Luminescence Properties of Nano Zinc Oxide Doped with Al(III) Ions Obtained in Microwave-Assisted Hydrothermal Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Hydrothermal Synthesis of Nano Zinc Oxide Doped with Al(III) Ions
2.2. Methods
3. Results and Discussion
Measurement of Luminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R.; Credico, B.; Arienzo, D. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. [Google Scholar] [CrossRef] [Green Version]
- Perera, W.P.T.D.; Dissanayake, R.K.; Ranatunga, U.I.; Hettiarachchi, N.M.; Perera, K.D.C.; Unagolla, J.M.; De Silva, R.T.; Pahalagedara, L.R. Curcumin loaded zinc oxide nanoparticles for activity-enhanced antibacterial and anticancer applications. RSC Adv. 2020, 10, 30785–30795. [Google Scholar] [CrossRef]
- Jiang, J.; Pi, J.; Cai, J. The Advancing of Zinc Oxide Nanoparticles for Biomedical Applications. Bioinorg. Chem. Appl. 2018, 2018, 1062562. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Gun’Ko, Y.; Vallet-Regí, M. ZnO Nanostructures for Drug Delivery and Theranostic Applications. Nanomaterials 2018, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Chimupala, Y.; Phromma, C.; Yimklan, S.; Semakul, N.; Ruankham, P. Dye wastewater treatment enabled by piezo-enhanced photocatalysis of single-component ZnO nanoparticles. RSC Adv. 2020, 10, 28567–28575. [Google Scholar] [CrossRef]
- Lau, G.E.; Abdullah, C.A.C.; Ahmad, W.A.N.W.; Assaw, S.; Zheng, A.L.T. Eco-Friendly Photocatalysts for Degradation of Dyes. Catalysts 2020, 10, 1129. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Betke, U.; Scheffler, M. Reticulated Open-Celled Zinc Oxide Ceramic Foams: Manufacturing, Microstructure, Mechanical, and Thermal Properties. Adv. Mater. Sci. Eng. 2019, 2019, 6570180. [Google Scholar] [CrossRef] [Green Version]
- Moradpoor, H.; Safaei, M.; Mozaffari, H.R.; Sharifi, R.; Imani, M.M.; Golshah, A.; Bashardoust, N. An overview of recent progress in dental applications of zinc oxide nanoparticles. RSC Adv. 2021, 11, 21189–21206. [Google Scholar] [CrossRef]
- Chebor, L.J. Characterization of Synthesized ZnO Nanoparticles and their Application in Photodegradation of Methyl Orange Dye Under Fluorescent Lamp Irradiation. Int. J. Sci. Eng. Sci. 2018, 2, 2456–7361. [Google Scholar]
- Hembram, K.; Rao, T.; Srinivasa, R.; Kulkarni, A. High performance varistors prepared from doped ZnO nanopowders made by pilot-scale flame spray pyrolyzer: Sintering, microstructure and properties. J. Eur. Ceram. Soc. 2015, 35, 3535–3544. [Google Scholar] [CrossRef]
- Xie, P.; Hu, J. Influence of Sintering Temperature and ZrO2 Dopants on the Microstructure and Electrical Properties of Zinc Oxide Varistors. IEEE Access 2019, 7, 140126–140133. [Google Scholar] [CrossRef]
- Ali, M.; Tit, N.; Yamani, Z.H. Role of defects and dopants in zinc oxide nanotubes for gas sensing and energy storage applications. Int. J. Energy Res. 2020, 44, 10926–10936. [Google Scholar] [CrossRef]
- Patil, V.L.; Kumbhar, S.S.; Vanalakar, S.A.; Tarwal, N.L.; Mali, S.S.; Kim, J.H.; Patil, P.S. Gas sensing properties of 3D mesoporous nanostructured ZnO thin films. New J. Chem. 2018, 42, 13573–13580. [Google Scholar] [CrossRef]
- Zhang, C.; Xing, H.; Li, C.; Cai, R.; Lv, D. Micro-Degradation Characteristics and Mechanism of ZnO Varistors under Multi-Pulse Lightning Strike. Energies 2020, 13, 2620. [Google Scholar] [CrossRef]
- Masai, H.; Toda, T.; Ueno, T.; Takahashi, Y.; Fujiwara, T. ZnO glass-ceramics: An alternative way to produce semiconductor materials. Appl. Phys. Lett. 2009, 94, 151908. [Google Scholar] [CrossRef]
