Porous Oxygen-Doped g-C3N4 with the Different Precursors for Excellent Photocatalytic Activities under Visible Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Preparation
2.3. Characterization
2.4. Photocatalytic Degradation of BPA
3. Result and Discussion
3.1. Photocatalytic Performances Test
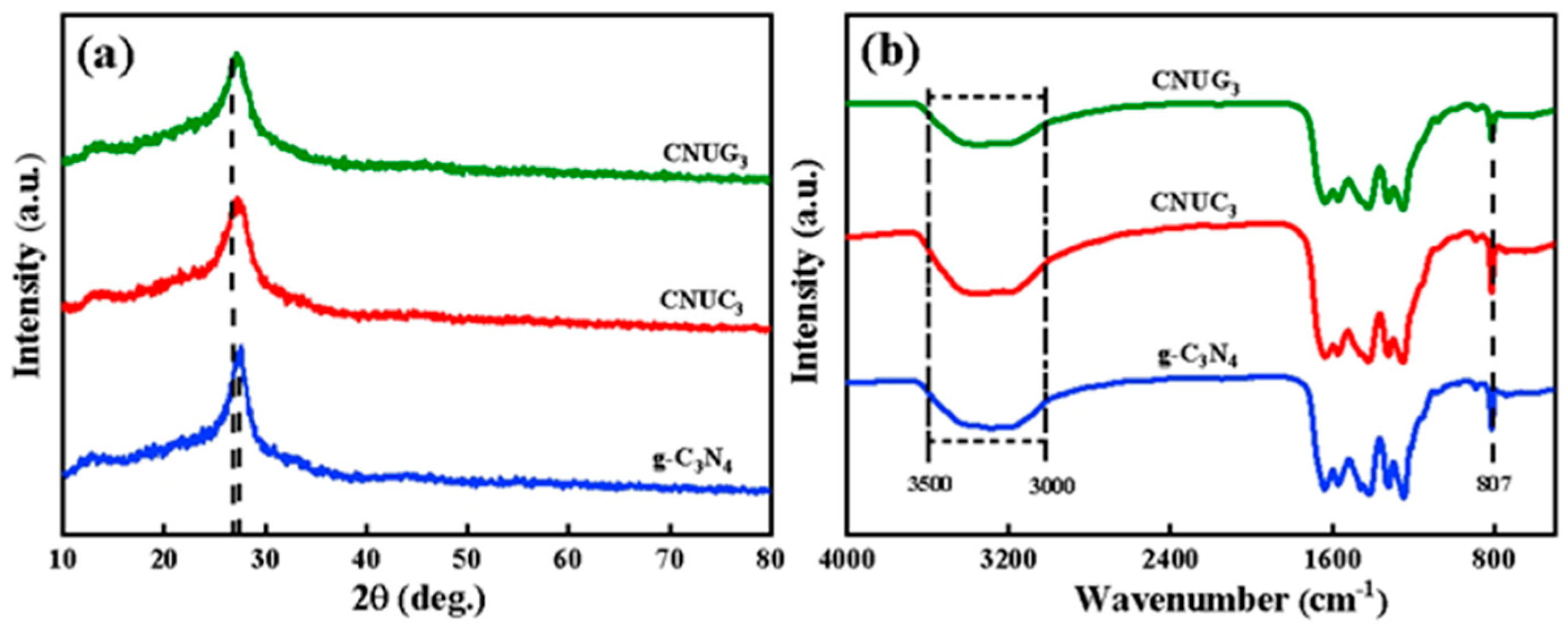
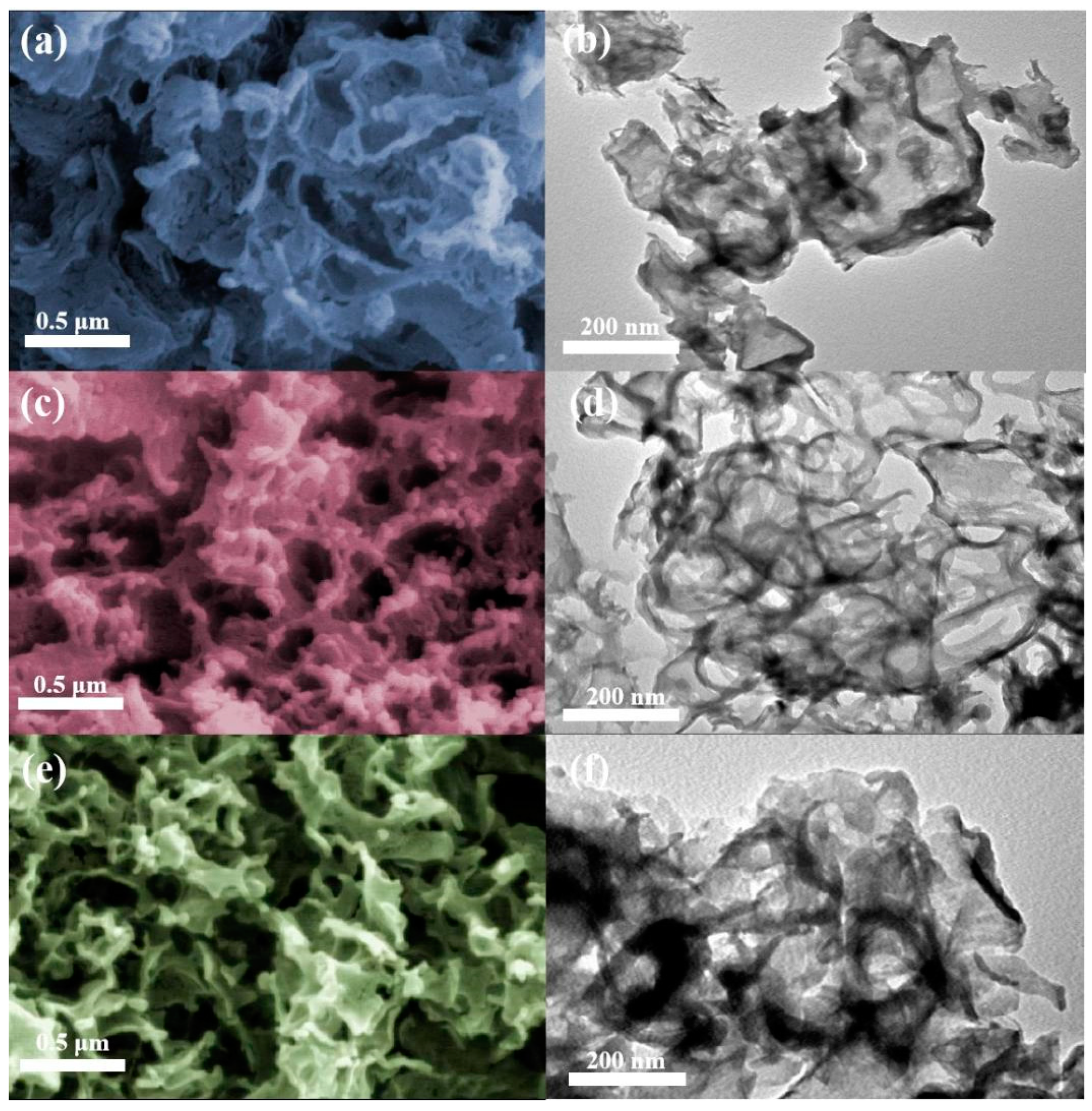
3.2. Characterization of Photocatalysts
3.2.1. Catalysis Structure and Morphology Analysis
3.2.2. Optical and Electrochemical Properties
