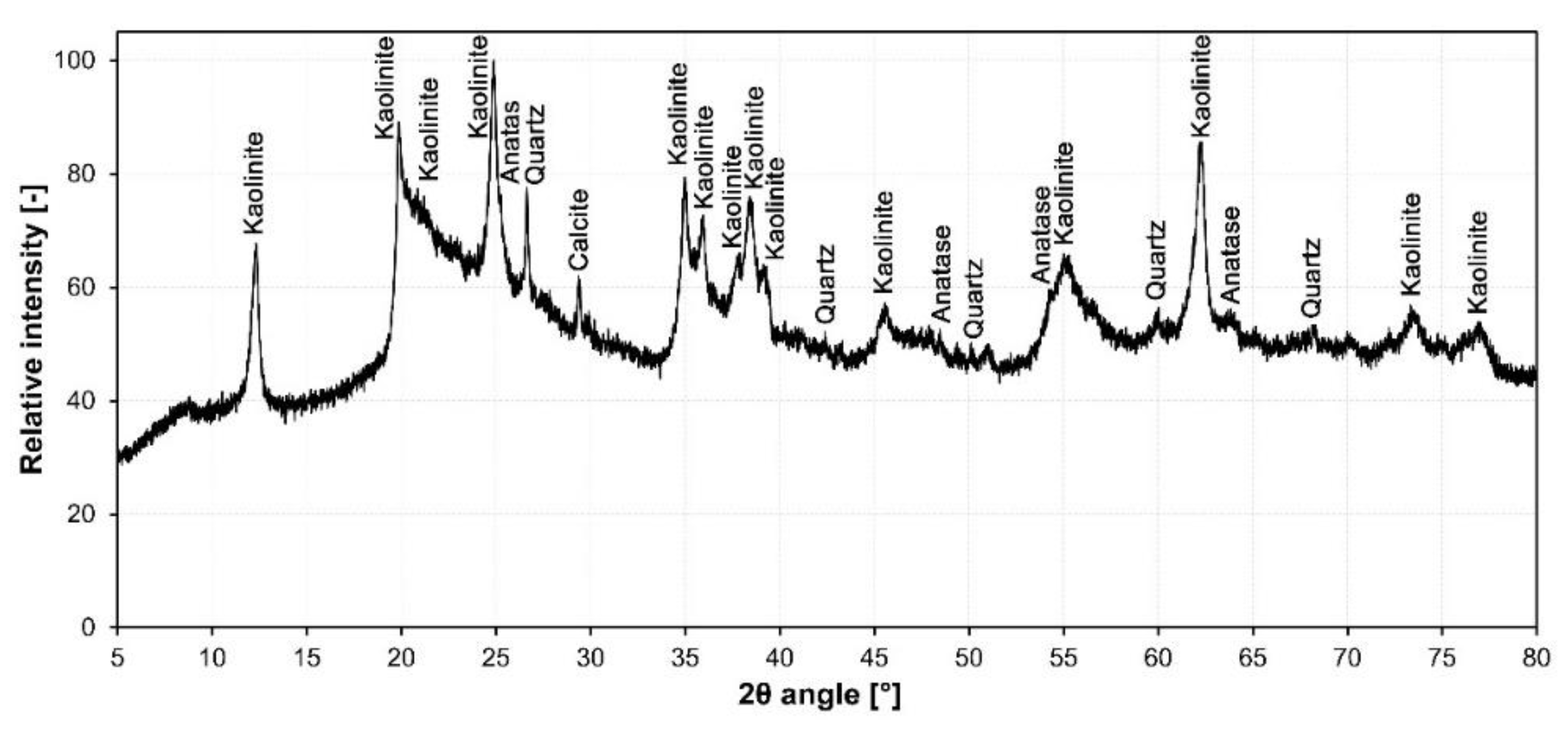
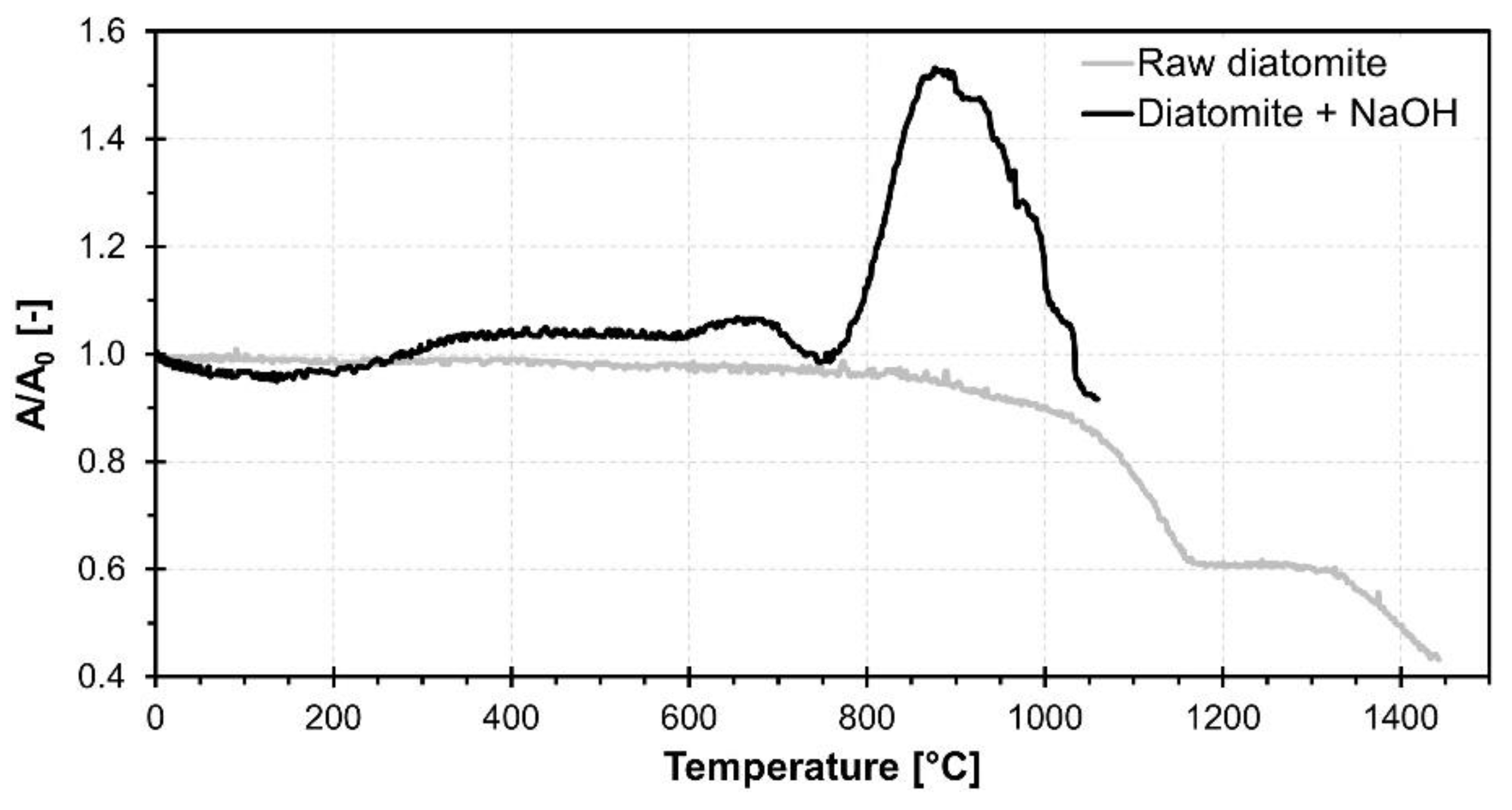
3.1. Raw Material Characterization
3.2. Foam Preparation and Characterization Results
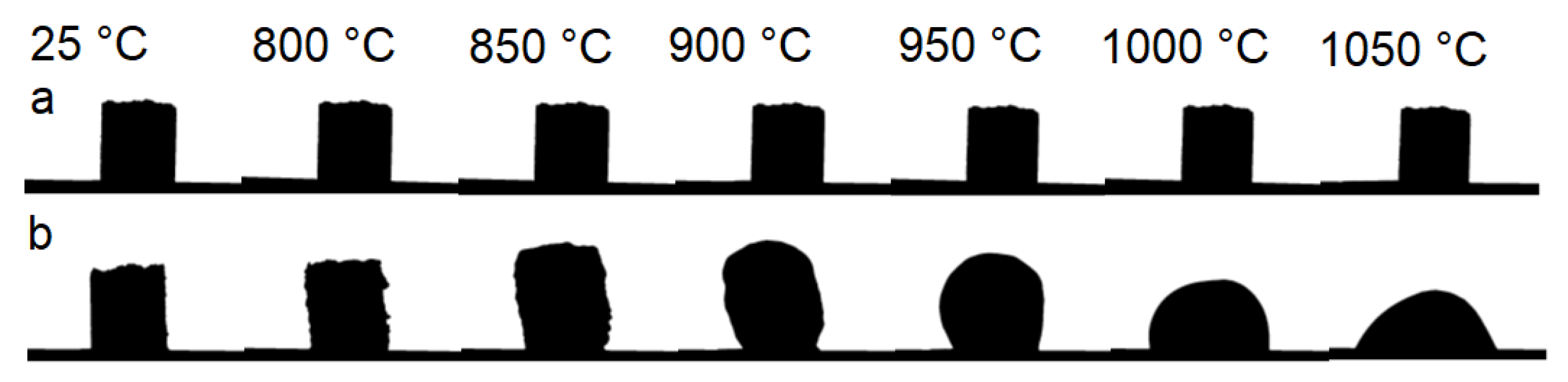
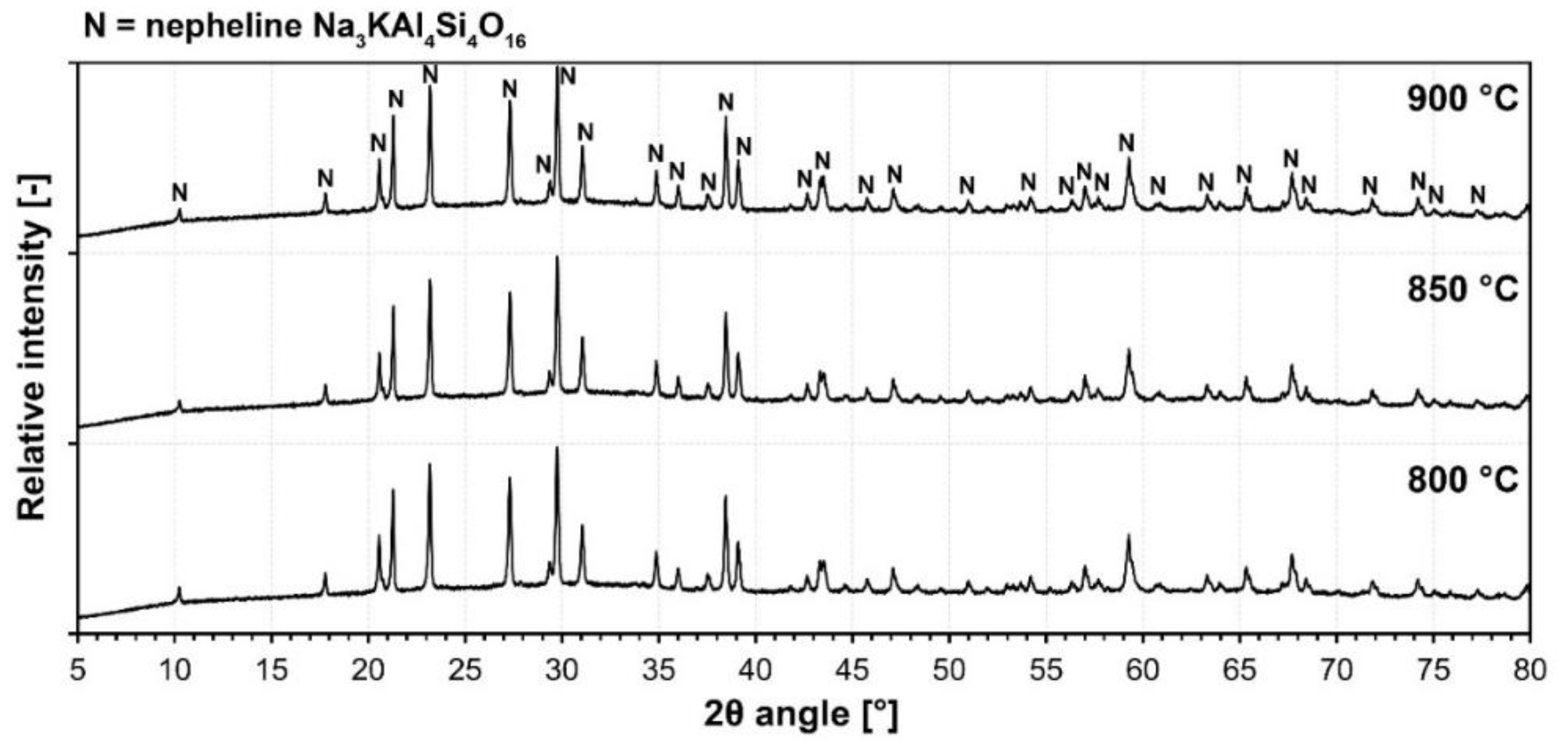
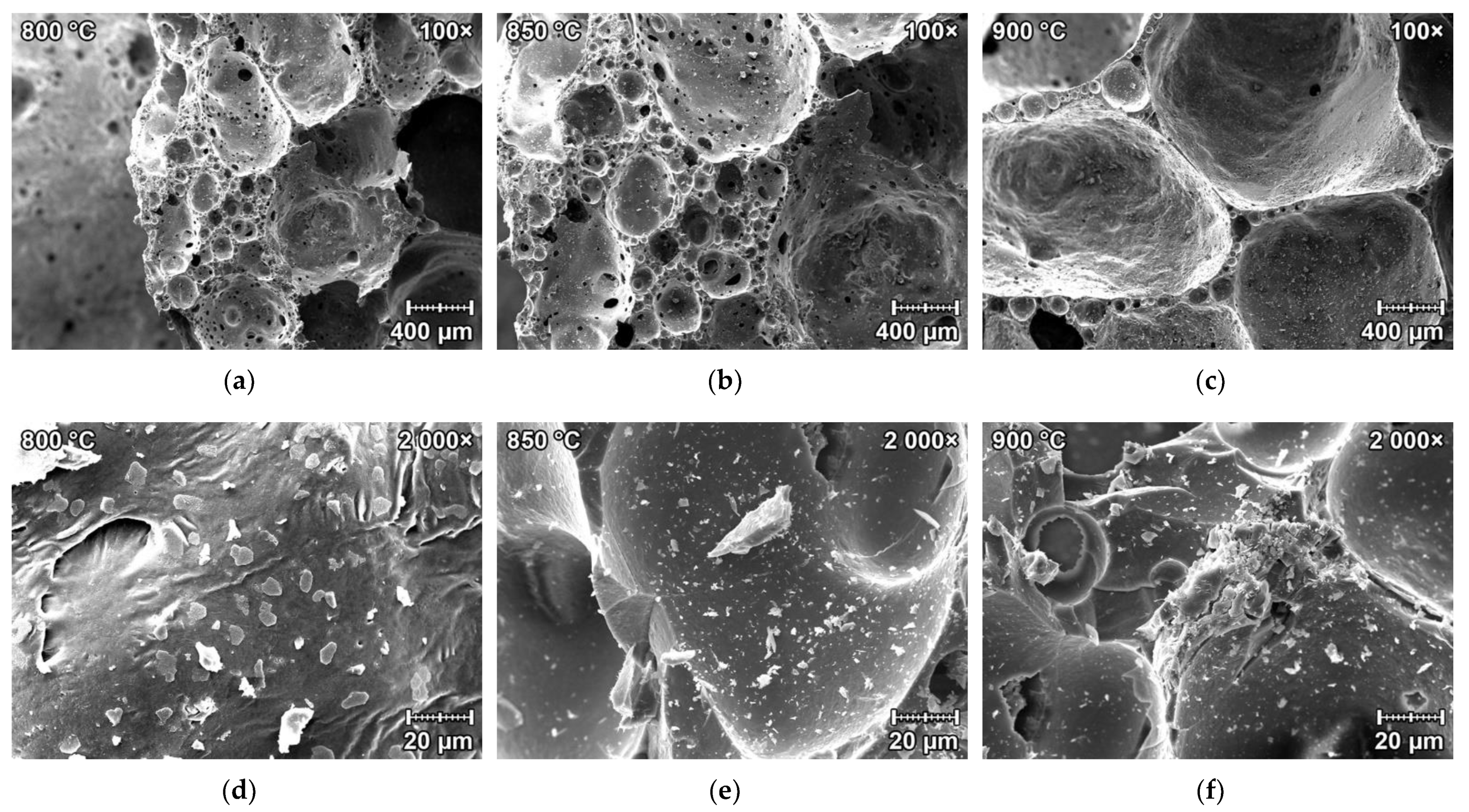
3.2.1. Structural Study