- Alexandrov, A.; Zvaigzne, M.; Lypenko, D.; Nabiev, I.; Samokhvalov, P. Al-, Ga-, Mg-, or Li-doped zinc oxide nanoparticles as electron transport layers for quantum dot light-emitting diodes. Sci. Rep. 2020, 10, 7496. [Google Scholar] [CrossRef]
- AlKahlout, A. A Comparative Study of Spin Coated Transparent Conducting Thin Films of Gallium and Aluminum Doped ZnO Nanoparticles. Phys. Res. Int. 2015, 2015, 238123. [Google Scholar] [CrossRef] [Green Version]
- Ade, R.; Kumar, S.S.; Valanarasu, S.; Sasikumar, S.; Ganesh, V.; Bitla, Y.; Algarni, H.; Yahia, I. Enhanced optoelectronic properties of Ti-doped ZnO nanorods for photodetector applications. Ceram. Int. 2021, 47, 24031–24038. [Google Scholar] [CrossRef]
- Ammaih, Y.; Lfakir, A.; Hartiti, B.; Ridah, A.; Thevenin, P.; Siadat, M. Structural, optical and electrical properties of ZnO:Al thin films for optoelectronic applications. Opt. Quantum Electron. 2013, 46, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Ragupathi, V.; Babu, M.M.; Panigrahi, P.; Subramaniam, N.G. Enhanced Electrical and Optical properties of Al doped and ZnO nanoparticles for Optoelectronic Application: Eco-friendly Green Route. J. Phys. Conf. Ser. 2020, 1495, 012040. [Google Scholar] [CrossRef]
- Arif, M.; Shkir, M.; AlFaify, S.; Ganesh, V.; Sanger, A.; Algarni, H.; Vilarinho, P.M.; Singh, A. A structural, morphological, linear, and nonlinear optical spectroscopic studies of nanostructured Al-doped ZnO thin films: An effect of Al concentrations. J. Mater. Res. 2019, 34, 1309–1317. [Google Scholar] [CrossRef]
- Mageswari, S.; Palanivel, B. Influence of Al, Ta Doped ZnO Seed Layer on the Structure, Morphology and Optical Properties of ZnO Nanorods. Curr. Smart Mater. 2019, 4, 45–58. [Google Scholar] [CrossRef]
- Nateq, M.H.; Ceccato, R. Enhanced Sol-Gel Route to Obtain a Highly Transparent and Conductive Aluminum-Doped Zinc Oxide Thin Film. Materials 2019, 12, 1744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carofiglio, M.; Barui, S.; Cauda, V.; Laurenti, M. Doped Zinc Oxide Nanoparticles: Synthesis, Characterization and Potential Use in Nanomedicine. Appl. Sci. 2020, 10, 5194. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.U.; Deen, K.M.; Ahmad, A.; Akram, M.A.; Aslam, M.; Akhtar, W. Formation of Al-doped ZnO thin films on glass by sol–gel process and characterization. Appl. Nanosci. 2015, 6, 235–241. [Google Scholar] [CrossRef]
- Ganesh, T.; Rajesh, S.; Xavier, F.P. Sol-Gel Preparation, Deposition and Characterization of Nanostructured Aluminium Doped Zinc Oxide. J. Nano Res. 2013, 24, 96–106. [Google Scholar] [CrossRef]
- Munawaroh, H.; Wahyuningsih, S.; Ramelan, A.H. Synthesis and Characterization of Al doped ZnO (AZO) by Sol-gel Method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 12049. [Google Scholar] [CrossRef] [Green Version]
- Strachowski, T.; Grzanka, E.; Lojkowski, W.; Presz, A.; Godlewski, M.; Yatsunenko, S.; Matysiak, H.; Piticescu, R.-R.; Monty, C.J. Morphology and luminescence properties of zinc oxide nanopowders doped with aluminum ions obtained by hydrothermal and vapor condensation methods. J. Appl. Phys. 2007, 102, 073513. [Google Scholar] [CrossRef]
- Vyas, S.; Singh, S.; Chakrabarti, P.; Sumit, V.; Shaivalini, S.; Chakrabarti, P. Tailoring Energy Bandgap of Al Doped ZnO Thin Films Grown by Vacuum Thermal Evaporation Method. J. Nanosci. Nanotechnol. 2015, 15, 9636–9642. [Google Scholar] [CrossRef]
- Anandh, B.; Ganesh, A.S.; Thangarasu, R.; Sakthivel, R.; Kannusamy, R.; Tamilselvan, K. Structural, Morphological and Optical Properties of Aluminium Doped ZnO Thin Film by Dip-Coating Method. Orient. J. Chem. 2018, 34, 1619–1624. [Google Scholar] [CrossRef]
- Sahu, D.; Lin, S.-Y.; Huang, J.-L. Improved properties of Al-doped ZnO film by electron beam evaporation technique. Microelectron. J. 2007, 38, 245–250. [Google Scholar] [CrossRef]