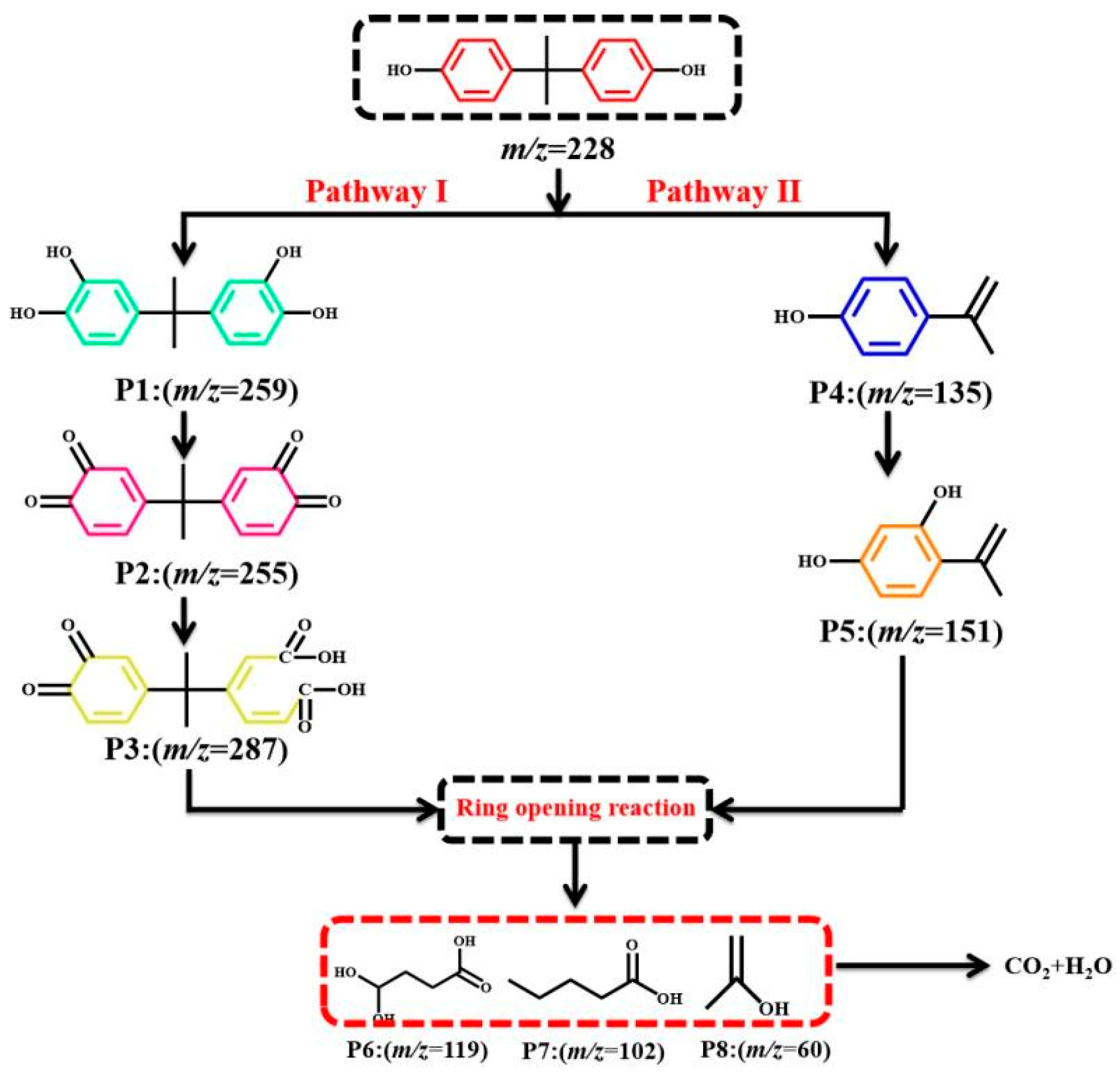
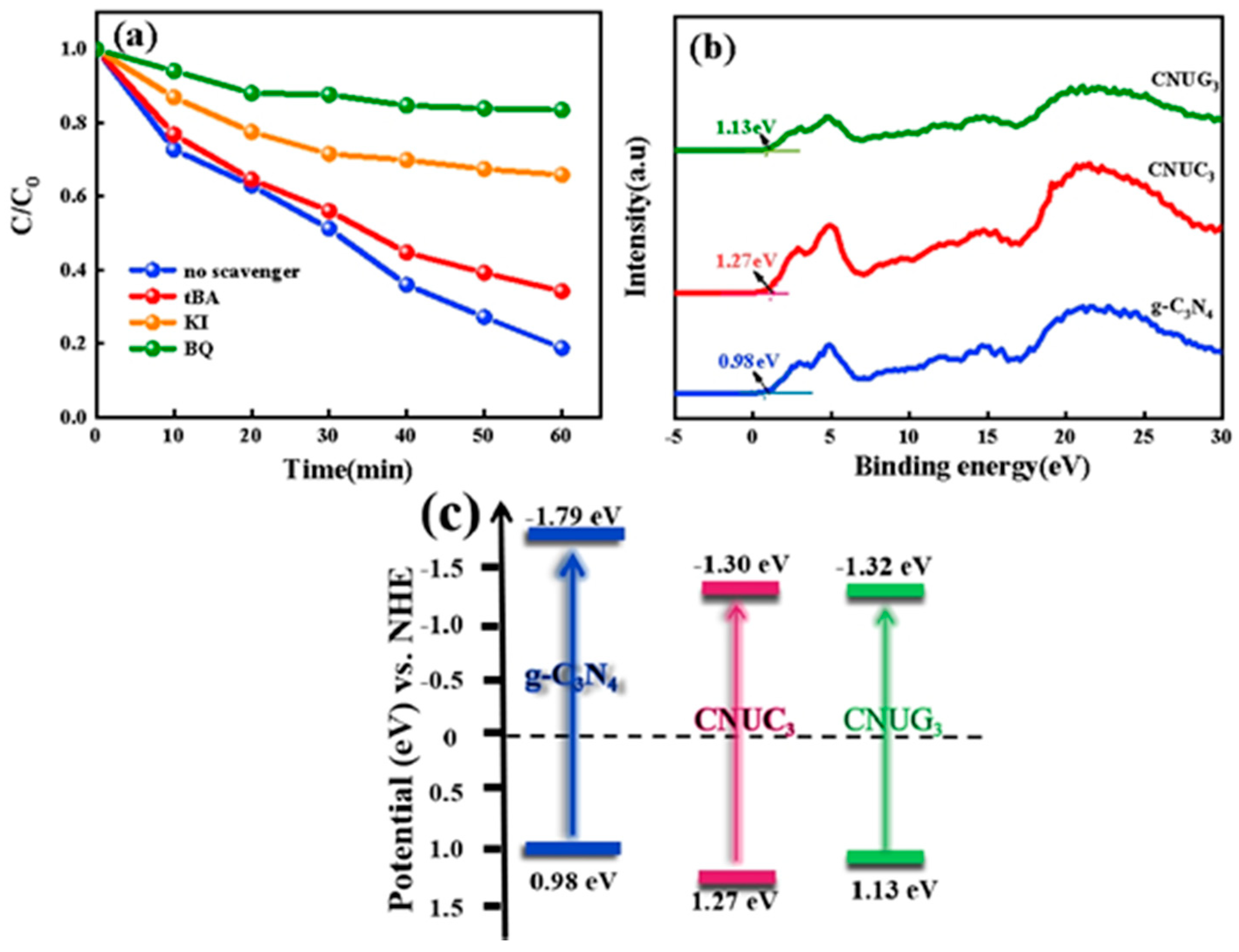
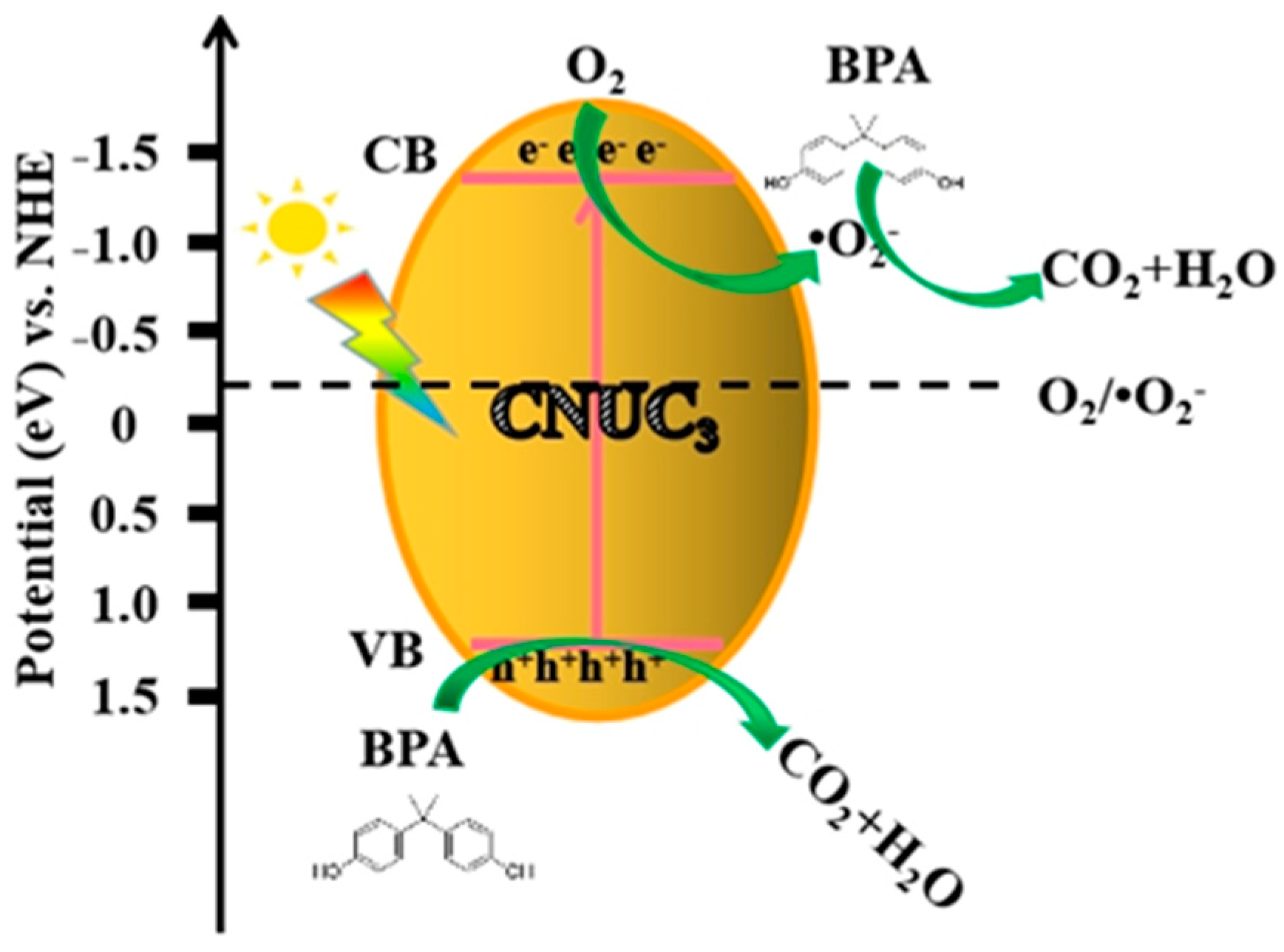
3.2.3. Mechanisms of Enhanced Photocatalytic Performance
4. Conclusions
- (1)
- Under visible light irradiation, the optimum photocatalytic efficiency of CNUC3 and CNUG3 nanosheets was enhanced by nearly seven and five times, respectively, more than that of g-C3N4 for BPA degradation in 60 min. CNUC3 was found to present high stability and excellent recycling in the photocatalytic degradation processes.
- (2)
- Free radical capture experiments have confirmed that h+ and •O2− were the main active species that degrade BPA, and •O2− could reduce BPA to produce CO2 and H2O. Meanwhile, h+ had strong oxidizing ability and could directly mineralize BPA into small molecular substances.
