Alkali-Treated Alumina and Zirconia Powders Decorated with Hydroxyapatite for Prospective Biomedical Applications
Abstract
:1. Introduction
2. Materials and Methods
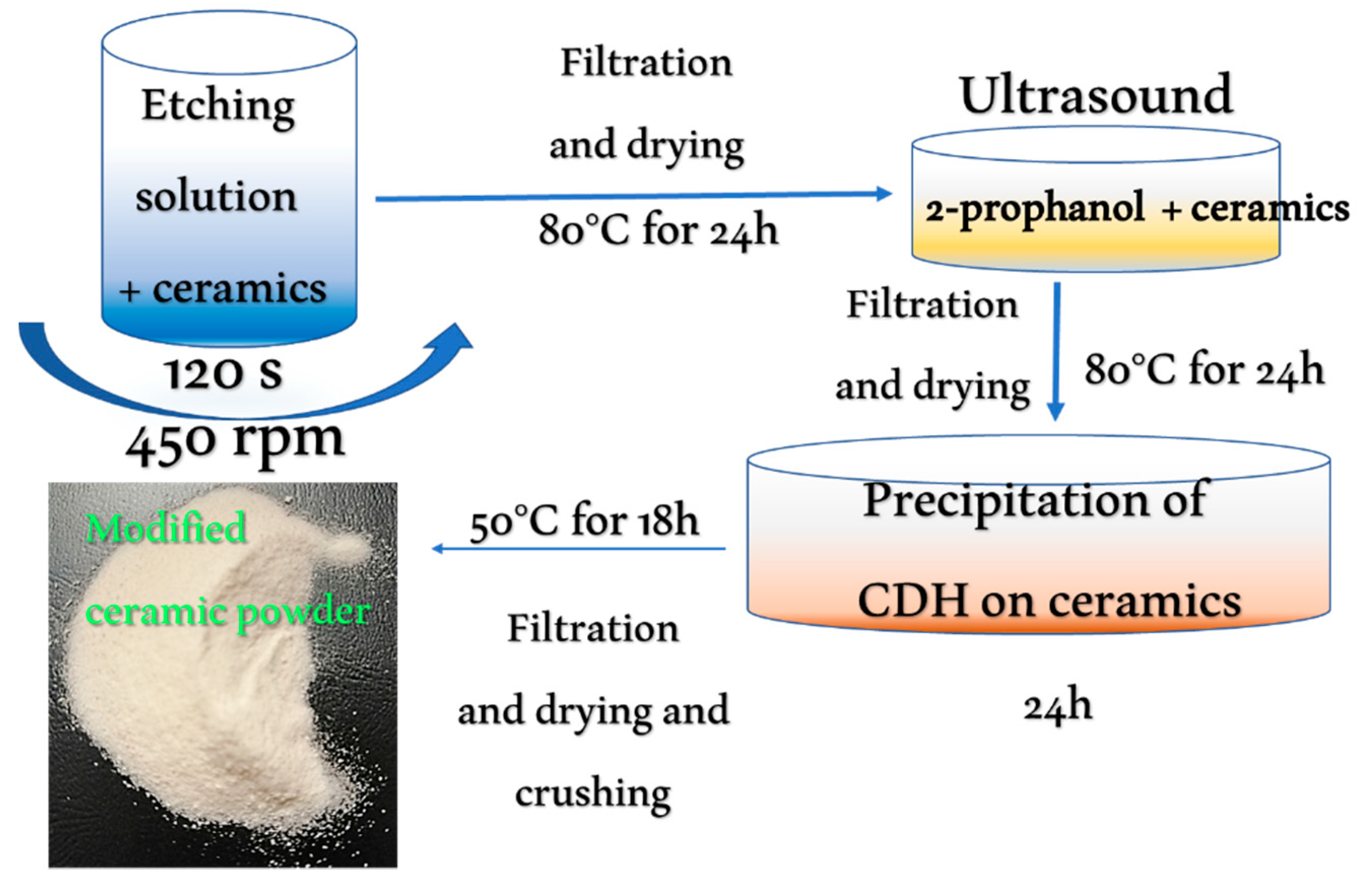
2.1. Sample Preparation
2.2. Materials Characterization
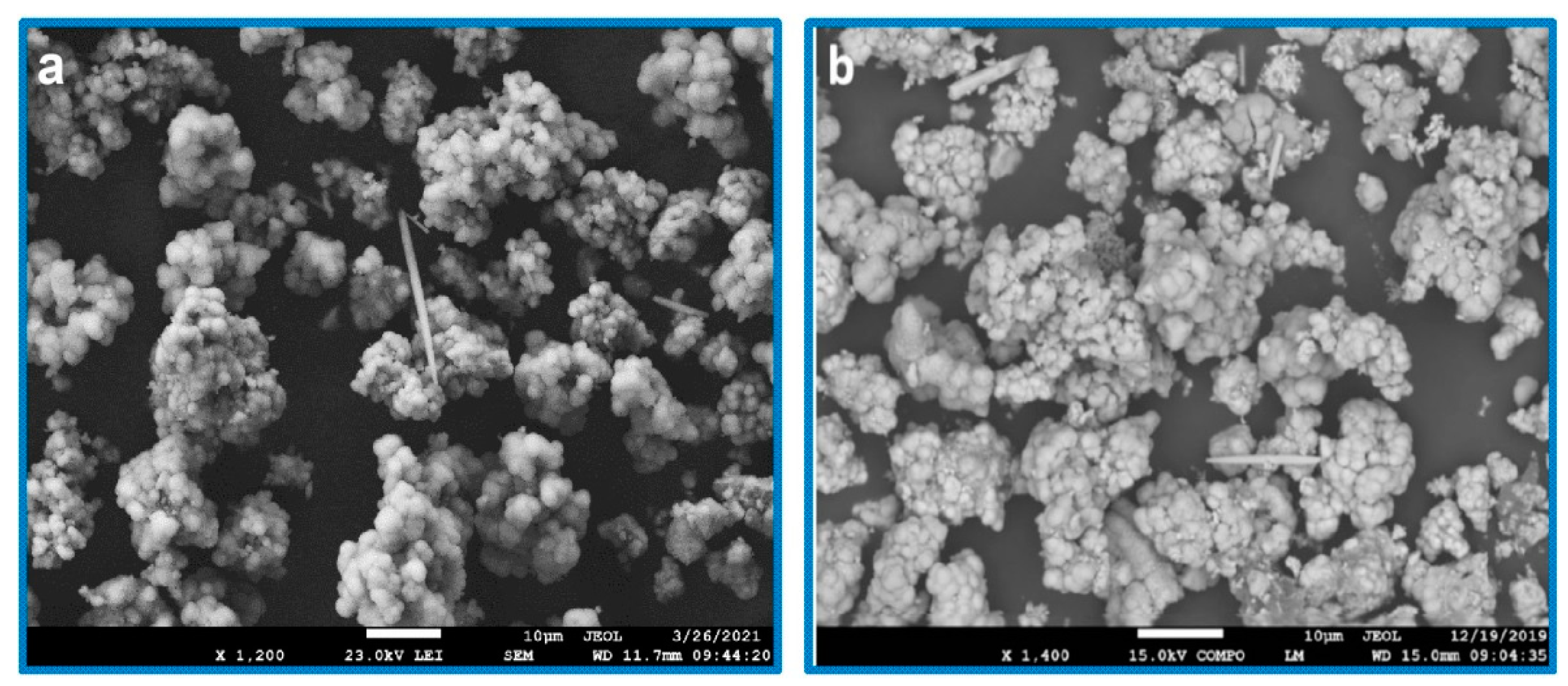
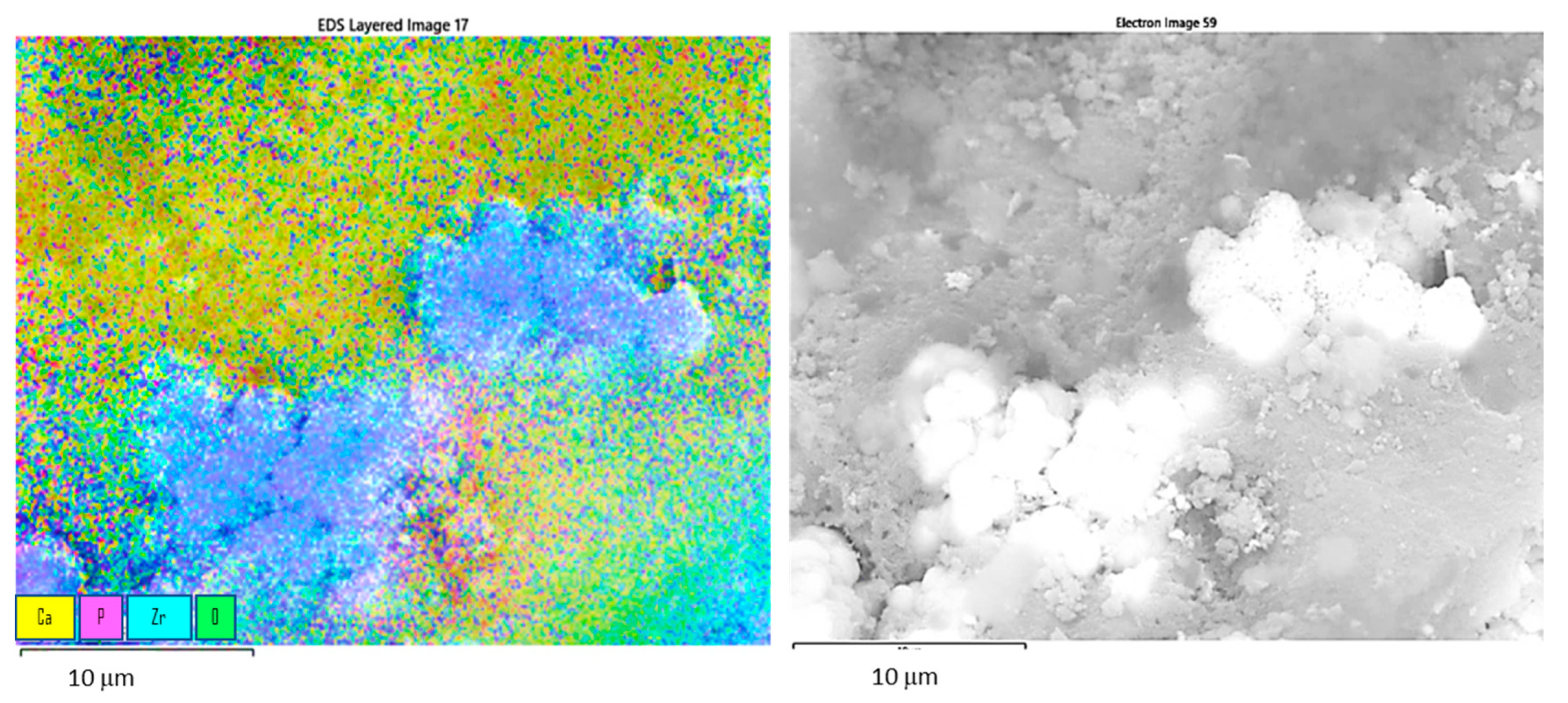
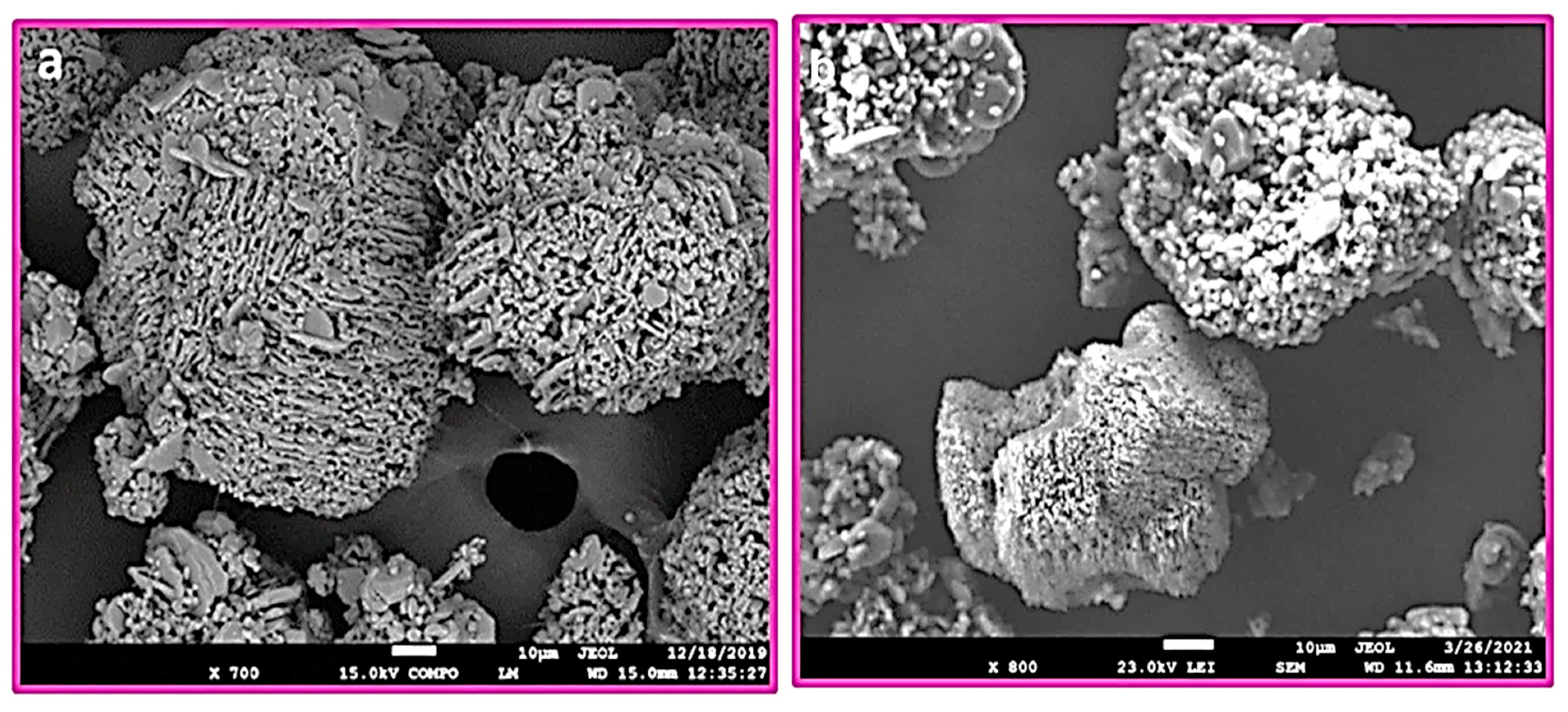
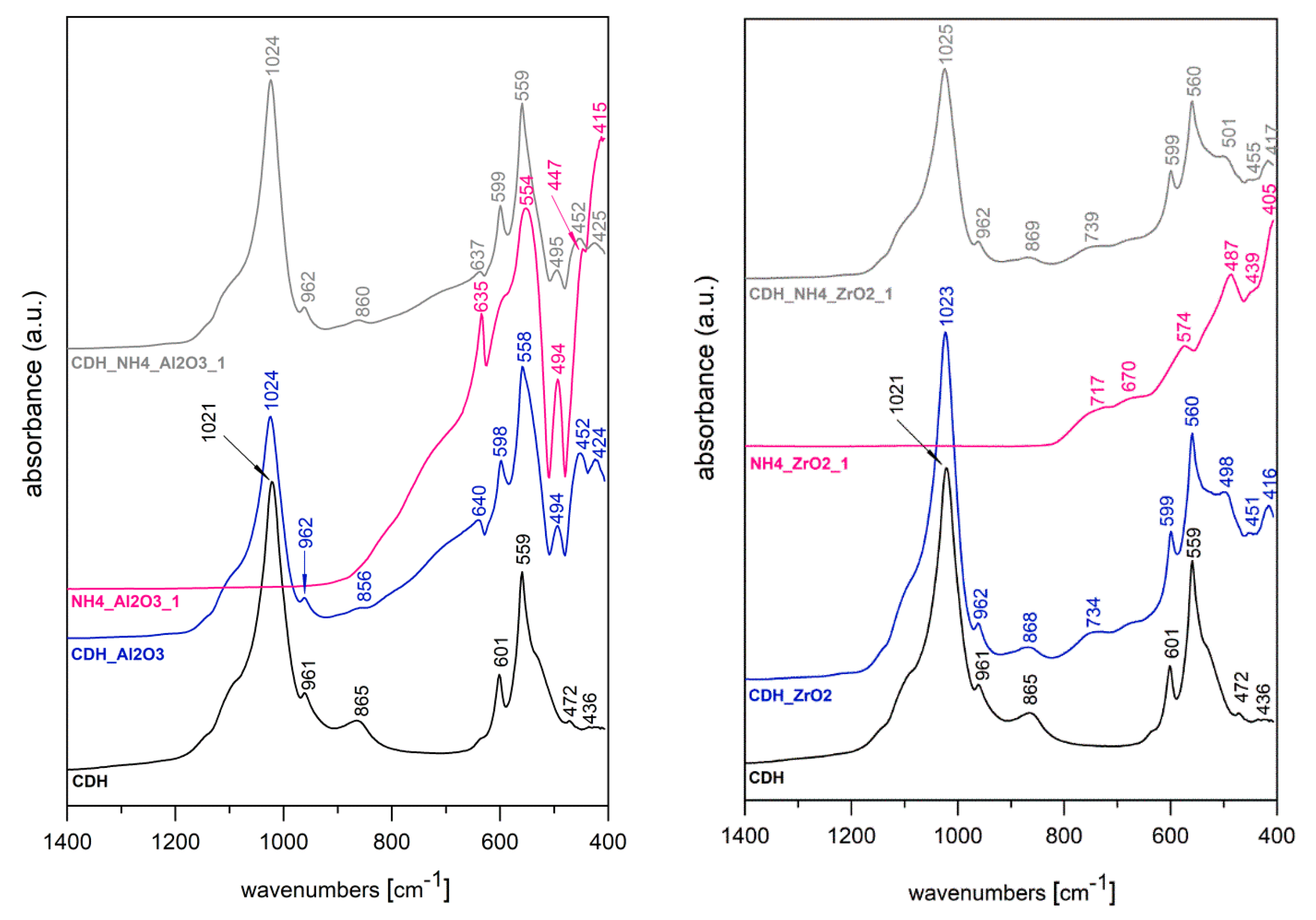
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakonieczny, D.S.; Ziębowicz, A.; Paszenda, Z.; Krawczyk, C. Trends and perspectives in modification of zirconium oxide for a dental prosthetic applications—A review. Biocybern. Biomed. Eng. 2017, 37, 229–245. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, H.W.; Bae, D.K.; Park, C.H. High incidence of tibial component loosening after total knee arthroplasty using ceramic titanium-nitride-coated mobile bearing prosthesis in moderate to severe varus deformity: A matched-pair study between ceramic-coated mobile bearing and fixed bearing prostheses. J. Arthroplast. 2020, 35, 1003–1008. [Google Scholar]
- Šupová, M.; Suchý, T.; Sucharda, Z.; Filová, E.; Kinderen, J.N.L.M. The comprehensive in vitro evaluation of eight different calcium phosphates: Significant parameters for cell behaviour. J. Am. Ceram. Soc. 2019, 102, 2882–2904. [Google Scholar] [CrossRef]
- Pazourková, L.; Reli, M.; Hundáková, M.; Pazdziora, E.; Predoi, D.; Simha Martynková, G.; Lafdi, K. Study of the structure and antimicrobial activity of Ca-deficient ceramics on chlorhexidine nanoclay substrate. Materials 2019, 12, 2996. [Google Scholar] [CrossRef] [Green Version]