- AlKahlout, A.; Al Dahoudi, N.; Grobelsek, I.; Jilavi, M.; De Oliveira, P.W. Synthesis and Characterization of Aluminum Doped Zinc Oxide Nanostructures via Hydrothermal Route. J. Mater. 2014, 2014, 235638. [Google Scholar] [CrossRef] [Green Version]
- Zan, L.; Wei, Q.; Xiaohong, W. Controllable hydrothermal synthesis of Al-doped ZnO with different microstructures, growth mechanisms, and gas sensing properties. RSC Adv. 2015, 5, 56325–56332. [Google Scholar] [CrossRef]
- Burunkaya, E.; Kiraz, N.; Kesmez, O.; Camurlu, H.E.; Asiltürk, M.; Arpaç, E. Preparation of aluminum-doped zinc oxide (AZO) nano particles by hydrothermal synthesis. J. Sol Gel Sci. Technol. 2010, 55, 171–176. [Google Scholar] [CrossRef]
- Piticescu, R.R.; Piticescu, R.M.; Monty, C.J. Synthesis of Al-doped ZnO nanomaterials with controlled luminescence. J. Eur. Ceram. Soc. 2006, 26, 2979–2983. [Google Scholar] [CrossRef]
- Naderi, M.; Shoushtari, M.Z.; Kazeminezhad, I.; Ahmadi, M.; Roghabadi, F.A. Hydrothermal synthesized AZO Nanorods layer as a high potential buffer layer for inverted polymer solar cell. Ceram. Int. 2018, 44, 15660–15665. [Google Scholar] [CrossRef]
- Aimable, A.; Strachowski, T.; Wolska, E.; Lojkowski, W.; Bowen, P. Comparison of two innovative precipitation systems for ZnO and Al-doped ZnO nanoparticle synthesis. Process. Appl. Ceram. 2010, 4, 107–114. [Google Scholar] [CrossRef]
- Jayathilake, D.S.Y.; Peiris, T.A.N.; Sagu, J.; Potter, D.B.; Wijayantha, K.G.U.; Carmalt, C.J.; Southee, D.J. Microwave-Assisted Synthesis and Processing of Al-Doped, Ga-Doped, and Al, Ga Codoped ZnO for the Pursuit of Optimal Conductivity for Transparent Conducting Film Fabrication. ACS Sustain. Chem. Eng. 2017, 5, 4820–4829. [Google Scholar] [CrossRef] [Green Version]
- Romeiro, F.D.C.; Marinho, J.; Silva, A.C.A.; Cano, N.; Dantas, N.; Lima, R.C. Photoluminescence and Magnetism in Mn2+-Doped ZnO Nanostructures Grown Rapidly by the Microwave Hydrothermal Method. J. Phys. Chem. C 2013, 117, 26222–26227. [Google Scholar] [CrossRef]
- Bernardo, M.; Villanueva, P.; Jardiel, T.; Calatayud, D.; Peiteado, M.; Caballero, A. Ga-doped ZnO self-assembled nanostructures obtained by microwave-assisted hydrothermal synthesis: Effect on morphology and optical properties. J. Alloy. Compd. 2017, 722, 920–927. [Google Scholar] [CrossRef]
- ERTEC Poland. Available online: http://www.ertec.pl/ (accessed on 1 January 2019).
- Lojkowski, W.; Opalinska, A.; Strachowski, T.; Presz, A.; Gierlotka, S.; Grzanka, E.; Palosz, B.; Strek, W.; Hreniak, D.; Grigorjeva, L.; et al.; et al. Microwave-Driven Hydrothermal Synthesis of Oxide Nanopowders for Applications in Optoelectronics. In The Nano-Micro Interface: Bridging the Micro and Nano Worlds; Fecht, H.-J., Werner, M., Eds.; Wiley-VCH: Weinheim, Germany, 2004; ISBN 3527309780. [Google Scholar]
- Strachowski, T.; Grzanka, E.; Palosz, B.F.; Presz, A.; Ślusarski, L.; Łojkowski, W. Microwave Driven Hydrothermal Synthesis of Zinc Oxide Nanopowders. Solid State Phenom. 2003, 94, 189–192. [Google Scholar] [CrossRef]
- Opalińska, A.; Leonelli, C.; Lojkowski, W.; Pielaszek, R.; Grzanka, E.; Chudoba, T.; Matysiak, H.; Wejrzanowski, T.; Kurzydlowski, K.J. Effect of Pressure on Synthesis of Pr-Doped Zirconia Powders Produced by Microwave-Driven Hydrothermal Reaction. J. Nanomater. 2006, 2006, 098769. [Google Scholar] [CrossRef]
- Para, T.A.; Sarkar, S.K. Challenges in Rietveld Refinement and Structure Visualization in Ceramics; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Micromeritics. Available online: https://www.micromeritics.com/ (accessed on 1 January 2022).