- (3)
- Therefore, this work may open a new route to fabricate nonmetal-modified g-C3N4-based catalysts with high degradation efficiency under visible light irradiation. Moreover, it may provide new inspiration for improving the activities of photocatalysts.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Q.; Yang, H.; Yang, M.; Yu, Y.; Yan, M.; Zhou, L.; Liu, X.; Xiao, S.; Yan, Y.; Wang, Y.; et al. Toxic effects of bisphenol A on goldfish gonad development and the possible pathway of BPA disturbance in female and male fish reproduction. Chemosphere 2019, 221, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.Q.; Zhang, W.H.; Ding, W.C.; Zhang, T.T.; Zhang, Y.; Chi, N.P.; Wang, Q. Fabrication of electrochemically-modified BiVO4-MoS2-Co3O4 composite film for bisphenol A degradation. J. Environ. Sci. 2021, 102, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Meng, D.D.; Liang, Y.; Wang, Z.; Zhang, C.; Wang, S.B.; Zhang, Z.H. Cation deficiency tuned LaCoO3-δ perovskite for peroxymonosulfate activation towards bisphenol A degradation. Chem. Eng. J. 2020, 409, 128196. [Google Scholar] [CrossRef]
- Han, Q.; Wang, H.J.; Dong, W.Y.; Liu, T.Z.; Yin, Y.L.; Fan, H.K. Degradation of bisphenol A by ferrate (VI) oxidation: Kinetics, products and toxicity assessment. Chem. Eng. J. 2015, 262, 34–40. [Google Scholar] [CrossRef]
- Zhao, J.G.; Chen, X.R.; Liu, F.K.; Yang, N.; Huang, H.; Zhao, J. Mechanism of toxicity formation and spatial distribution in activated sludge treating synthetic effluent containing bisphenol A (BPA). Chem. Eng. J. 2014, 250, 91–98. [Google Scholar] [CrossRef]
- Guo, W.L.; Hu, W.; Pan, J.M.; Zhou, H.C.; Guan, W.; Wang, X.; Dai, J.D.; Xu, L.C. Selective adsorption and separation of BPA from aqueous solution using novel molecularly imprinted polymers based on kaolinite/Fe3O4 composites. Chem. Eng. J. 2011, 171, 603–611. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhu, G.Q.; Hojamberdiev, M.; Gao, J.Z.; Zhu, R.L.; Wang, C.H.; Wei, X.M.; Liu, P. Three-dimensional Ag2O/Bi5O7I p–n heterojunction photocatalyst harnessing UV–vis–NIR broad spectrum for photodegradation of organic pollutants. J. Hazard. Mater. 2018, 344, 42–54. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Al-Shahrani, T.; Al-hokbany, N.; Alshehri, S.M. Preparation of chitosan based magnetic nanocomposite for tetracycline adsorption: Kinetic and thermodynamic studie. Int. J. Biol. Macromol. 2020, 147, 258–267. [Google Scholar] [CrossRef]
- Wei, M.Y.; Gao, L.; Li, J.; Fang, J.; Cai, W.X.; Li, X.X.; Xu, A.H. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation. J. Hazard. Mater. 2016, 316, 60–68. [Google Scholar] [CrossRef]
- Wang, X.C.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Li, F.; Zhang, D.N.; Liao, Y.L.; Zhou, H.P. Plasma-based surface modification of g-C3N4 nanosheets for highly efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 495, 143520. [Google Scholar] [CrossRef]
- Tian, L.; Li, J.Y.; Liang, F.; Wang, J.K.; Li, S.S.; Zhang, H.J.; Zhang, S.W. Molten salt synthesis of tetragonal carbon nitride hollow tubes and their application for removal of pollutants from wastewater. Appl. Catal. B Environ. 2018, 225, 307–313. [Google Scholar] [CrossRef]
- Yu, W.W.; Zhang, T.; Zhao, Z.K. Garland-like intercalated carbon nitride prepared by an oxalic acid-mediated assembly strategy for highly-efficient visible-light-driven photoredox catalysis. Appl. Catal. B Environ. 2020, 278, 119342. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, C.; Huang, D.L.; Zeng, G.M.; Huang, J.H.; Lai, C.; Zhou, C.Y.; Wang, W.J.; Guo, H.; Xue, W.J. Boron nitride quantum dots decorated ultrathin porous g-C3N4: Intensified exciton dissociation and charge transfer for promoting visible-light-driven molecular oxygen activation. Appl. Catal. B Environ. 2018, 245, 87–99. [Google Scholar] [CrossRef]