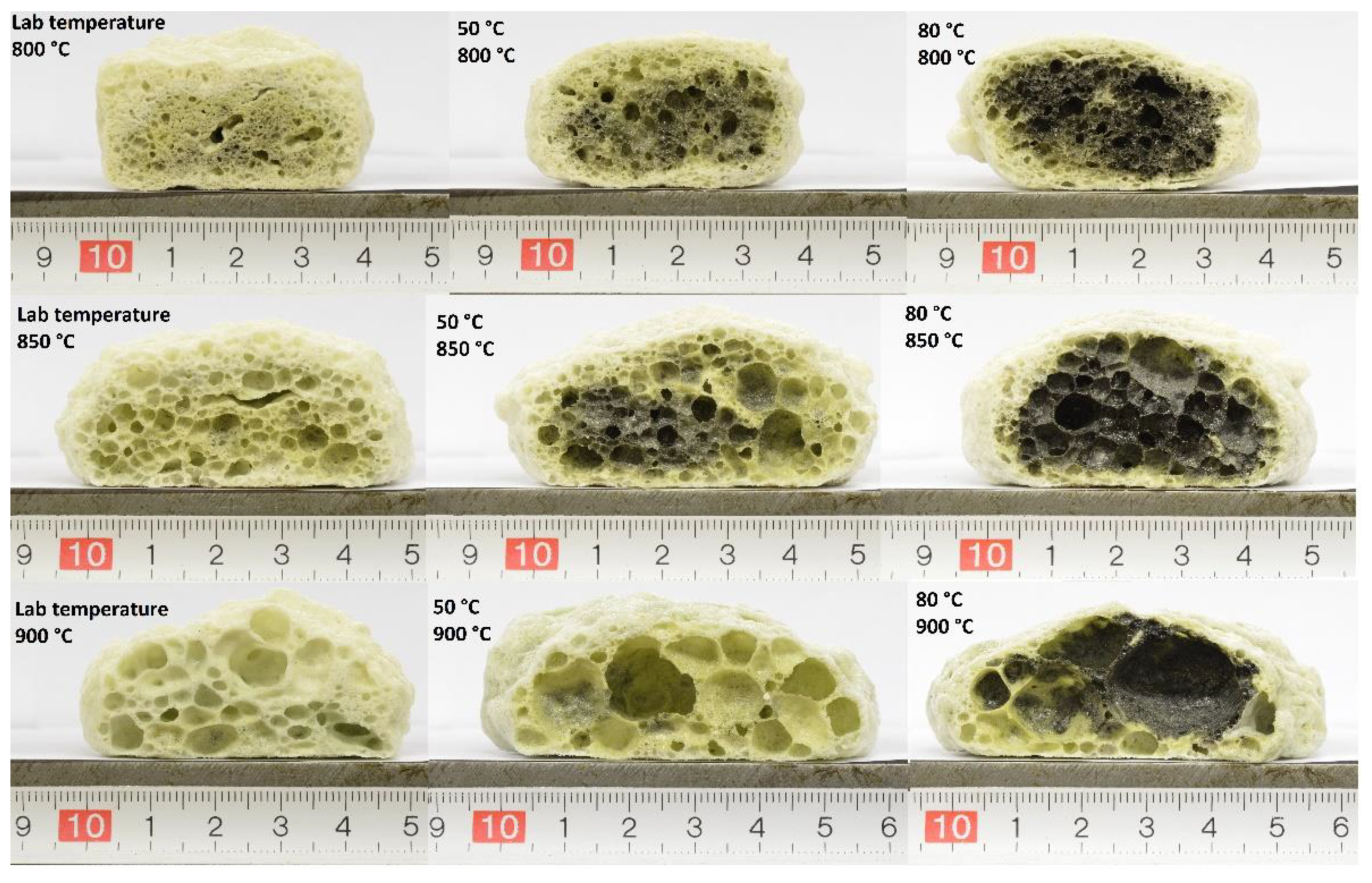
3.2.2. Bulk Density and Compressive Strength
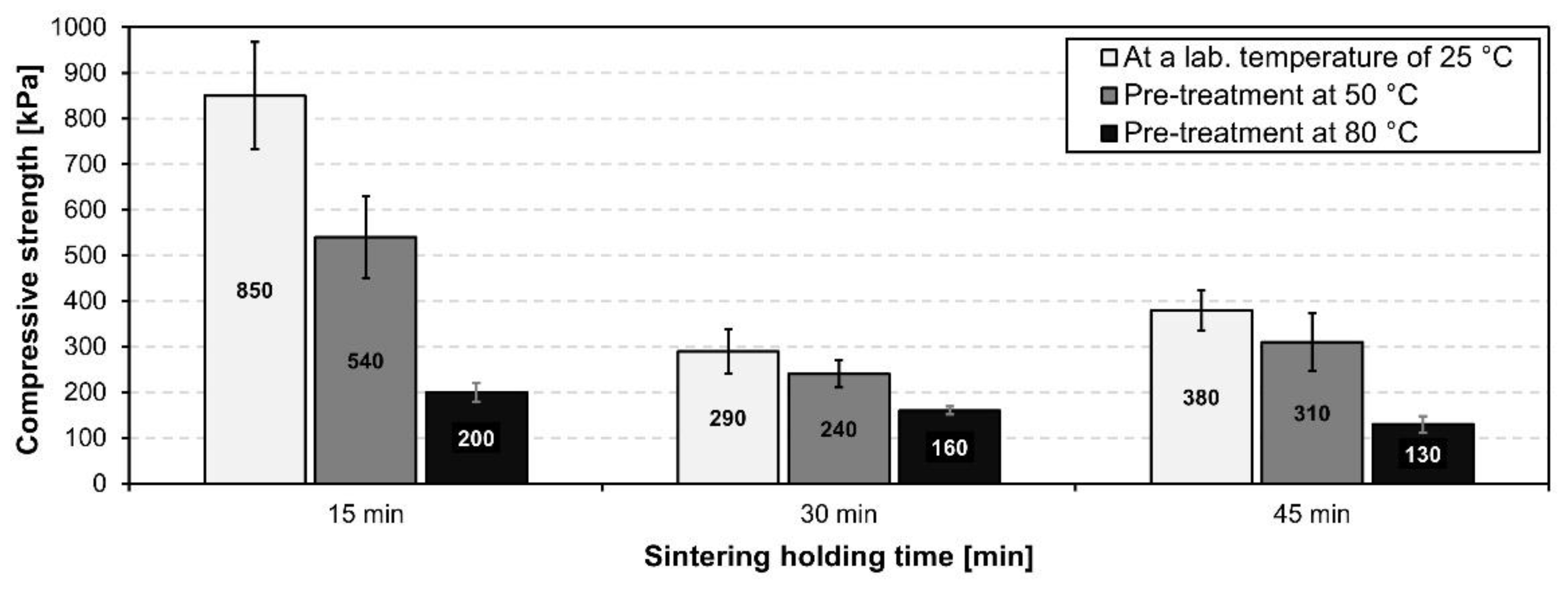
3.2.3. Influence of Sintering Holding Time on the Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scarinci, G.; Brusatin, G.; Bernado, E. Glass Foam. In Cellular Ceramics: Structure, Manufacturing, Properties