- He, K.; Chen, N.; Wang, C.; Wei, L.; Chen, J. Method for Determining Crystal Grain Size by X-Ray Diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Narjis, A.; El Aakib, H.; Boukendil, M.; El Hasnaoui, M.; Nkhaili, L.; Aberkouks, A.; Outzourhit, A. Controlling the structural properties of pure and aluminum doped zinc oxide nanoparticles by annealing. J. King Saud Univ. Sci. 2020, 32, 1074–1080. [Google Scholar] [CrossRef]
- Yathisha, R.O.; Nayaka, Y.A. Structural, Optical and Electrical Properties of ZnO Nanostructures Synthesized under Different Microwave Power. Russ. J. Electrochem. 2021, 57, 784–794. [Google Scholar] [CrossRef]
- Ganesh, R.S.; Navaneethan, M.; Mani, G.K.; Ponnusamy, S.; Tsuchiya, K.; Muthamizhchelvan, C.; Kawasaki, S.; Hayakawa, Y. Influence of Al doping on the structural, morphological, optical, and gas sensing properties of ZnO nanorods. J. Alloy. Compd. 2017, 698, 555–564. [Google Scholar] [CrossRef]
- Wei, S.; Lian, J.; Wu, H. Annealing effect on the photoluminescence properties of ZnO nanorod array prepared by a PLD-assistant wet chemical method. Mater. Charact. 2010, 61, 1239–1244. [Google Scholar] [CrossRef]