- Bfaqeer, A.; Tahir, M.; Amin, N.A.S. Well-designed ZnV2O6/g-C3N4 2D/2D nanosheets heterojunction with faster charges separation via pCN as mediator towards enhanced photocatalytic reduction of CO2 to fuels. Appl. Catal. B Environ. 2019, 242, 312–326. [Google Scholar] [CrossRef]
- Li, K.; Xie, X.; Zhang, W.D. Porous Graphitic carbon nitride derived from melamine–ammonium oxalate stacking sheets with excellent photocatalytic hydrogen evolution activity. ChemCatChem 2016, 8, 2128–2135. [Google Scholar] [CrossRef]
- Jiang, L.B.; Yuan, X.Z.; Zeng, G.M.; Liang, J.; Wu, Z.B.; Yu, H.B.; Mo, D.; Wang, H.; Xiao, Z.H.; Zhou, C.Y. Nitrogen self-doped g-C3N4 nanosheets with tunable band structures for enhanced photocatalytic tetracycline degradation. J. Colloid Interface Sci. 2019, 536, 17–29. [Google Scholar] [CrossRef]
- Chen, H.; Yao, J.H.; Qiu, P.X.; Xu, C.M.; Jiang, F.; Wang, X. Facile surfactant assistant synthesis of porous oxygen-doped graphitic carbon nitride nanosheets with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2017, 91, 42–48. [Google Scholar] [CrossRef]
- Zhu, D.D.; Zhou, Q.X. Nitrogen doped g-C3N4 with the extremely narrow band gap for excellent photocatalytic activities under visible light. Appl. Catal. B Environ. 2020, 281, 119474. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Sulfur-Doped Mesoporous carbon nitride decorated with Cu particles for efficient photocatalytic degradation under visible-light irradiation. J. Phys. Chem. C 2017, 121, 19239–19253. [Google Scholar] [CrossRef]
- Tong, Z.W.; Yang, D.; Li, Z.; Nan, Y.H.; Ding, F.; Shen, Y.C.; Jiang, Z.Y. Thylakoid-Inspired multishell g-C3N4 nanocapsules with Enhanced visible-light harvesting and electron transfer properties for high-efficiency photocatalysis. Acs Nano 2017, 11, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Zheng, Y.J.; Zheng, H.S. A 2D/3D g-C3N4/BiOI heterostructure nano-sphere with oxygen-doped for enhanced visible light−driven photocatalytic activity in environmental remediation. J. Alloys Compd. 2021, 897, 163044. [Google Scholar] [CrossRef]
- Li, J.H.; Shen, B.A.; Hong, Z.H.; Lin, B.Z.; Gao, B.F.; Chen, Y.L. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity. Chem. Comm. 2012, 48, 12017–12019. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.L.; Zhu, B.C.; Cheng, B.; Yu, J.G.; Zhou, M.H.; Ho, W.K. Photocatalytic H2 evolution on graphdiyne/g-C3N4 hybrid nanocomposites. Appl. Catal. B Environ. 2019, 255, 117770. [Google Scholar] [CrossRef]
- Wang, L.J.; Zhou, G.; Tian, Y.; Yan, L.K.; Deng, M.X.; Yang, B.; Kang, Z.H.; Sun, H.Z. Hydroxyl decorated g-C3N4 nanoparticles with narrowed bandgap for high efficient photocatalyst design. Appl. Catal. B Environ. 2019, 244, 262–271. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, J.J.; Yang, W.D.; Sun, X.Y.; Wang, R.J.; Jia, X. A step-by-step synergistic stripping approach toward ultra-thin porous g-C3N4 nanosheets with high conduction band position for photocatalystic CO2 reduction. J. Colloid Interface Sci. 2021, 589, 179–186. [Google Scholar] [CrossRef]
- Uddin, A.; Muhmood, T.; Guo, Z.C.; Gu, J.Y.; Chen, H.; Jiang, F. Hydrothermal synthesis of 3D/2D heterojunctions of ZnIn2S4/oxygen doped g-C3N4 nanosheet for visible light driven photocatalysis of 2,4-dichlorophenoxyacetic acid degradation. J. Alloys Compd. 2020, 845, 156206. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Liu, J.H.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Li, G.S.; Shi, J.L.; Zhang, G.; Fang, Y.X.; Anpo, M.; Wang, X.C. The facile synthesis of graphitic carbon nitride from amino acid and urea for photocatalytic H2 production. Res. Chem. Intermed. 2017, 43, 5137–5152. [Google Scholar] [CrossRef]