- Pazourková, L.; Peikertová, P.; Hundáková, M.; Martynková, G.S. Preparation of calcium deficient hydroxyapatite on the montmorillonite substrate: Structure and morphology. Mater. Today Proc. 2019, 37, 35–41. [Google Scholar] [CrossRef]
- Li, L.; Hei, H.; Wang, Y.; Zheng, K.; Ma, Y.; Gao, J.; Zhou, B.; He, Z.; Zong, J.; Yu, S.; et al. Mictrostructure and properties of Ta coatings on the 3Y-TZP ceramic fabricated by plasma alloying technique. J. Alloys Comp. 2019, 805, 1135–1143. [Google Scholar] [CrossRef]
- Ju, L.; Yang, J.; Hao, A.; Daniel, J.; Morales, J.; Nguyen, S.; Andrei, P.; Liang, R.; Hellstrom, E.; Xu, C. A hybrid ceramic-polymer composite fabricated by co-curing lay-up process for a strong bonding and enhanced transient thermal protection. Ceram. Int. 2018, 44, 11497–11504. [Google Scholar] [CrossRef]
- Silva, C.S.; Henriques, B.; Novaes de Oliveira, A.P.; Silva, F.; Gomes, J.R.; Souza, J.C.M. Micro-scale abrasion and sliding wear of zirconium-lithium silicate glass-ceramic and polymer-infiltrated ceramic network used in dentistry. Wear 2020, 448–449, 202314. [Google Scholar] [CrossRef]
- Soon, G.; Pingguan-Murphy, B.; Wee La, K.; Akbar, S.A. Review of zirconia-based bioceramic: Surface modification and cellular response. Ceram. Int. 2016, 42, 12543–12555. [Google Scholar] [CrossRef]
- Pazourková, L.; Peikertová, P.; Hundakova, M.; Simha Martynková, G. Preparation of calcium deficient hydroxyapatite on vermiculite from China and Africa deposits. Mater. Today Proc. 2018, 5, S38–S44. [Google Scholar] [CrossRef]