- Secu, C.E.; Sima, M. Photoluminescence and thermoluminescence of ZnO nano-needle arrays and films. Opt. Mater. 2009, 31, 876–880. [Google Scholar] [CrossRef]
- Naddaf, M.; Saad, M. Comparative study of structural and visible luminescence properties of AZO thin film deposited on GaAs and porous GaAs substrates. Vacuum 2015, 122, 36–42. [Google Scholar] [CrossRef]
- Hauser, M.; Hepting, A.; Hauschild, R.; Zhou, H.; Fallert, J.; Kalt, H.; Klingshirn, C. Absolute external luminescence quantum efficiency of zinc oxide. Appl. Phys. Lett. 2008, 92, 211105. [Google Scholar] [CrossRef]
- Jha, J.K.; Santos-Ortiz, R.; Sun, W.; Du, J.; Sheperd, N.D. Surface Modification of Aluminum Doped Zinc Oxide (AZO) Anodes with CFx Plasma Treatment and Nanoscale WOx Layers for Enhanced Electro-Optical Performance in OLEDs; TechConnect Briefs: Washington, DC, USA, 2016; pp. 294–297. ISBN 9780997511710. [Google Scholar]

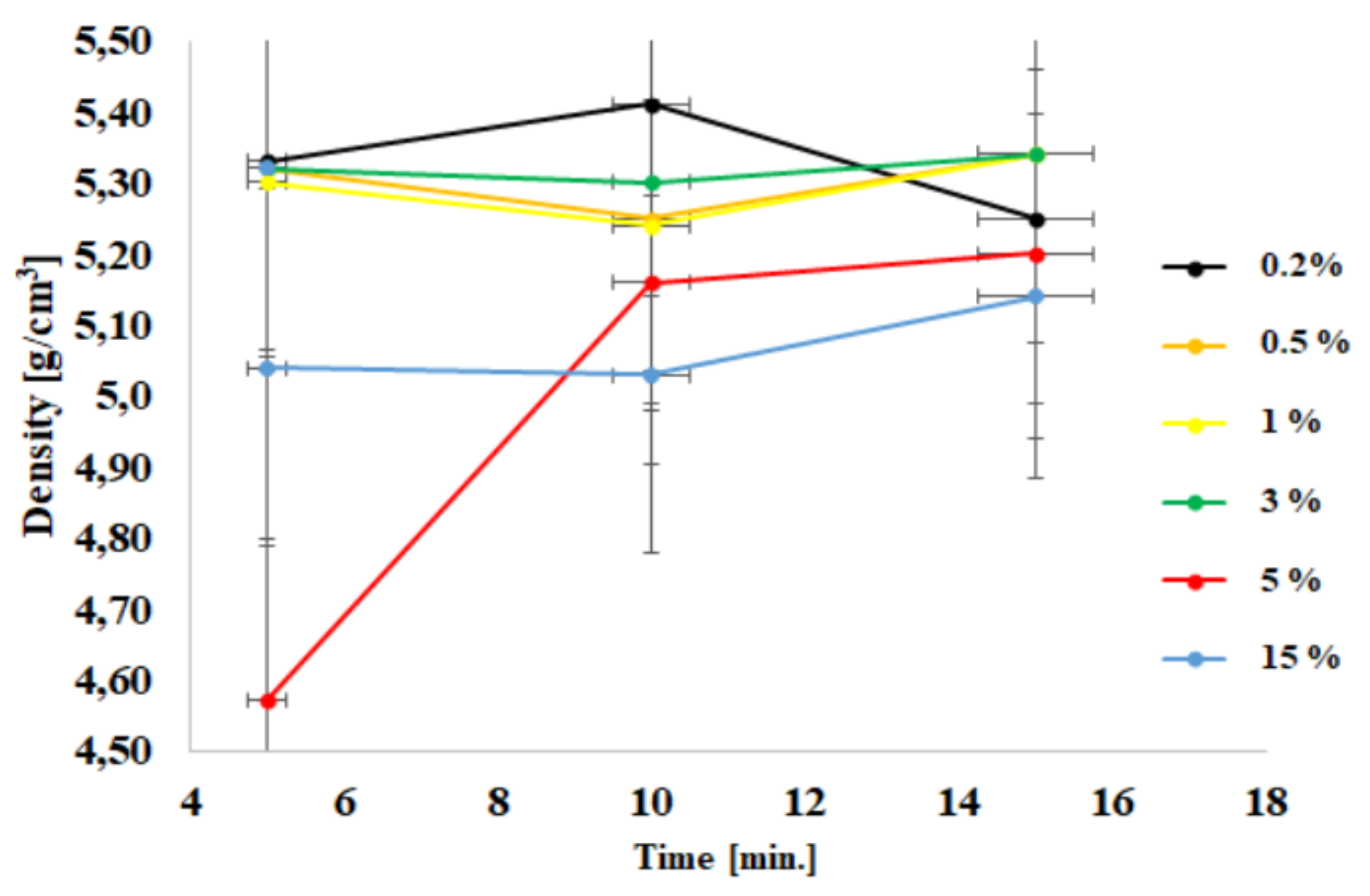
