Finite Boundary Conditions Due to the Bar Presence in the Model of Chloride Penetration
Abstract
:1. Introduction
- -
- General models are reviewed and commented in [22,23,24] being the models proposed of increasing complexity, as they are more detailed. The complexity appears due to the multiple variables concerned in the material, adding its evolution in time and the modifications introduced by the interaction with the cycling and evolutionary environment. All parameters together mean that the number of basic phenomena influencing each other, and of the model input parameters and constitutive laws to be fulfilled, is very high. Thus, Ref. [22] analyses several different models mentioning their “accuracy and precision” while the results are limited in time, logically because they are not validated in the long term, which makes the “precision” a non-homogeneous concept as each model has been calibrated with its own results. The work in [23] has developed a comprehensive model, but whose input parameters are very difficult to be obtained in normal experiments and also has not been calibrated beyond short term results. In [24] is presented also a model based in thermodynamic equilibria data and surface complexation which is promising, as it seems to model well the chloride binding isotherms, but again it has not been validated in real conditions in the long term. Identifying that present models have a limited amount of variables but the process is more complex, in [25] the authors extended through “Hermite polynomials non-constant multifactorial” to a more complete model, taking into account the water/cement ratio, chloride binding, temperature, age, deformation, damage, and humidity. A similar study was made through another numerical tool [26], through a reactive–transport model.
- -
- With respect to the input parameters as is the chloride surface concentration, in some cases it is found that the more superficial zone of the concrete performs differently to the bulk. This is named as concrete “skin” with a different diffusion coefficient [2,18,27]. This superficial (skin) zone having different diffusion properties than the bulk, in [18] is named the “convection” zone, in spite of convection is not the only mechanism responsible for the effect, because also the concrete composition is different in that zone near the surface, due to the “wall effect” of the mould, which generally induces a higher amount of mortar and paste than of large aggregates in the that “skin” zone. The mechanism supporting the development of such a skin zone has been studied from different assumptions. Thus:
- ○
- In [28] by introducing an empirical manner to take into account that the chloride profile may have the maximum chloride concentration in the interior of the concrete and not on its surface. This work, however, does not give a model on how this maximum evolves in time, which is a central question for long term prediction. On this aspect of the maximum of chloride concentration [29], studies the effect of rainfalls in the chloride concentration at the concrete surface, concluding that the concentration decreases with time (instead of increasing); however, other simultaneous effects are not included in the study. In [30] is considered the convection–diffusion transport in the external parts of the concrete under reverse water pressure and makes a model through FEM, while [31] studies the effect of capillary action on the chloride transport in that zone. Another perspective is taken to explain the maximum by [32,33,34] because they consider the action of the carbonation as a promoter of the decrease in the chloride binding capacity and then, on changing the shape of the chloride profile.
- -
- With respect to the bound and free chlorides, in [35] is studied several cases of chloride isotherms, resulting that a solid solution is found between the Friedel’s salt and hemicarboaluminate, together with the known fact that chlorides are adsorbed on the calcium silicate gel, pointing out that the method of measurement of the free and bound chlorides are a key aspect for the correct quantification. Authors in [36] also studied the effect of the testing condition defining that the isotherms have been studied in a diluted solution which affects the equilibrium of free/bound chlorides and also modifies the pH of the solution. Moreover, Ref. [37] studied the chloride adsorption on the C-S-H, but attributing to the electrostatic charges the binding ability, which changes with the nature of the cation. However, Ref. [38] emphasizes on the subject the effect on the adsorption of aluminium’s incorporation into C-S-H gel. The cation effect is as well addressed by [39] and they found that more chlorides were bound when the associated cation was Mg2+ or Ca2+ compared to Na+. Additionally, Ref. [40] addressed the effect of the cation but with the simultaneous change in pH of simulated pore solutions. In this subject, Ref. [41] investigated the effect of the temperature on Friedel’s salt decomposition.
- -
- Finally, with respect to the chloride threshold, several reviews were made collecting data of the literature [17,42,43,44,45,46]; however, due to the several influences acting simultaneously, until present, the best consists in the prediction of the chloride developing corrosion through a statistical distribution [14,16].
- -
- x is the distance from the concrete surface (cm),
- -
- y is the time (s),
- -
- J(x) is the chloride flow (g/cm2·s),
- -
- ∂C(x)/∂t is the variation of the chloride concentration at a precise distance from the surface (g/cm3),
- -
- Dap is the diffusion coefficient (cm2/sg),
- -
- ∂2C/∂x2 is the derivative or variation of the concentration with the distance (g/cm3).
- -
- x is the distance reached by the chloride concentration defined as Cx (cm). If a “skin” superficial zone is considered, the term “x” is replaced by (x − Δx) [18] being Δx the thickness of that skin layer,
- -
- Cx is the concentration of chlorides at a distance in a time. When Cx reaches the threshold value, the steel depassivates (% by cement weight),
- -
- Cs is the concrete surface concentration of chlorides in the first mm of the concrete surface (% by cement weight),
- -
- t is the time (s).
2. Experimental
- -
- GMSH: an automatic 3D finite element grid generator post-processor.
- -
- OOFEM—free finite element code from Czech Technical University, Prague, Czech Republic, that was modified with the author’s authorisation.
- -
- bar diameter,
- -
- cover depth,
- -
- value of the diffusion coefficient,
- -
- service life duration.