and Applications; Scheffler, M., Colombo, P., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 158–176. [Google Scholar]
- Karandashova, N.S.; Goltsman, B.M.; Yatsenko, E.A. Analysis of Influence of Foaming Mixture Components on Structure and Properties of Foam Glass. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012020. [Google Scholar] [CrossRef]
- Siddika, A.; Hajimohammadi, A.; Sahajwalla, V. Powder sintering and gel casting methods in making glass foam using waste glass: A review on parameters, performance, and challenges. Ceram. Int. 2022, 48, 1494–1511. [Google Scholar] [CrossRef]
- Khamidulina, D.D.; Nekrasova, S.A.; Voronin, K.M. Foam Glass Production from Waste Glass by Compression. IOP Conf. Ser. Mater. Sci. Eng. 2017, 262, 012008. [Google Scholar] [CrossRef]
- Manevich, V.E.; Subbotin, K.Y. Foam glass and problems of energy conservation. Glass Ceram. 2008, 65, 105–108. [Google Scholar] [CrossRef]
- Foam Glass Market by Type (Open Cell and Closed Cell), Process (Physical and Chemical), Application (Building & Industrial Insulation and Chemical Processing Systems), End-Use Industry (Building & Construction and Industrial)—Global Forecast to 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/foam-glass-market-3506071.html (accessed on 11 November 2021).