- Bellis, C.A.; Addison, O.; Nobbs, A.H.; Duckworth, P.F.; Holder, J.A.; Barbour, M.E. Glass ionomer cements with milled, dry chlorhexidine hexametaphosphate filler particles to provide long-term antimicrobial properties with recharge capacity. Dent. Mater. 2018, 34, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, G.; Cao, Y.; Liu, C.; Jin, Y.; Wang, Y.; Yang, L.; Guo, J.; Zhu, L. Self-assembling peptide and nHA/CTS composite scaffolds promote bone regeneration through increasing seed cell adhesion. Mater. Sci. Eng. C 2018, 93, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.; Gao, Y.; Jin, T.; Luo, X.; Zeng, Q.; Shang, Z. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2020, 26, 6550–6559. [Google Scholar] [CrossRef]
- Kohal, R.J.; Bächle, M.; Att, W.; Chaar, S.; Altmann, B.; Renz, A.; Butz, F. Osteoblast and bone tissue response to surface modified zirconia and titanium implants materials. Dent. Mater. 2013, 29, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Langhoff, J.D.; Voelter, K.; Scharnweber, D.; Schnabelrauch, M.; Schlottig, F.; Hefti, T.; Kalchofner, K.; Nuss, K.; Rechenberg, B. Comparison of chemically and pharmaceutically modified titanium and zirconia implant surfaces in dentistry: A study in sheep. Int. J. Oral Maxillofac. Surg. 2008, 37, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Bayram, L.; Guler, M. An ultra-sensitive non-enzymatic hydrogen peroxide sensor based on SiO2-APTES supported Au nanoparticles modified glassy carbon electrode. Prog. Nat. Sci. Mater. Int. 2019, 29, 390–396. [Google Scholar] [CrossRef]
- Rokicka-Konieczna, P.; Wanag, A.; Sienkiewicz, A.; Kusiak-Nejman, E.; Morawski, W. Antibacterial effect of TiO2 nanoparticles modified with APTES. Catal. Commun. 2020, 134, 105862. [Google Scholar] [CrossRef]