3. Results
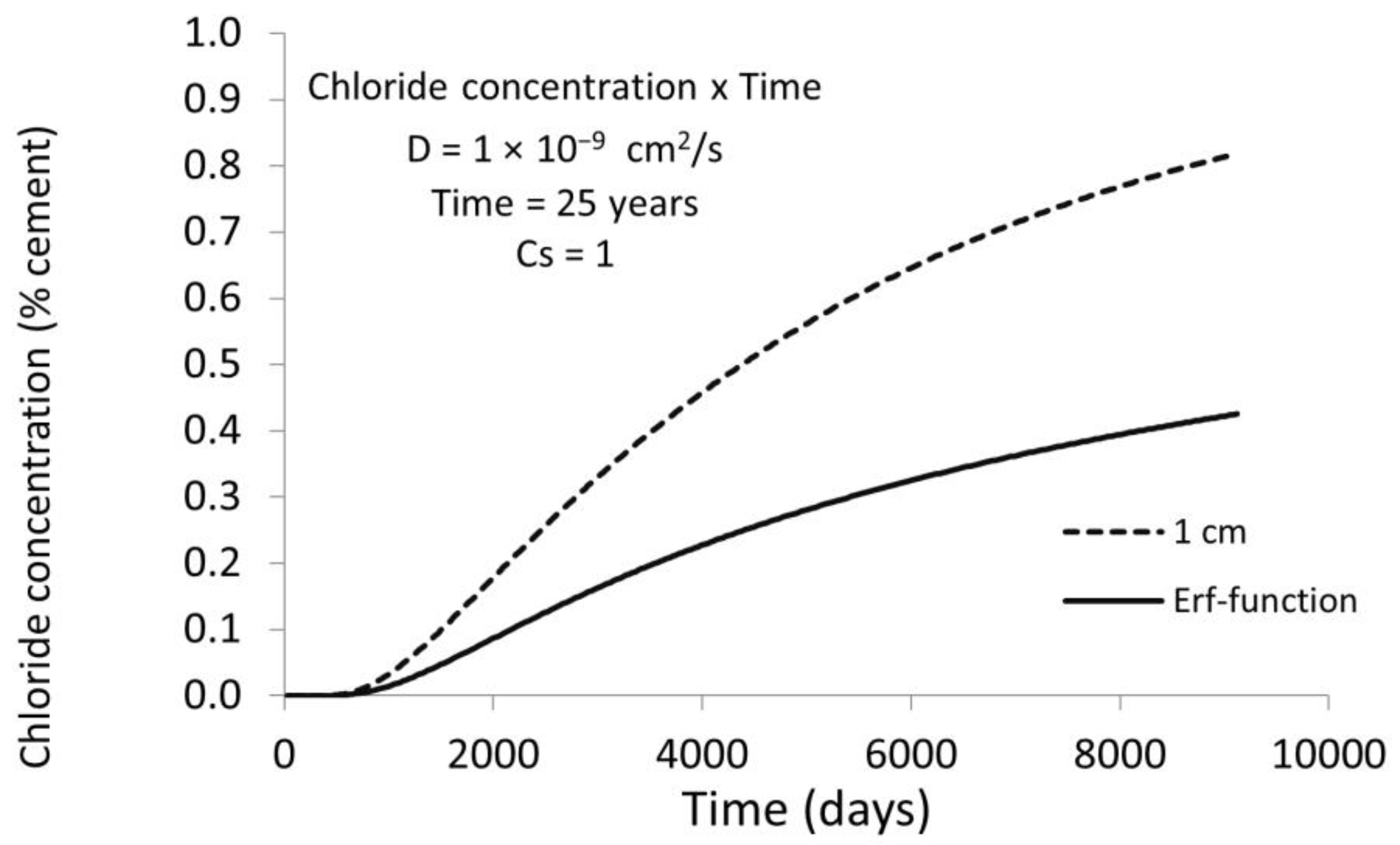
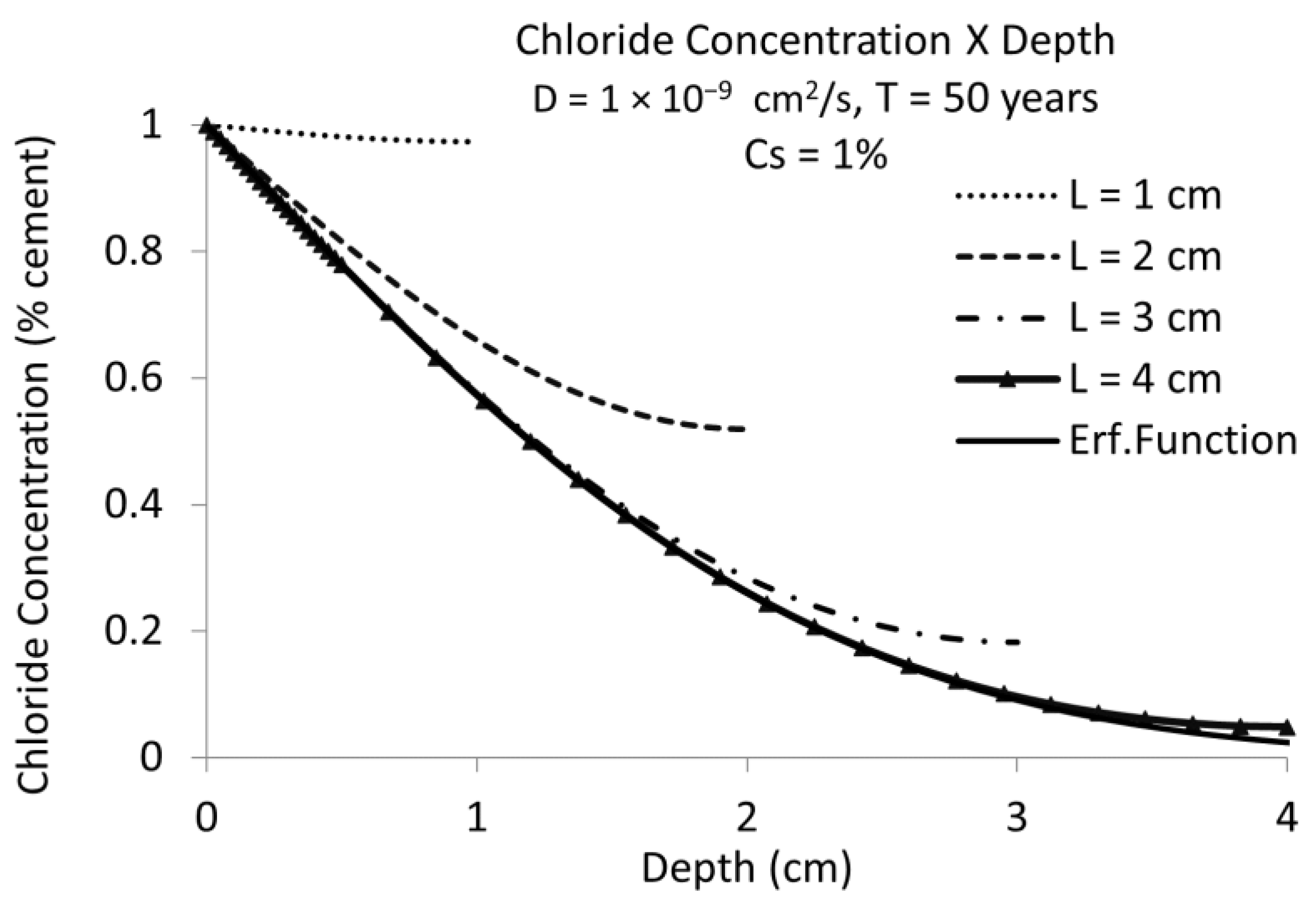
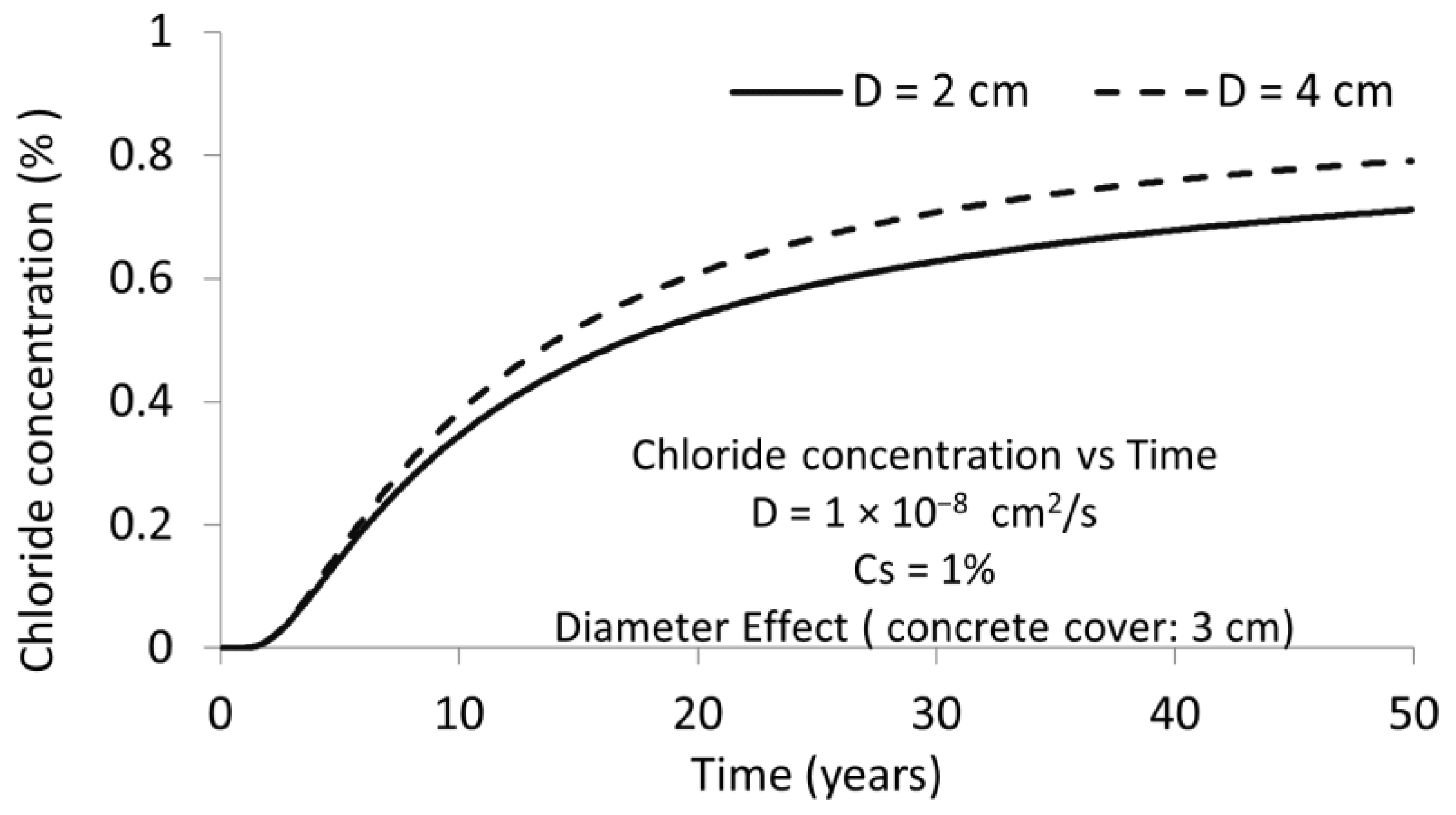
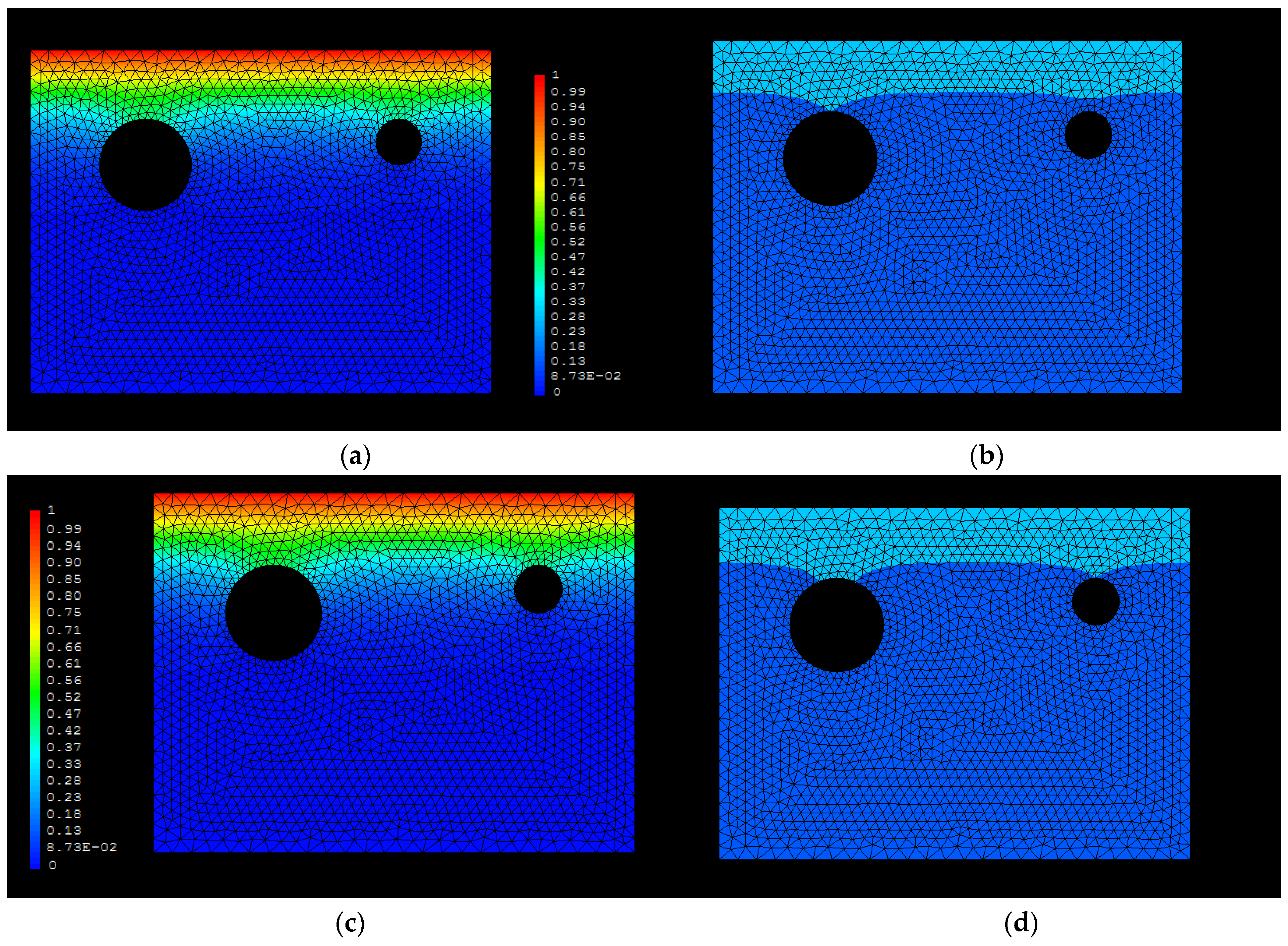