- Yatsenko, E.A.; Goltsman, B.M.; Klimova, L.V.; Yatsenko, L.A. Peculiarities of foam glass synthesis from natural silica-containing raw materials. J. Therm. Anal. Calorim. 2020, 142, 119–127. [Google Scholar] [CrossRef]
- König, J.; Petersen, R.R.; Yue, Y. Fabrication of highly insulating foam glass made from CRT panel glass. Ceram. Int. 2015, 41, 9793–9800. [Google Scholar] [CrossRef]
- Hao, Y.; Wu, H.; Guan, J. Synthesis of Foam Glass-Ceramic from CRT Panel Glass using One-step Powder Sintering. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 012020. [Google Scholar] [CrossRef]
- Sassi, M.; Ibrahim, J.F.M.; Simon, A. Characterization of foam glass produced from waste CRT glass and aluminium dross. J. Phys. Conf. Ser. 2020, 1527, 012037. [Google Scholar] [CrossRef]
- Bai, J.; Yang, X.; Xu, S.; Jing, W.; Yang, J. Preparation of foam glass from waste glass and fly ash. Mater. Lett. 2014, 136, 52–54. [Google Scholar] [CrossRef]
- Chen, B.; Luo, Z.; Lu, A. Preparation of sintered foam glass with high fly ash content. Mater. Lett. 2011, 65, 3555–3558. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Tulyaganov, D.U.; Ferreira, J.M.F. Production and characterisation of glass ceramic foams from recycled raw materials. Adv. Appl. Ceram. 2013, 108, 9–13. [Google Scholar] [CrossRef]
- Ye, Z.N.; Wang, Y.W.; Jiang, H.; Li, N.; Liu, S.Q. Production and characterisation of glass ceramic foams from recycled raw materials. Key Eng. Mater. 2013, 575–576, 461–464. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Guan, L.; Liu, P.; Wu, T.; Li, Z.; Lu, A. Low-cost and environment-friendly ceramic foams made from lead–zinc mine tailings and red mud: Foaming mechanism, physical, mechanical and chemical properties. Ceram. Int. 2016, 42, 1733–1739. [Google Scholar] [CrossRef]
- Kurtulus, C.; Kurtulus, R.; Kavas, T. Foam glass derived from ferrochrome slag and waste container glass: Synthesis and extensive characterizations. Ceram. Int. 2021, 47, 24997–25008. [Google Scholar] [CrossRef]
- Yan, Z.; Feng, K.; Tian, J.; Liu, Y. Effect of high titanium blast furnace slag on preparing foam glass–ceramics for sound absorption. J. Porous Mater. 2019, 26, 1209–1215. [Google Scholar] [CrossRef]
- Kazantseva, L.K.; Yusupov, T.S.; Lygina, T.Z.; Shumskaya, L.G.; Tsyplakov, D.S. Foam Glass from Mechanoactivated Zeolite-Poor Rock. Glass Ceram. 2014, 70, 360–364. [Google Scholar] [CrossRef]
- Vereshagin, V.I.; Sokolova, S.N.; Lygina, T.Z.; Shumskaya, L.G.; Tsyplakov, D.S. Granulated foam glass–ceramic material from zeolitic rocks. Constr. Build. Mater. 2008, 22, 999–1003. [Google Scholar] [CrossRef]
- Yatsenko, E.A.; Goltsman, B.M.; Ryabova, A.V. Complex Protection of Pipelines Using Silicate Materials Based on Local Raw Materials of the Far East. Mater. Sci. Forum 2019, 945, 46–52. [Google Scholar] [CrossRef]
- Ivanov, K.S.; Goltsman, B.M.; Ryabova, A.V. Preparation and Properties of Foam Glass-ceramic from Diatomite. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2018, 33, 273–277. [Google Scholar] [CrossRef]
- Petersen, R.R.; König, J.; Yue, Y. The mechanism of foaming and thermal conductivity of glasses foamed with MnO2. J. Non-Cryst. Solids 2015, 425, 74–82. [Google Scholar] [CrossRef]
- Davraz, M.; Koru, M.; Akdağ, A.E.; Kılınçarslan, Ş.; Delikanlı, Y.E.; Çabuk, M. An investigation of foaming additives and usage rates in the production of ultra-light foam glass. J. Therm. Anal. Calorim. 2015, 425, 74–82. [Google Scholar] [CrossRef]