- Sandomierski, M.; Strzemiecka, B.; Grams, J. Diazonium-modified zeolite fillers. Effect of diazonium substituent position on the filler surface modification and the mechanical properties of phenolic/zeolite composites. Int. J. Adhes. Adhes. 2018, 85, 157–164. [Google Scholar] [CrossRef]
- Zhu, P.; Meier, S.; Saravanamurugan, S.; Riisager, A. Modification of commercial Y zeolites by alkaline-treatment for improved performance in the isomerization of glucose to fructose. Mol. Catal. 2021, 510, 111686. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.; Valencia-Saavedra, W.; Mejia de Gutirrez, R. Recycling of concrete, ceramic, and masonry waste via alkaline activation: Obtaining and characterization of hybrid cements. J. Build. Eng. 2022, 46, 103698. [Google Scholar] [CrossRef]
- Shahapurkar, K.; Bapurao Chavan, V.; Doddmani, M.; Kumar, G.C.M. Influence of surface modification on wear behaviour of fly ash cenosphere/epoxy syntactic foam. Wear 2018, 414–415, 327–340. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, J.; Zeng, X.; Cao, Y. UV laser microprocessing and post chemical etching on ultrathin Al2O3 ceramic substrate. J. Eur. Ceram. Soc. 2011, 31, 1631–1639. [Google Scholar] [CrossRef]
- Alford, W.J.; Stephens, D.L. Chemical polishing and etching techniques for Al2O3 single crystals. J. Am. Ceram. Soc. 1963, 46, 193–194. [Google Scholar] [CrossRef]