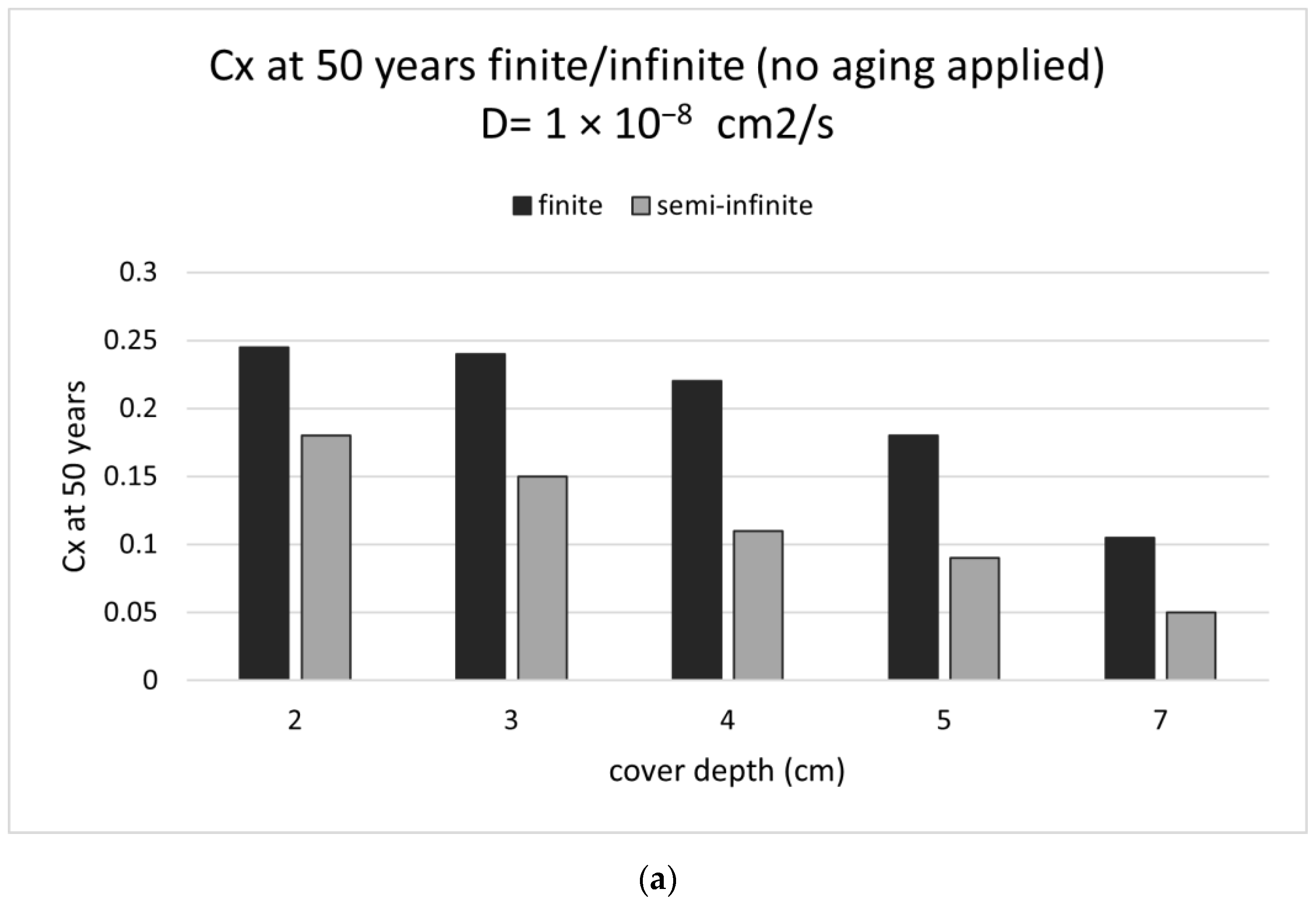
3.1. D Representations
3.2. D Representations
- -
- D (t) is the diffusion coefficient when it decreases with time (cm2/s),
- -
- D0 is the Diffusion coefficient at the testing time t0 (cm2/s),
- -
- t0 is the time at testing the chloride diffusion (s),
- -
- ti is the time for the service life (s),
- -
- m is the aging factor (-).