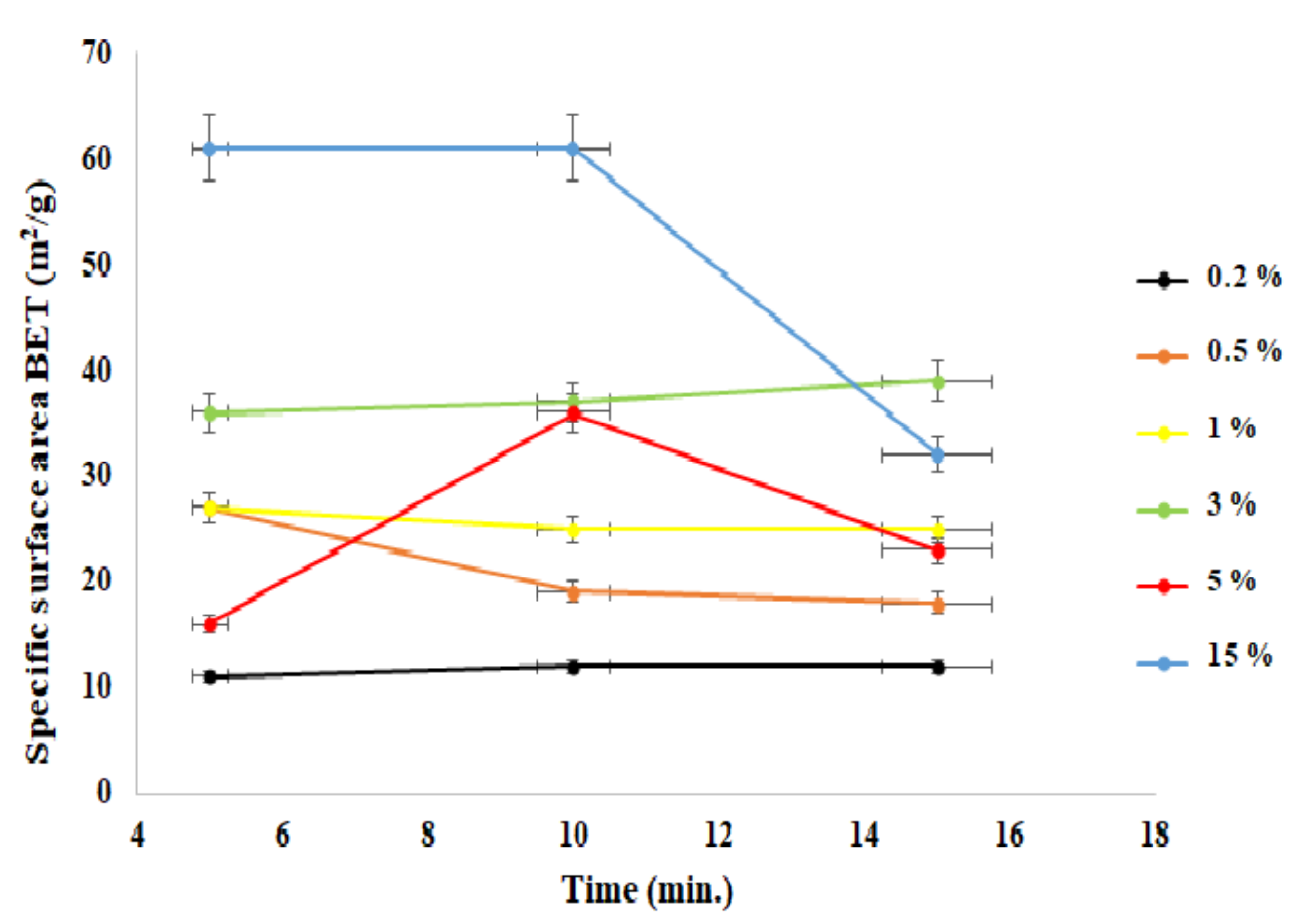
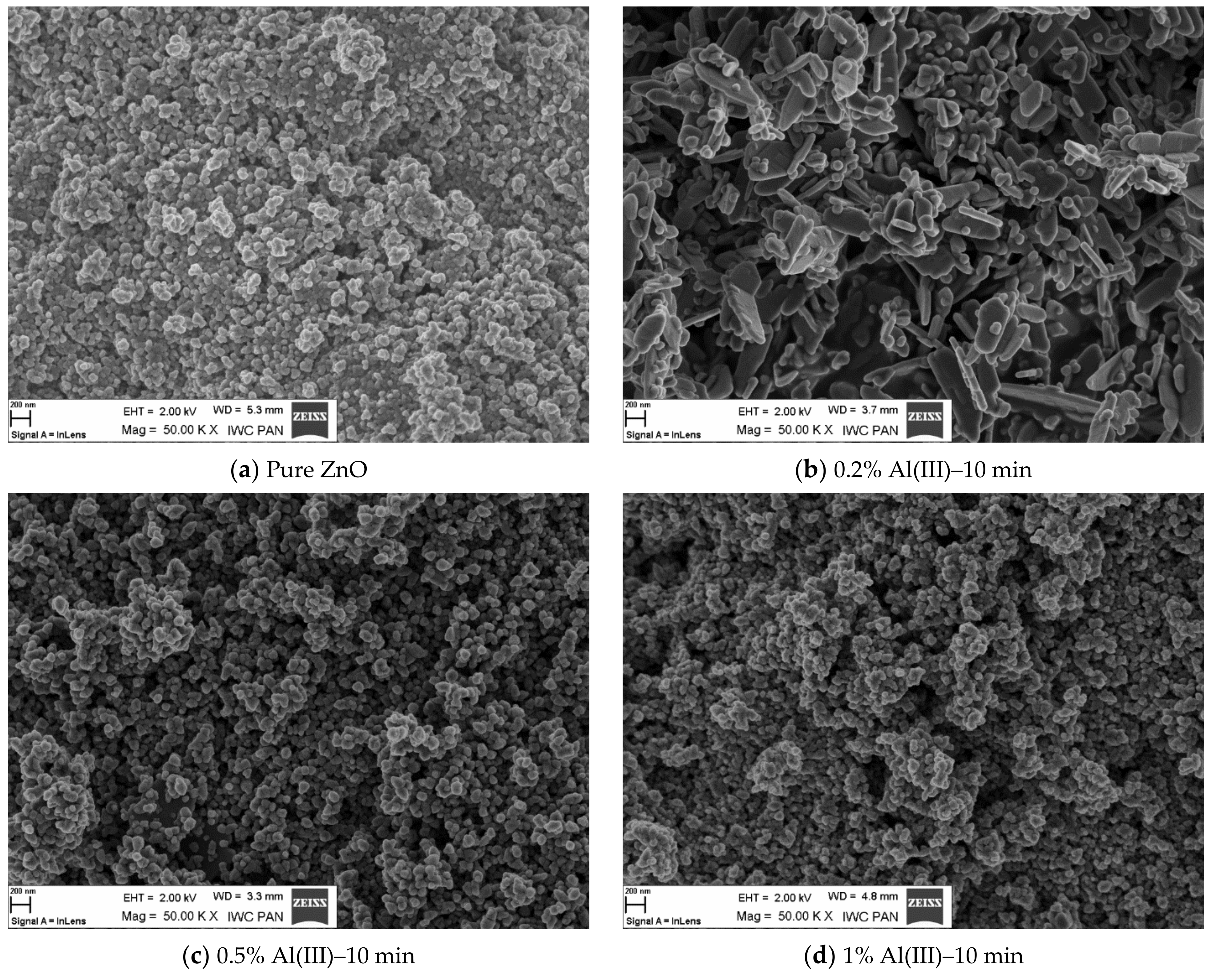

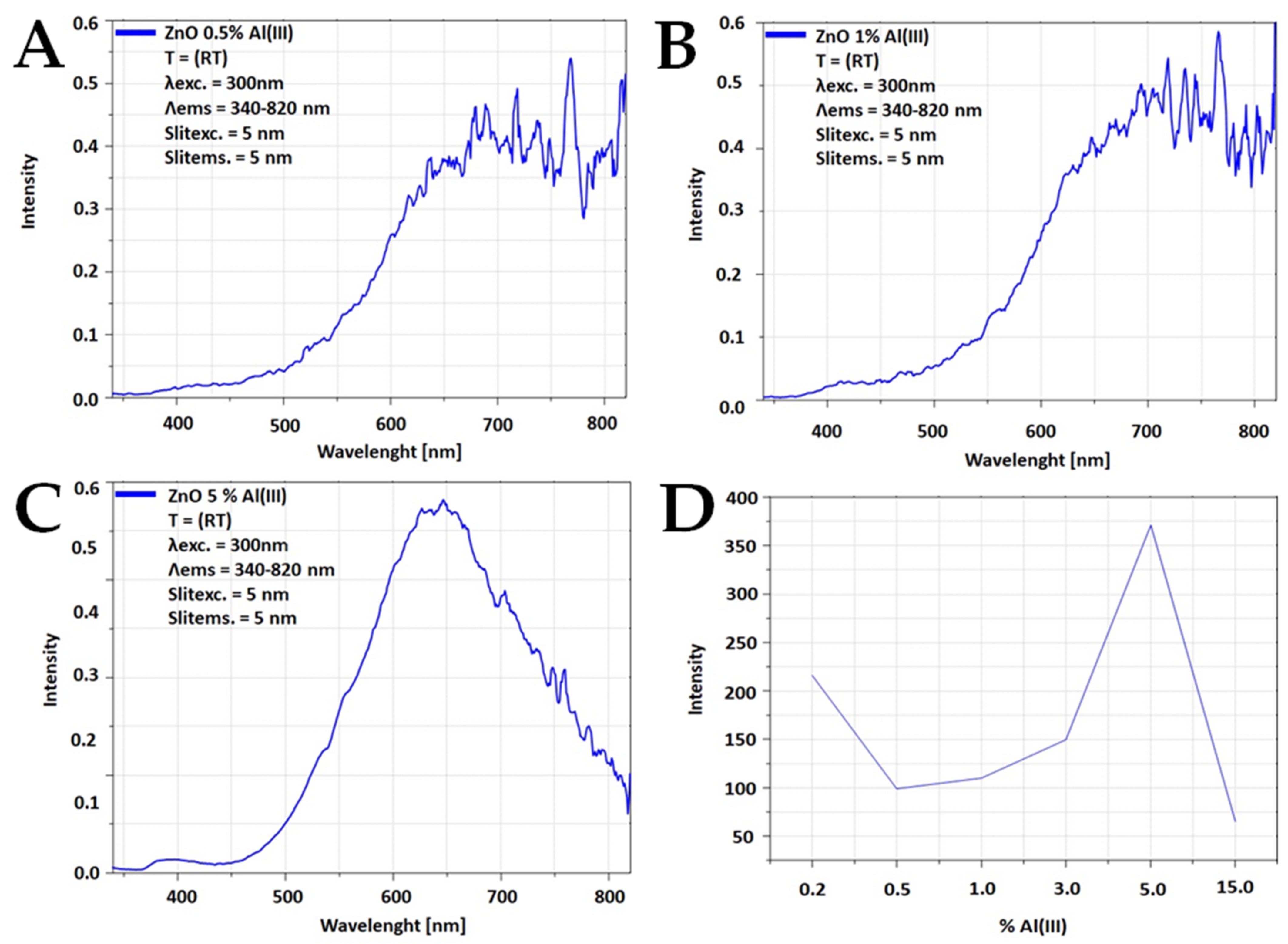
| Sample | Time [min] | Density [g/cm3] | BET [m2/g] | Average Grain size [nm] | Phase Composition | |
|---|---|---|---|---|---|---|
| 1 | ZnO + 0.2% | 5 | 5.33 ± 0.03 | 11 ± 1 | 93.67 ± 2.89 | ZnO |
| ZnO + 0.2% | 10 | 5.41 ± 0.02 | 12 ± 1 | ZnO | ||
| ZnO + 0.2% | 15 | 5.25 ± 0.02 | 12 ± 1 | ZnO | ||
| 2 | ZnO + 0.5% | 5 | 5.32 ± 0.03 | 27 ± 1 | 52.33 ± 6.89 | ZnO |
| ZnO + 0.5% | 10 | 5.25 ± 0.02 | 19 ± 2 | ZnO | ||
| ZnO + 0.5% | 15 | 5.34 ± 0.03 | 18 ± 2 | ZnO | ||
| 3 | ZnO + 1% | 5 | 5.30 ± 0.03 | 27 ± 2 | 42.33 ± 1.11 | ZnO |
| ZnO + 1% | 10 | 5.24 ± 0.02 | 25 ± 2 | ZnO | ||
| ZnO + 1% | 15 | 5.34 ± 0.03 | 25 ± 2 | ZnO | ||
| 4 | ZnO + 3% | 5 | 5.32 ± 0.03 | 36 ± 2 | 30.00 ± 0.67 | ZnO |
| ZnO + 3% | 10 | 5.30 ± 0.03 | 37 ± 2 | ZnO | ||
| ZnO + 3% | 15 | 5.34 ± 0.03 | 39 ± 2 | ZnO | ||
| 5 | ZnO + 5% | 5 | 4.57 ± 0.04 | 16 ± 2 | 53.33 ± 17.11 | ZnO |
| ZnO + 5% | 10 | 5.16 ± 0.04 | 36 ± 3 | ZnO + ZnAl2O4 | ||