- Elsaka, S.E. Influence of surface treatments on the surface properties of different zirconia cores and adhesion of zirconia-veneering ceramic systems. Dent. Mater. 2013, 29, e239–e251. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Chiu, Y.-C.; Yang, C.-S.; Chen, M. Oxide layers characteristics and interfacial analysis of porcelain fused to high-gold alloy using multitechnique analysis methods. J. Dent. Sci. 2017, 12, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef] [Green Version]
- Šupová, M. Isolation and preparation of nanoscale bioapatites from natural sources: A review. J. Nanosci. Nanotechnol. 2014, 14, 546–563. [Google Scholar] [CrossRef]
- Atif Faiz Afzala, M.; Kesarwani, P.; Madhav Reddy, K.M.; Kalmodia, S.; Basu, B.; Balani, K. Functionally graded hydroxyapatite-alumina-zirconia biocomposite: Synergy of toughness and biocompatibility. Mater. Sci. Eng. C 2012, 32, 1164–1173. [Google Scholar] [CrossRef]
- Huaxia, J.I.; Ponton, C.B.; Marquis, P.M. Microstructural characterization of hydroxyapatite coating on titanium. J. Mater. Sci. Mater. Med. 1992, 3, 283–287. [Google Scholar]
- Stango, S.A.X.; Vijayalakshmi, U. Electrochemical deposition of HAP/f-MWCNTs and HAP/GO composite layers on 316L SS implant with excellent corrosion resistance performance. Matter. Lett. 2021, 304, 130666. [Google Scholar] [CrossRef]
- Liu, X.; Miao, Y.; Liang, H.; Dia, J.; Hao, L.; Shi, Z.; Zhao, N.; Wang, Y. 3D-printed bioactive ceramic scaffolds with biomimetic micro/nano-Hap surfaces mediated cell fate and promoted bone augmentation of the bone-implant interface in vivo. Bioact. Mater. 2022, 12, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Kocyło, E.; Franchin, G.; Colombo, P.; Chmielarz, A.; Potoczek, M. Hydroxyapatite-coated ZrO2 scaffolds with a fluorapatite intermediate layer produced by direct ink writing. J. Eur. Ceram. 2021, 41, 920–928. [Google Scholar] [CrossRef]