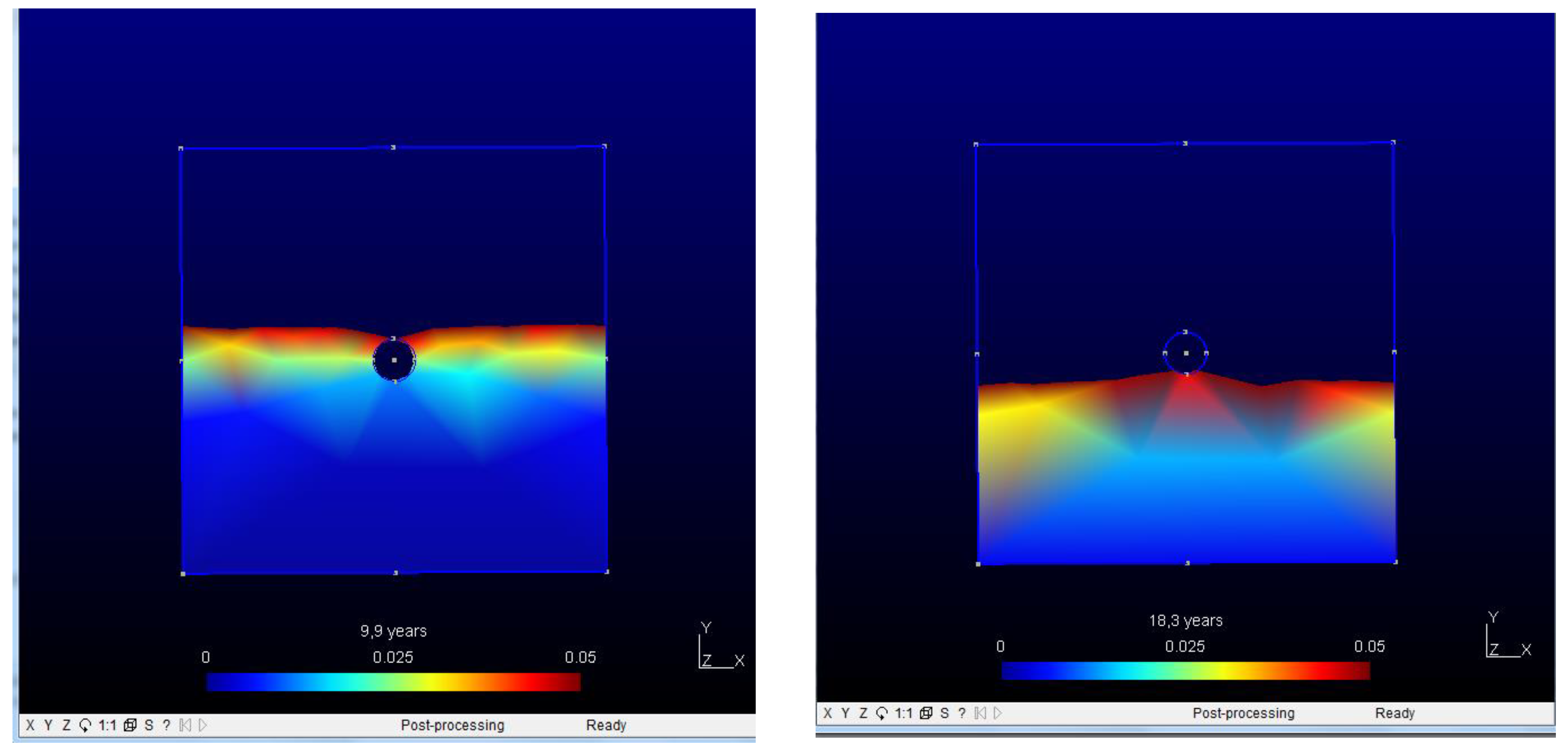
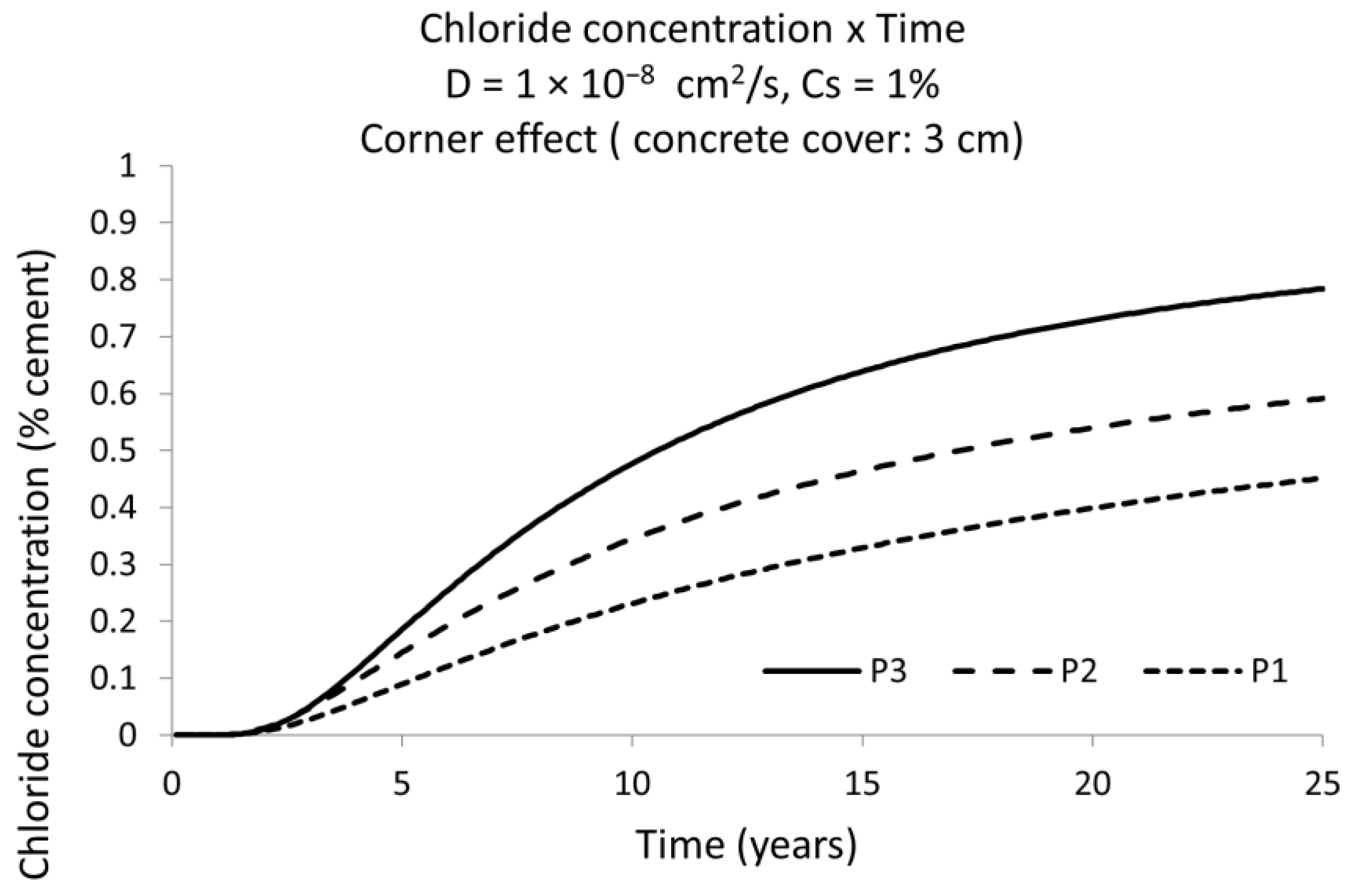
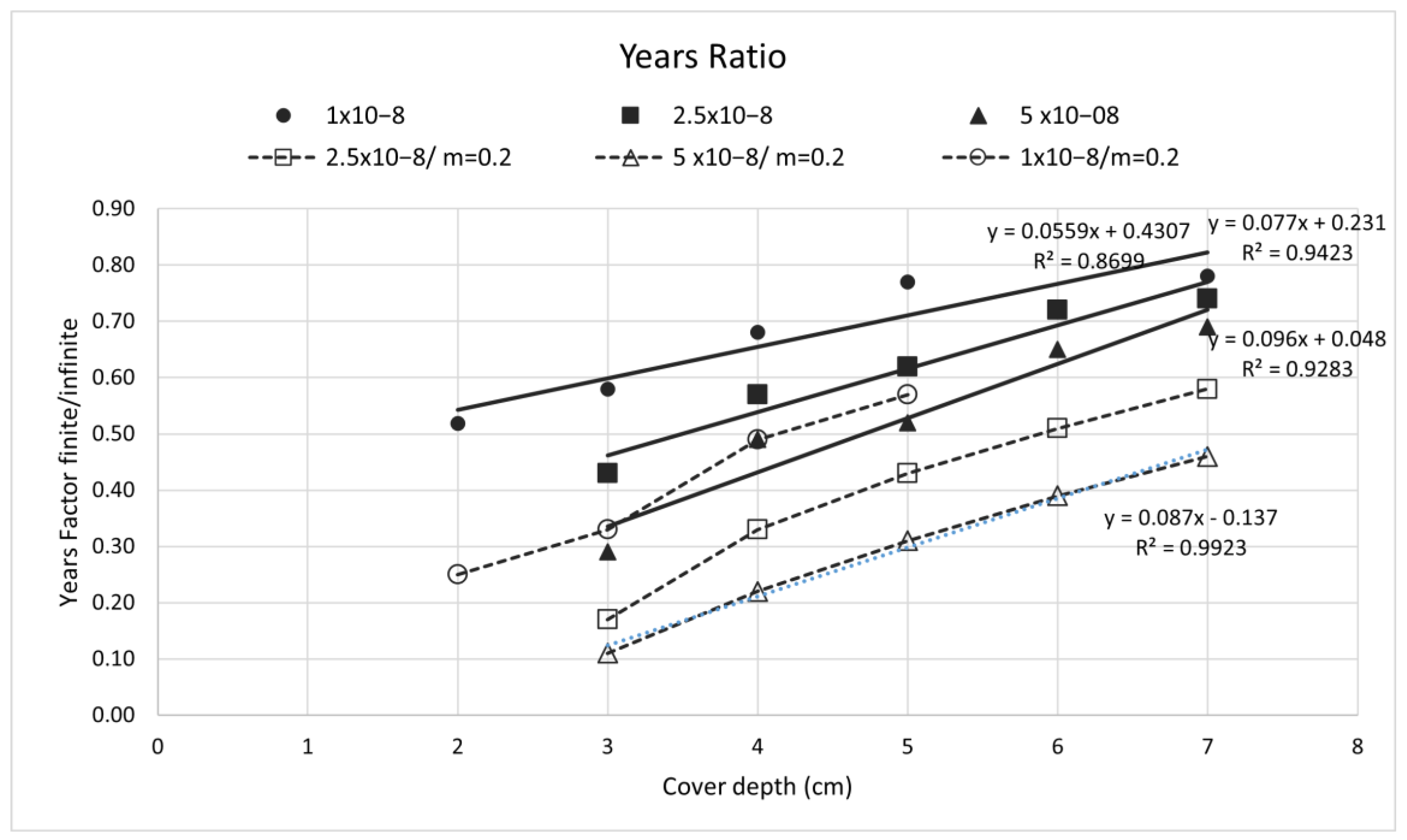
4. Discussion
5. Conclusions
- -
- if the cover is thinner,
- -
- if the diffusion coefficient is higher,
- -
- if the service life is longer,
- -
- and if the bar is thicker.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Page, C.L.; Treadaway, K.W.J. Aspects of the electrochemistry of steel in concrete. Nature 1982, 297, 109–115. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Institute (CBI): Stockholm, Sweden, 1982; pp. 4–82. [Google Scholar]
- Hausmann, D.A. A probability model for steel corrosion in concrete. Mater. Perform. 1996, 37, 64–68. [Google Scholar]
- Atkinson, A.; Nickerson, A.K. The diffusion of ions through water-saturated cement. J. Mater. Sci. 1984, 19, 3068–3078. [Google Scholar] [CrossRef]
- Bamforth, P.; Chapman-Andrews, J. Long term performance of RC elements under UK coastal exposure conditions. In Proceedings of the International Conference on Corrosion and Corrosion Protection of Steel in Concrete, Sheffield, UK, 24–28 July 1994; pp. 139–156. [Google Scholar]
- Martin-Perez, B.; Pantazopoulou, S.; Thomas, M. Numerical solution of mass transport equations in concrete structures. Comput. Struct. 2001, 79, 1251–1264. [Google Scholar] [CrossRef] [Green Version]
- Saetta, A.; Scotta, R.; Vitaliani, R. Analysis of chloride diffusion into partially saturated concrete. ACI Mater. J. 1993, 90, 441–451. [Google Scholar]
- Sagüés, A.A.; Kranc, S.C.; Lau, K. Service life forecasting for reinforced concrete incorporating potential-dependent chloride threshold. Corrosion 2009, 70, 942–957. [Google Scholar] [CrossRef] [Green Version]
- Andrade, C.; Tavares, F.; Castellote, M.; Petre-Lazars, I.; Climent, M.A.; Vera, G. Comparison of the chloride models: The importance of surface concentration. In Proceedings of the RILEM 60th Annual Week 2006 & International Conference on Concrete and Reinforced Concrete, Quebec City, QC, Canada, 11–13 September 2006. [Google Scholar]