- Shi, H.; Feng, K.; Wang, H.; Chen, C.; Zhou, H.; Çabuk, M. Influence of aluminium nitride as a foaming agent on the preparation of foam glass-ceramics from high-titanium blast furnace slag. Int. J. Miner. Metall. Mater. 2016, 23, 595–600. [Google Scholar] [CrossRef]
- Yot, P.G.; Méar, F.O. Lead extraction from waste funnel cathode-ray tubes glasses by reaction with silicon carbide and titanium nitride. J. Hazard. Mater. 2009, 172, 117–123. [Google Scholar] [CrossRef]
- da Silva, R.C.; Kubaski, E.T.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Tebcherani, S.M. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda-lime glass matrix. J. Non-Cryst. Solids 2019, 511, 177–182. [Google Scholar] [CrossRef]
- Éidukyavichus, K.K.; Matseikene, V.R.; Balkyavichus, V.V.; Shpokauskas, A.A.; Laukaitis, A.A.; Kunskaite, L.Y. Use of Cullet of Different Chemical Compositions in Foam Glass Production: Study on the reaction mechanism in soda-lime glass matrix. Glass Ceram. 2004, 61, 77–80. [Google Scholar] [CrossRef]
- Reka, A.A.; Pavlovski, B.; Fazlija, E.; Berisha, A.; Pacarizi, M.; Daghmehchi, M.; Sacalis, C.; Jovanovski, G.; Makreski, P.; Oral, A. Diatomaceous Earth: Characterization, thermal modification, and application. Open Chem. 2021, 19, 451–461. [Google Scholar] [CrossRef]
- Ptáček, P.; Frajkorová, F.; Šoukal, F.; Opravil, T. Kinetics and mechanism of three stages of thermal transformation of kaolinite to metakaolinite. Powder Technol. 2014, 264, 439–445. [Google Scholar] [CrossRef]
- Mos, Y.M.; Vermeulen, A.C.; Buisman, C.J.N.; Weijma, J. X-ray Diffraction of Iron Containing Samples: The Importance of a Suitable Configuration. Geomicrobiol. J. 2018, 35, 511–517. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, K.S.; Radaev, S.S.; Selezneva, O.I.; Yatsenko, L.A. Diatomites in Granular Foam-Glass Technology. Glass Ceram. 2014, 71, 119–127. [Google Scholar] [CrossRef]





| Oxide | SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | K2O | CuO | LE |
|---|---|---|---|---|---|---|---|---|
| Amount (wt.%) | 63.9 | 18.1 | 3.3 | 0.8 | 0.7 | 0.3 | 0.2 | 12.7 |
| SD (wt.%) | 0.1 | 0.1 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.1 |
| LOD (wt.%) | LOI (wt.%) | Density (g·cm−3) |
|---|---|---|
| 45.1 | 10.7 | 0.7988 |
| Phase | wt.% |
|---|---|
| Kaolinite | 59.9 |
| Quartz low | 0.2 |
| Anatase | 0.5 |
| Calcite | 0.2 |
| Amorphous | 39.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedlačík, M.; Nguyen, M.; Opravil, T.; Sokolář, R. Preparation and Characterization of Glass-Ceramic Foam from Clay-Rich Waste Diatomaceous Earth. Materials 2022, 15, 1384. https://doi.org/10.3390/ma15041384
Sedlačík M, Nguyen M, Opravil T, Sokolář R. Preparation and Characterization of Glass-Ceramic Foam from Clay-Rich Waste Diatomaceous Earth. Materials. 2022; 15(4):1384. https://doi.org/10.3390/ma15041384
Chicago/Turabian StyleSedlačík, Martin, Martin Nguyen, Tomáš Opravil, and Radomír Sokolář. 2022. "Preparation and Characterization of Glass-Ceramic Foam from Clay-Rich Waste Diatomaceous Earth" Materials 15, no. 4: 1384. https://doi.org/10.3390/ma15041384
APA StyleSedlačík, M., Nguyen, M., Opravil, T., & Sokolář, R. (2022). Preparation and Characterization of Glass-Ceramic Foam from Clay-Rich Waste Diatomaceous Earth. Materials, 15(4), 1384. https://doi.org/10.3390/ma15041384