| ZnO + 5% | 15 | 5.20 ± 0.04 | 23 ± 3 | ZnO + ZnAl2O4 | ||
| 6 | ZnO + 15% | 5 | 5.04 ± 0.06 | 61 ± 2 | 24.67 ± 7.56 | ZnO + ZnAl2O4 |
| ZnO + 15% | 10 | 5.03 ± 0.06 | 61 ± 3 | ZnO + ZnAl2O4 | ||
| ZnO + 15% | 15 | 5.14 ± 0.05 | 32 ± 2 | ZnO + ZnAl2O4 |
| Time (min) | Aluminum Ions Content (%mol) | Lattice Parameter a (Å) | Lattice Parameter c (Å) | |
|---|---|---|---|---|
| 0 | 5 10 15 | 0 | 3.251 ± 0.001 3.252 ± 0.002 3.251 ± 0.002 | 5.210 ± 0.001 5.211 ± 0.002 5.210 ± 0.002 |
| 1 | 5 10 15 | 0.2 | 3.252 ± 0.002 3.252 ± 0.002 3.251 ± 0.001 | 5.211 ± 0.002 5.210 ± 0.002 5.211 ± 0.001 |
| 2 | 5 10 15 | 0.5 | 3.255 ± 0.001 3.256 ± 0.002 3.256 ± 0.002 | 5.209 ± 0.001 5.208 ± 0.002 5.209 ± 0.002 |
| 3 | 5 10 15 | 1 | 3.253 ± 0.002 3.255 ± 0.002 3.257 ± 0.002 | 5.207 ± 0.002 5.206 ± 0.002 5.206 ± 0.002 |
| 4 | 5 10 15 | 3 | 3.250 ± 0.002 3.252 ± 0.002 3.257 ± 0.003 | 5.210 ± 0.002 5.211 ± 0.002 5.209 ± 0.002 |
| 5 | 5 10 15 | 5 | 3.251 ± 0.002 3.253 ± 0.001 3.251 ± 0.002 | 5.209 ± 0.002 5.209 ± 0.001 5.210 ± 0.002 |