- Gulikers, J. Critical evaluation of service life models applied on an existing marine concrete structure. In Proceedings of the NORECON Seminar 2004: Repair and Maintenance of Concrete Structures, Copenhagen, Denmark, 19–20 April 2004. [Google Scholar]
- Page, C.L.; Vennesland, O. Pore solution composition and chloride binding capacity of silica-fume cement pastes. Mater. Constr. 1983, 16, 19–25. [Google Scholar] [CrossRef]
- Mangat, P.S.; Molloy, B.T. Predicting of long term chloride concentration in concrete. Mater. Struct. 1994, 27, 338–346. [Google Scholar] [CrossRef]
- Helland, S.; Aarstein, R.; Maage, M. In-field performance of north sea hsc/hpc offshore platforms with regard to chloride resistance. In Proceedings of the 8th International Symposium on Utilization of High-Strength and High-Performance Concrete, Tokyo, Japan, 27–29 October 2008. [Google Scholar]
- Markeset, G. Critical chloride content and its influence on service life predictions. Mater. Corros. 2009, 60, 593–596. [Google Scholar] [CrossRef]
- Page, C.L.; Lambert, P.; Vassie, P.R.W. Investigations of reinforcement corrosion, 2: Electrochemical monitoring of steel in chloride-contaminated concrete. Mater. Struct. 1991, 24, 351–358. [Google Scholar] [CrossRef]
- Izquierdo, D.; Alonso, C.; Andrade, C.; Castellote, M. Potentiostatic determination of chloride threshold values for rebar depassivation. Electrochim. Acta 2004, 49, 2731–2739. [Google Scholar] [CrossRef]
- Alonso, C.; Castellote, M.; Andrade, C. Chloride threshold dependence of pitting potential of reinforcements. Electrochim. Acta 2002, 47, 3469–3481. [Google Scholar] [CrossRef]
- fib Model Code 2010; International Federation for Structural Concrete; fib: Lausanne, Switzerland, 2012.