- Jiang, Z.F.; Qian, K.; Zhu, C.Z.; Sun, H.L.; Wan, W.M.; Xie, J.M.; Li, H.M.; Wong, P.K.; Yuan, S.Q. Carbon nitride coupled with CdS-TiO2 nanodots as 2D/0D ternary composite with enhanced photocatalytic H2 evolution: A novel efficient three-level electron transfer process. Appl. Catal. B Environ. 2017, 210, 194–204. [Google Scholar] [CrossRef]
- Deng, Y.C.; Tang, L.; Zeng, G.M.; Zhu, Z.J.; Yan, M.; Zhou, Y.Y.; Wang, J.J.; Liu, Y.N. Insight into highly efficient simultaneous photocatalytic removal of Cr(VI) and 2,4-diclorophenol under visible light irradiation by phosphorus doped porous ultrathin g-C3N4 nanosheets from aqueous media: Performance and reaction mechanism. Appl. Catal. B Environ. 2017, 203, 343–354. [Google Scholar] [CrossRef]
- Li, G.X.; Li, Y.L.; Liu, H.B.; Guo, Y.B.; Li, Y.J.; Zhu, D.B. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef] [PubMed]
- She, X.J.; Wu, J.J.; Zhong, J.; Xu, H.; Yang, Y.C.; Vajtai, R.; Lou, J.; Liu, Y.; Du, D.L.; Li, H.M. Oxygenated monolayer carbon nitride for excellent photocatalytic hydrogen evolution and external quantum efficiency. Nano Energy 2016, 27, 138–146. [Google Scholar] [CrossRef]
- Oh, J.; Yoo, R.J.; Kim, S.Y.; Lee, Y.J.; Kim, D.W.; Park, S. Oxidized carbon nitrides: Water-dispersible, atomically thin carbon nitride-based nanodots and their performances as bioimaging probes. Chem. Eur. J. 2015, 21, 6241–6246. [Google Scholar] [CrossRef]
- Shi, Y.H.; Huang, J.H.; Zeng, G.M.; Cheng, W.J.; Yu, H.B.; Gu, Y.L.; Shi, L.X.; Yi, K.X. Stable, metal-free, visible-light-driven photocatalyst for efficient removal of pollutants: Mechanism of action. J. Colloid Interface Sci. 2018, 531, 433–443. [Google Scholar] [CrossRef]
- Yang, L.Q.; Huang, J.F.; Shi, L.; Cao, L.Y.; Yu, Q.; Jie, Y.N.; Fei, J.; Ouyang, H.B.; Ye, J.H. A surface modification resultant thermally oxidized porous g-C3N4 with enhanced photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 204, 335–345. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, X.; Jin, P.; Dzakpasu, M.; Wang, X.; Zhang, Q.; Zhang, L.; Yang, L.; Ding, D.; Wang, W. MOF-templated synthesis of CoFe2O4 nanocrystals and its coupling with peroxymonosulfate for degradation of bisphenol A. Chem. Eng. J. 2018, 353, 329–339. [Google Scholar] [CrossRef]
- Yu, W.; Wan, S.; Yuan, D.; Sun, L.; Wang, Y. Microwave solvothermal-assisted calcined synthesis of Bi2WxMo1−XO6 solid solution photocatalysts for degradation and detoxification of bisphenol A under simulated sunlight irradiation. Sep. Purif. Technol. 2021, 275, 119175. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, L.; Gu, P.C.; Ma, R.; Wen, T.; Zhao, X.G. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies. Appl. Catal. B Environ. 2019, 248, 1–10. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zheng, Y.; Zheng, H.; Jing, T.; Zhao, Y.; Tian, J. Porous Oxygen-Doped g-C3N4 with the Different Precursors for Excellent Photocatalytic Activities under Visible Light. Materials 2022, 15, 1391. https://doi.org/10.3390/ma15041391
Zhang J, Zheng Y, Zheng H, Jing T, Zhao Y, Tian J. Porous Oxygen-Doped g-C3N4 with the Different Precursors for Excellent Photocatalytic Activities under Visible Light. Materials. 2022; 15(4):1391. https://doi.org/10.3390/ma15041391
Chicago/Turabian StyleZhang, Jiajing, Yongjie Zheng, Heshan Zheng, Tao Jing, Yunpeng Zhao, and Jingzhi Tian. 2022. "Porous Oxygen-Doped g-C3N4 with the Different Precursors for Excellent Photocatalytic Activities under Visible Light" Materials 15, no. 4: 1391. https://doi.org/10.3390/ma15041391