- Musyarofah Soontranon, S.; Limphirat, W.; Triwikantoro Pratapa, S. XRD, WAXS, FTIR, and XANES studies of silica-zirconia systems. Ceram. Int. 2019, 45, 15660–15670. [Google Scholar] [CrossRef]
- Nuckowski, P.M.; Wróbel, T. Mechanical properties and fracture analysis of AlCu4MgSi alloy ingots obtained by horizontal continuous casting. Arch. Metall. Mater. 2019, 64, 113–118. [Google Scholar]
- Yakout, S.M.; Hassan, H.S. Adsorption characteristics of sol gel-derived zirconia for cesium ions from aqueous solutions. Molecules 2014, 19, 9160–9172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Liu, Y.; Zhu, K.; Siu, G.; Xiong, Y.; Xiong, C. Infrared spectra of nanometre granular zirconia. J. Phys. Condens. Matter 1999, 11, 2035–2042. [Google Scholar] [CrossRef]
- Toledo, R.R.; Santoyo, V.; Sánchez, D.M.; Rosales, M.M. Effect of aluminum precursor on physicochemical properties of Al2O3 by hydrolysis/precipitation method. Nova Sci. 2018, 20, 83–99. [Google Scholar] [CrossRef]
- Reyes, J.M.; Perez Ramos, B.M.; Islas, C.S.; Arriaga, W.C.; Quintero, P.R.; Jacome, A.R. Chemical and morphological characteristics of ALD Al2O3 thin-film surfaces after immersion in pH buffer solutions. J. Elecrtrochem. Soc. 2013, 160, B201–B206. [Google Scholar] [CrossRef] [Green Version]