- Val, D.V.; Stewart, M.G. Life-cycle cost analysis of reinforced concrete structures in marine environments. Struct. Saf. 2003, 25, 343–362. [Google Scholar] [CrossRef]
- Izquierdo, D.; Andrade, C.; Arteaga, A.; Tanner, P. Economical optimization of appraising concrete degradation processes. In Proceedings of the 3rd International IABMAS Workshop on Life–Cycle Cost Analysis and Design of Civil Infrastructure Systems, Lausanne, Switzerland, 24–26 March 2003. [Google Scholar]
- Andrade, C. 2013 WR Whitney Award Lecture: Probabilistic Treatment of the Reinforcement Corrosion. Corrosion 2014, 70, 643–651. [Google Scholar] [CrossRef]
- Fenaux, M.; Reyes, E.; Gálvez, J.; Moragues, A. Modelling the transport of chloride and other ions in cement-based materials. Cem. Concr. Compos. 2018, 97, 33–42. [Google Scholar] [CrossRef]
- Shafikhani, M.; Chidiac, S. Quantification of concrete chloride diffusion coefficient—A critical review. Cem. Concr. Compos. 2019, 99, 225–250. [Google Scholar] [CrossRef]
- Tran, V.Q.; Soive, A.; Baroghel-Bouny, V. Modelisation of chloride reactive transport in concrete including thermodynamic equilibrium, kinetic control and surface complexation. Cem. Concr. Res. 2018, 110, 70–85. [Google Scholar] [CrossRef]
- Zacchei, E.; Nogueira, C.G. Calibration of boundary conditions correlated to the diffusivity of chloride ions: An accurate study for random diffusivity. Cem. Concr. Compos. 2021, 126, 104346. [Google Scholar] [CrossRef]
- Jasielec, J.J.; Stec, J.; Szyszkiewicz-Warzecha, K.; Łagosz, A.; Deja, J.; Lewenstam, A.; Filipek, R. Effective and Apparent Diffusion Coefficients of Chloride Ions and Chloride Binding Kinetics Parameters in Mortars: Non-Stationary Diffusion–Reaction Model and the Inverse Problem. Materials 2020, 13, 5522. [Google Scholar] [CrossRef]
- Andrade, C.; Díez, J.M.; Alonso, C. Mathematical modelling of a concrete surface “skin effect” on Diffusion in chloride con-taminated media. Adv. Cem. Based Mater. 1997, 6, 39–44. [Google Scholar] [CrossRef]
- Balestra, C.E.T.; Reichert, T.A.; Vizzotto, A.L.P.; Pansera, W.A.; Savaris, G. General model for service life analysis of reinforced concrete structures subject to chloride penetration. Constr. Build. Mater. 2021, 305, 124727. [Google Scholar] [CrossRef]
- Liu, J.; Liao, C.; Jin, H.; Jiang, Z.; Zhou, H. Numerical and experimental research on the effect of rainfall on the transporting behavior of chloride ions in concrete. Constr. Build. Mater. 2021, 302, 124160. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, D.; Jiang, M.; Xiao, L.; Mei, G. Experimental and numerical analysis of chloride transport in finite concrete under reverse water pressure. Constr. Build. Mater. 2021, 304, 124576. [Google Scholar] [CrossRef]
- Sanchez, T.; Conciatori, D.; Laferriere, F.; Sorelli, L. Modelling capillary effects on the reactive transport of chloride ions in cementitious materials. Cem. Concr. Res. 2020, 131, 106033. [Google Scholar] [CrossRef]
- Andrade, C.; Chang, H. Andrade Chloride profile induced by wet-dry cycles and carbonation. In Proceedings of the 4th International Conference on Service Life Design for Infrastructures (SLD4-Rilem), Delft, The Netherlands, 27–30 August 2018. [Google Scholar]
- Li, K.; Zhao, F.; Zhang, Y. Influence of carbonation on the chloride ingress into concrete: Theoretical analysis and application to durability design. Cem. Concr. Res. 2019, 123, 105788. [Google Scholar] [CrossRef]
- Liu, J.; Qiu, Q.; Chen, X.; Xing, F.; Han, N.; He, Y.; Ma, Y. Understanding the interacted mechanism between carbonation and chloride aerosol attack in ordinary Portland cement concrete. Cem. Concr. Res. 2017, 95, 217–225. [Google Scholar] [CrossRef]