| 6 | 5 10 15 | 15 | 3.250 ± 0.002 3.253 ± 0.002 3.251 ± 0.002 | 5.209 ± 0.002 5.209 ± 0.002 5.207 ± 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strachowski, T.; Grzanka, E.; Mizeracki, J.; Chlanda, A.; Baran, M.; Małek, M.; Onyszko, K.; Januszewski, B.; Przybysz, M. Luminescence Properties of Nano Zinc Oxide Doped with Al(III) Ions Obtained in Microwave-Assisted Hydrothermal Synthesis. Materials 2022, 15, 1403. https://doi.org/10.3390/ma15041403
Strachowski T, Grzanka E, Mizeracki J, Chlanda A, Baran M, Małek M, Onyszko K, Januszewski B, Przybysz M. Luminescence Properties of Nano Zinc Oxide Doped with Al(III) Ions Obtained in Microwave-Assisted Hydrothermal Synthesis. Materials. 2022; 15(4):1403. https://doi.org/10.3390/ma15041403
Chicago/Turabian StyleStrachowski, Tomasz, Ewa Grzanka, Jan Mizeracki, Adrian Chlanda, Magdalena Baran, Marcin Małek, Klaudia Onyszko, Bartosz Januszewski, and Mirosław Przybysz. 2022. "Luminescence Properties of Nano Zinc Oxide Doped with Al(III) Ions Obtained in Microwave-Assisted Hydrothermal Synthesis" Materials 15, no. 4: 1403. https://doi.org/10.3390/ma15041403