- Nakonieczny, D.S.; Slíva, A.; Paszenda, Z.; Hundáková, M.; Kratošová, G.; Holešová, S.; Majewska, J.; Kałużyński, P.; Sathish, S.K.; Simha Martynková, G. Simple approach to medical grade alumina and zirconia ceramics surface alteration via acid etching treatment. Crystals 2021, 11, 1232. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Kern, F.; Antonowicz, M.; Dufner, L.; Matus, K. Alumina and zirconia-reinforced polyamide PA-12 composites for biomedical additive manufacturing. Materials 2021, 14, 6201. [Google Scholar] [CrossRef] [PubMed]
- Hodásová, L.; Brenda, J.S.; Molina, G.; Alemán, C.; Llanes, L.; Fargas, G.; Armelin, E. Polymer infiltrated ceramic networks with biocompatibilie adhesive and 3D-printed highly porous scaffolds. Add. Manuf. 2021, 39, 101850. [Google Scholar]
- Kim, D.H.; Son, J.S.; Kwon, T.Y. Antibactirial effect of chlorohexidine releasing porous hydroxyapatite scaffold incorporated with human serum albumin nanoparticles. Matter Lett. 2020, 266, 127479. [Google Scholar] [CrossRef]
- Nakonieczny, D.S.; Antonowicz, M.; Paszenda, Z.K.; Radko, T.; Drewaniak, S.; Bogacz, W.; Krawczyk, C. Experimental investigations of particle size distribution and morphology of alumina-yttria-ceria-zirconia powders obtained via sol-gel route. Biocybern. Biomed. Eng. 2018, 38, 535–543. [Google Scholar] [CrossRef]
- Guo, Y.J.; Long, T.; Chen, W.; Ning, C.Q.; Zhu, Z.A.; Guo, Y.P. Bactericidal property and biocompatibility of gentamicin-loaded mesoporous carbonated hydroxyapatite microspheres. Mater. Sci. Eng. C 2013, 33, 3583–3591. [Google Scholar] [CrossRef]





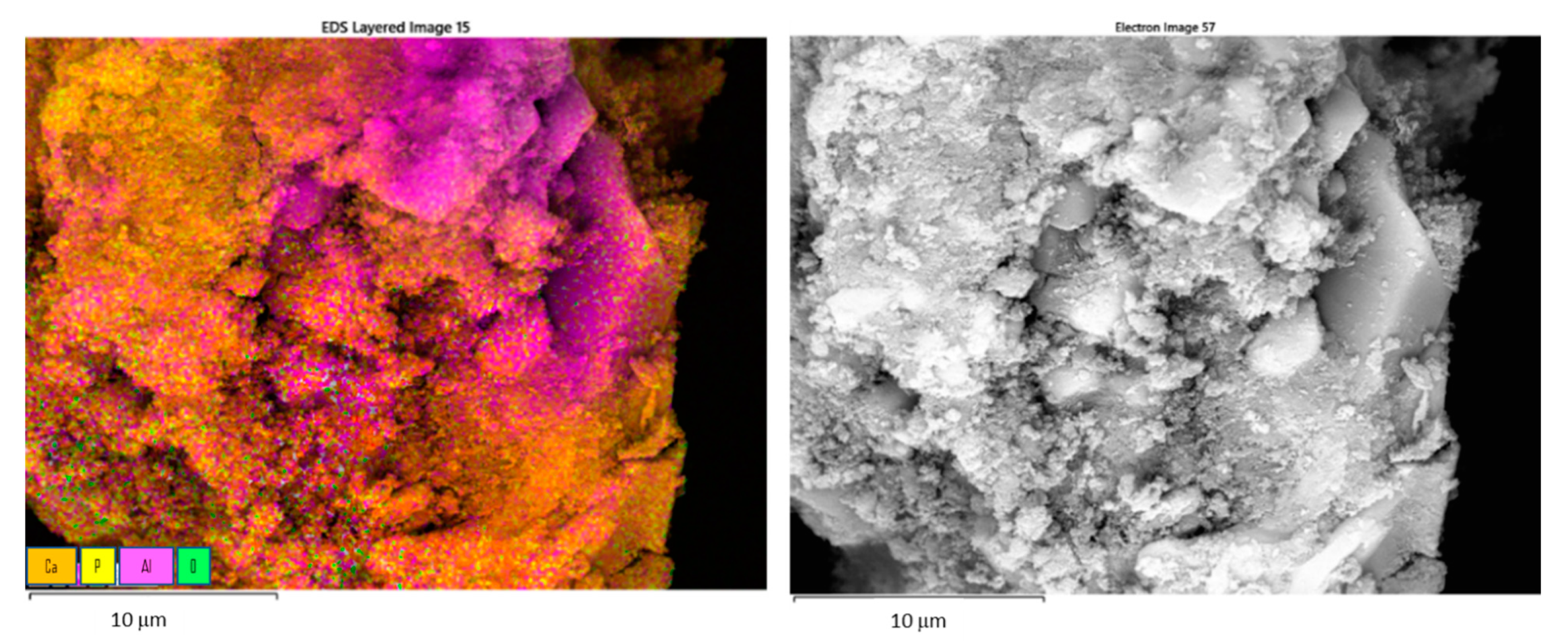
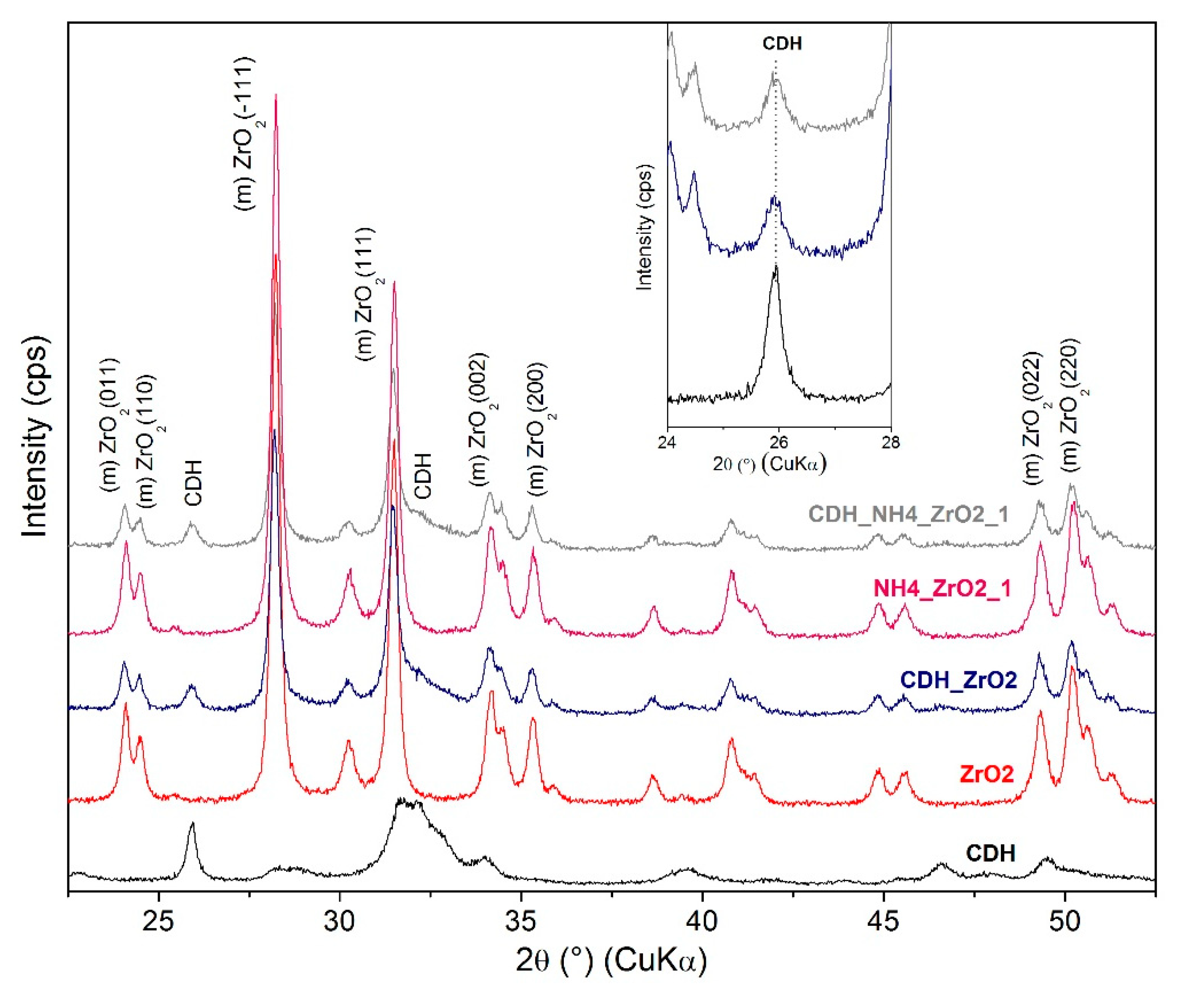
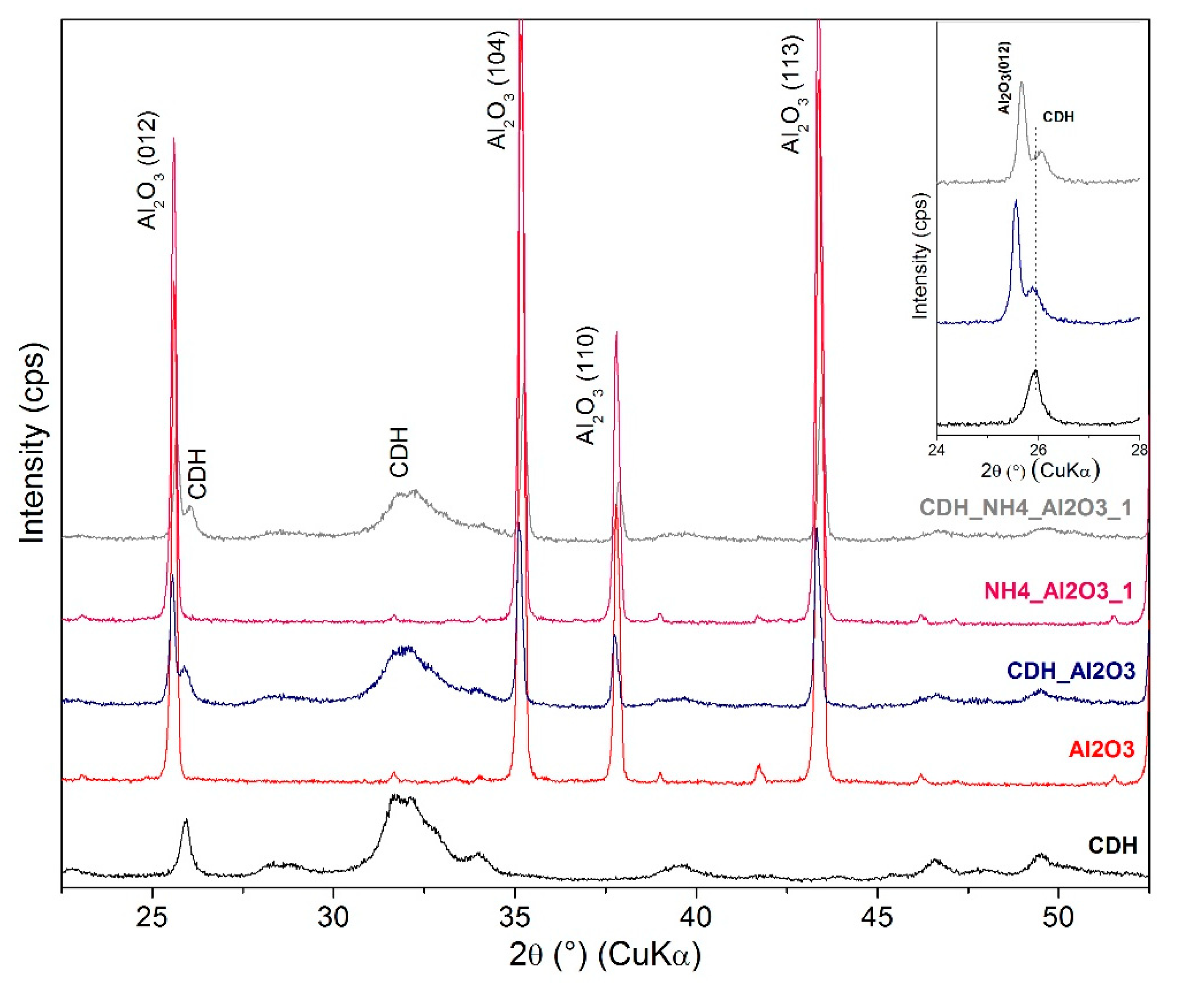
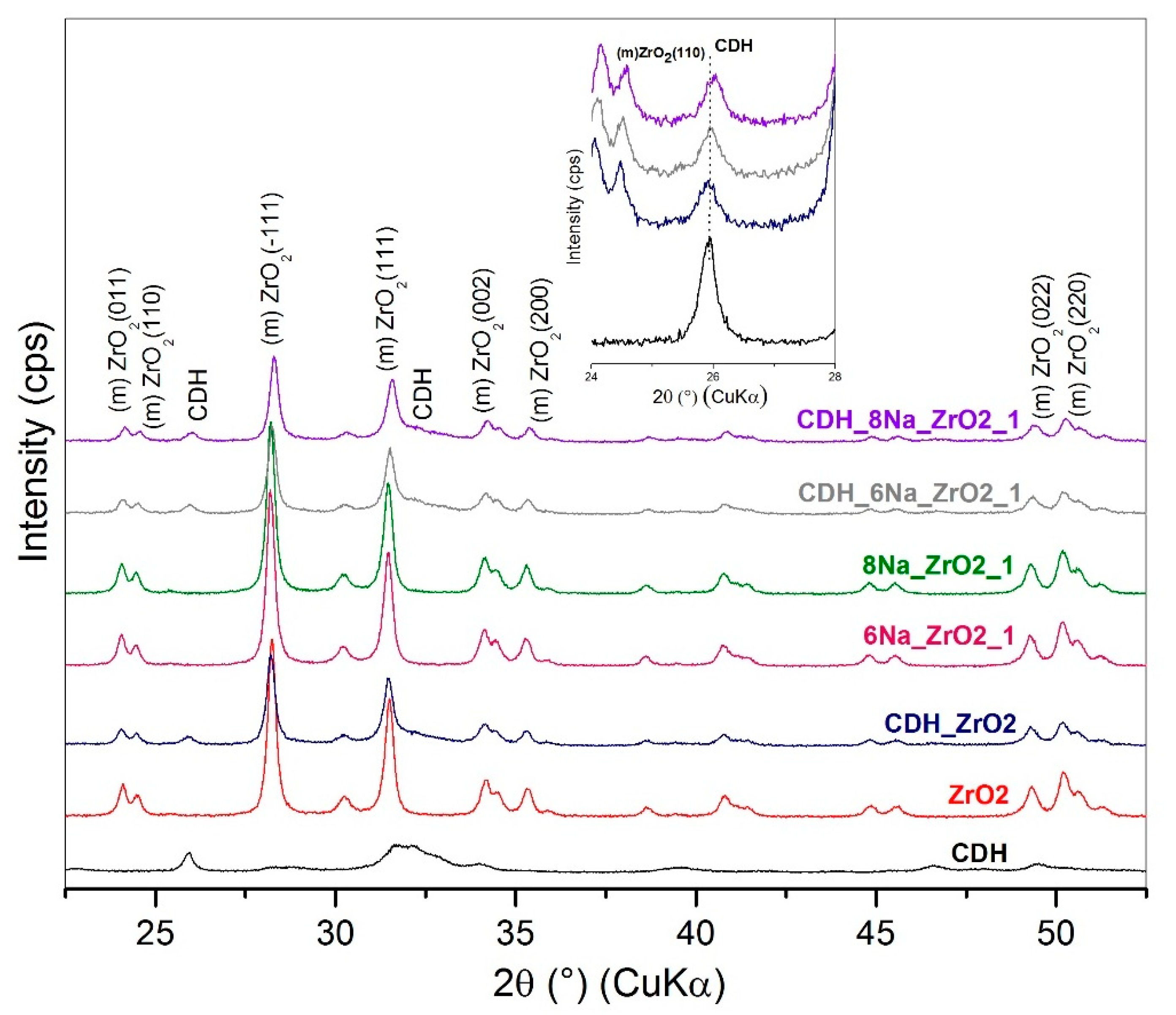

| Etching Agent | Reagent’s Volume Ratio | Name of the Samples | |