- Georget, F.; Bénier, C.; Wilson, W.; Scrivener, K.L. Chloride sorption by C-S-H quantified by SEM-EDX image analysis. Cem. Concr. Res. 2021, 152, 106656. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Influence of pH on the chloride binding capacity of Limestone Calcined Clay Cements (LC3). Cem. Concr. Res. 2020, 131, 106031. [Google Scholar] [CrossRef]
- Teymouri, M.; Shakouri, M.; Vaddey, N.P. pH-dependent chloride desorption isotherms of Portland cement paste. Constr. Build. Mater. 2021, 312, 125415. [Google Scholar] [CrossRef]
- Yoshida, S.; Elakneswaran, Y.; Nawa, T. Electrostatic properties of C–S–H and C-A-S-H for predicting calcium and chloride adsorption. Cem. Concr. Compos. 2021, 121, 104109. [Google Scholar] [CrossRef]
- De Weerdt, K.; Colombo, A.; Coppola, L.; Justnes, H.; Geiker, M. Impact of the associated cation on chloride binding of Portland cement paste. Cem. Concr. Res. 2015, 68, 196–202. [Google Scholar] [CrossRef]
- Jain, A.; Gencturk, B.; Pirbazari, M.; Dawood, M.; Belarbi, A.; Sohail, M.; Kahraman, R. Influence of pH on chloride binding isotherms for cement paste and its components. Cem. Concr. Res. 2021, 143, 106378. [Google Scholar] [CrossRef]
- Balonis, M. Thermodynamic modelling of temperature effects on the mineralogy of Portland cement systems containing chloride. Cem. Concr. Res. 2019, 120, 66–76. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Cao, Y.; Gehlen, C.; Angst, U.; Wang, L.; Wang, Z.; Yao, Y. Critical chloride content in reinforced concrete—An updated review considering Chinese experience. Cem. Concr. Res. 2018, 117, 58–68. [Google Scholar] [CrossRef]
- Angst, U.M. Predicting the time to corrosion initiation in reinforced concrete structures exposed to chlorides. Cem. Concr. Res. 2018, 115, 559–567. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Ann Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Meira, G.; Andrade, C.; Vilar, E.; Nery, K. Analysis of chloride threshold from laboratory and field experiments in marine atmosphere zone. Constr. Build. Mater. 2014, 55, 289–298. [Google Scholar] [CrossRef]
- Kranc, S.C.; Sagues, A.A.; Presuel-Moreno, F.J. Decreased corrosion initiation time of steel in concrete due to reinforcing bar obstruction of diffusional flow. ACI Mater. J. 2002, 99, 51–53. [Google Scholar]
- Tavares, F.; Andrade, C.; Capra, B. Numerical Modelling of Chloride Ingress through Cracked Concrete. In Proceedings of the RILEM V International Ph.D. Student Workshop on Durability of Reinforced Concrete—From Composition to Service Life Design, Helsinki, Finland, 9–10 February 2012. [Google Scholar]



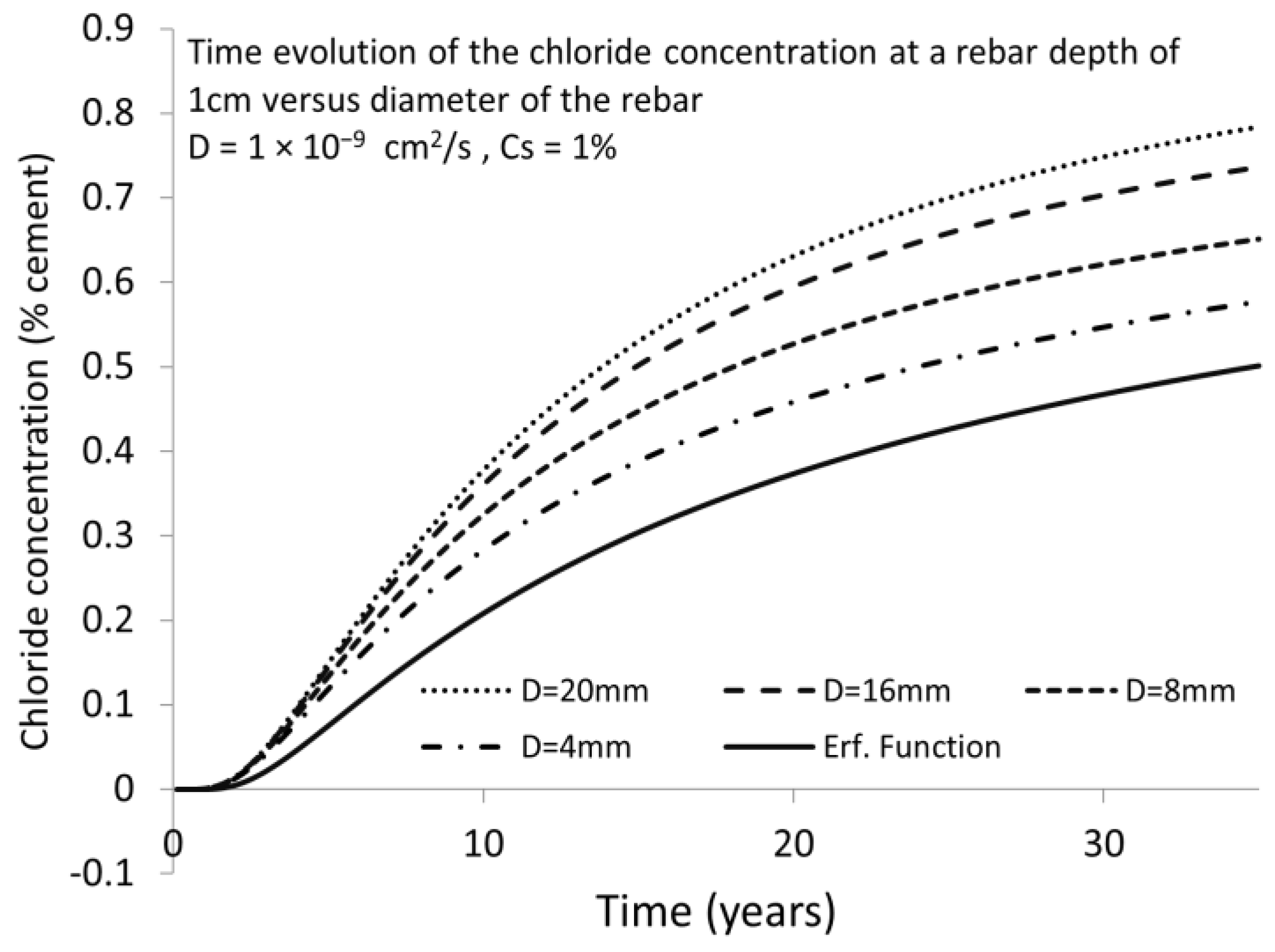


| Rebar Diameter | Time to Depassivation Ccritical = 0.4% | Porcentual Increase in Chloride Concentration | Underestimation of Chloride Concentration |
|---|---|---|---|
| Erf. Function (semi-infinite boundary) | 22.4 years | 0% | 100% |
| 4 mm (finite boundary) | 15.68 years | 42.86% | 30.00% |
| 8 mm (finite boundary) | 12.88 years | 73.91% | 42.50% |
| 16 mm (finite boundary) | 11.2 years | 100.00% | 50.00% |
| 20 mm (finite boundary) | 10.64 years | 110.53% | 52.50% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavares, F.; Andrade, C. Finite Boundary Conditions Due to the Bar Presence in the Model of Chloride Penetration. Materials 2022, 15, 1426. https://doi.org/10.3390/ma15041426
Tavares F, Andrade C. Finite Boundary Conditions Due to the Bar Presence in the Model of Chloride Penetration. Materials. 2022; 15(4):1426. https://doi.org/10.3390/ma15041426
Chicago/Turabian StyleTavares, Fabiano, and Carmen Andrade. 2022. "Finite Boundary Conditions Due to the Bar Presence in the Model of Chloride Penetration" Materials 15, no. 4: 1426. https://doi.org/10.3390/ma15041426