|---|---|---|---|
| ZrO2 | ZrO2 + CDH | ||
| 35% NH4OH 35% H2O2 | 1:1 | NH4_ZrO2_1 | CDH_NH4_ZrO2_1 |
| 1:3 | NH4_ZrO2_0.3 | CDH_NH4_ZrO2_0.3 | |
| 8 M NaOH 35% H2O2 | 1:1 | 8Na_ZrO2_1 | CDH_8Na_ZrO2_1 |
| 6 M NaOH 35% H2O2 | 1:1 | 6Na_ZrO2_1 | CDH_6Na_ZrO2_1 |
| Etching Agent | Reagent’s Volume Ratio | Name of the Samples | |
|---|---|---|---|
| Al2O3 | Al2O3 + CDH | ||
| 35% NH4OH 35% H2O2 | 1:1 | NH4_Al2O3_1 | CDH_NH4_Al2O3_1 |
| 1:3 | NH4_Al2O3_0.3 | CDH_NH4_Al2O3_0.3 | |
| 8 M NaOH 35% H2O2 | 1:1 | 8Na_Al2O3_1 | CDH_8Na_Al2O3_1 |
| 6 M NaOH 35% H2O2 | 1:1 | 6Na_Al2O3_1 | CDH_6Na_Al2O3_1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakonieczny, D.S.; Martynková, G.S.; Hundáková, M.; Kratošová, G.; Holešová, S.; Kupková, J.; Pazourková, L.; Majewska, J. Alkali-Treated Alumina and Zirconia Powders Decorated with Hydroxyapatite for Prospective Biomedical Applications. Materials 2022, 15, 1390. https://doi.org/10.3390/ma15041390
Nakonieczny DS, Martynková GS, Hundáková M, Kratošová G, Holešová S, Kupková J, Pazourková L, Majewska J. Alkali-Treated Alumina and Zirconia Powders Decorated with Hydroxyapatite for Prospective Biomedical Applications. Materials. 2022; 15(4):1390. https://doi.org/10.3390/ma15041390
Chicago/Turabian StyleNakonieczny, Damian S., Gražyna Simha Martynková, Marianna Hundáková, Gabriela Kratošová, Sylva Holešová, Jana Kupková, Lenka Pazourková, and Justyna Majewska. 2022. "Alkali-Treated Alumina and Zirconia Powders Decorated with Hydroxyapatite for Prospective Biomedical Applications" Materials 15, no. 4: 1390. https://doi.org/10.3390/ma15041390






