Evaluating the X-ray-Shielding Performance of Graphene-Oxide-Coated Nanocomposite Fabric
Abstract
:1. Introduction
2. Materials and Methods
2.1. Graphene Oxide (GO) Synthesis
2.2. Nanocomposite Fabric Preparation Method
2.3. X-ray Shielding Simulation of Nanocomposite Fabrics
2.4. Experimental Setup
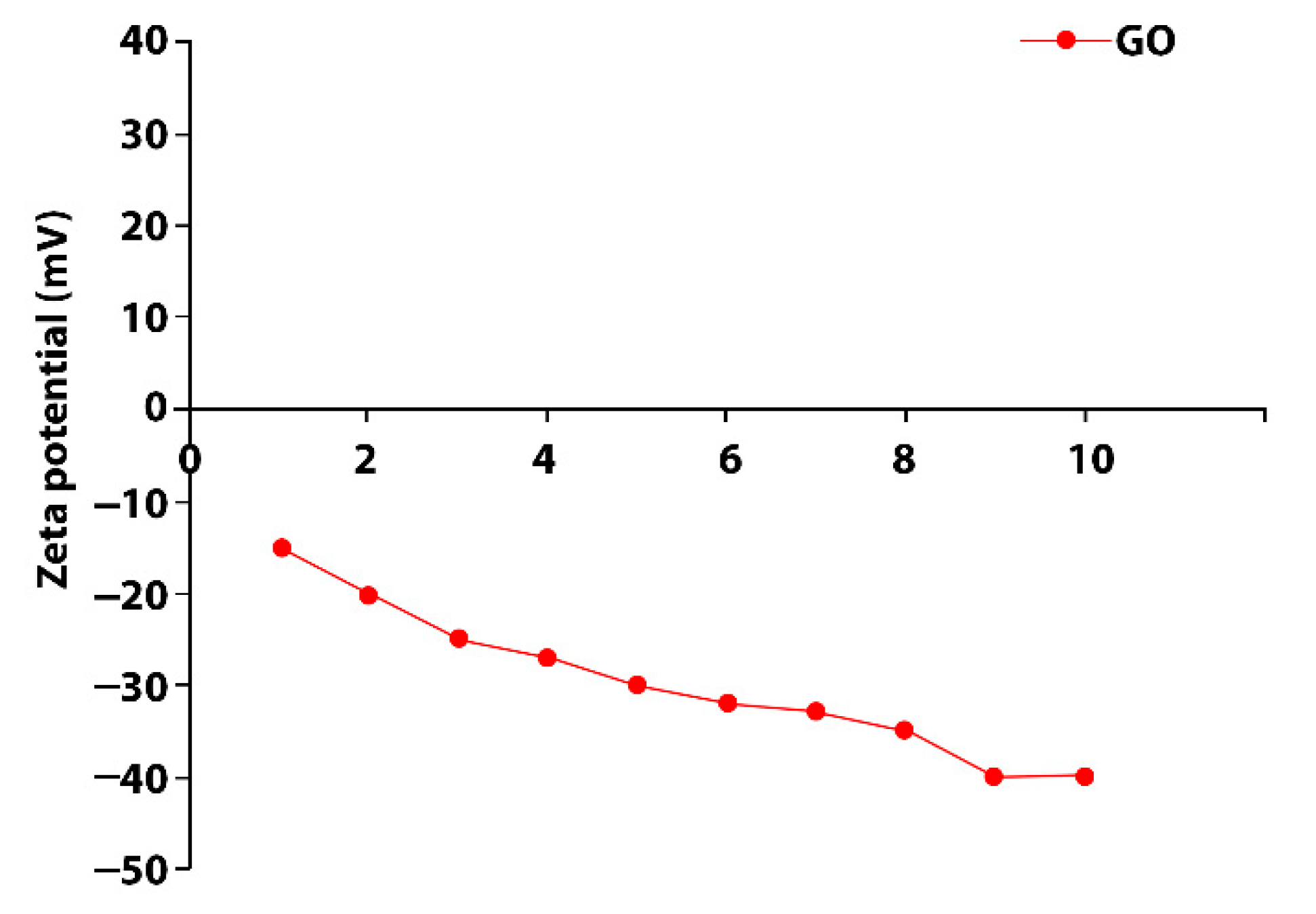
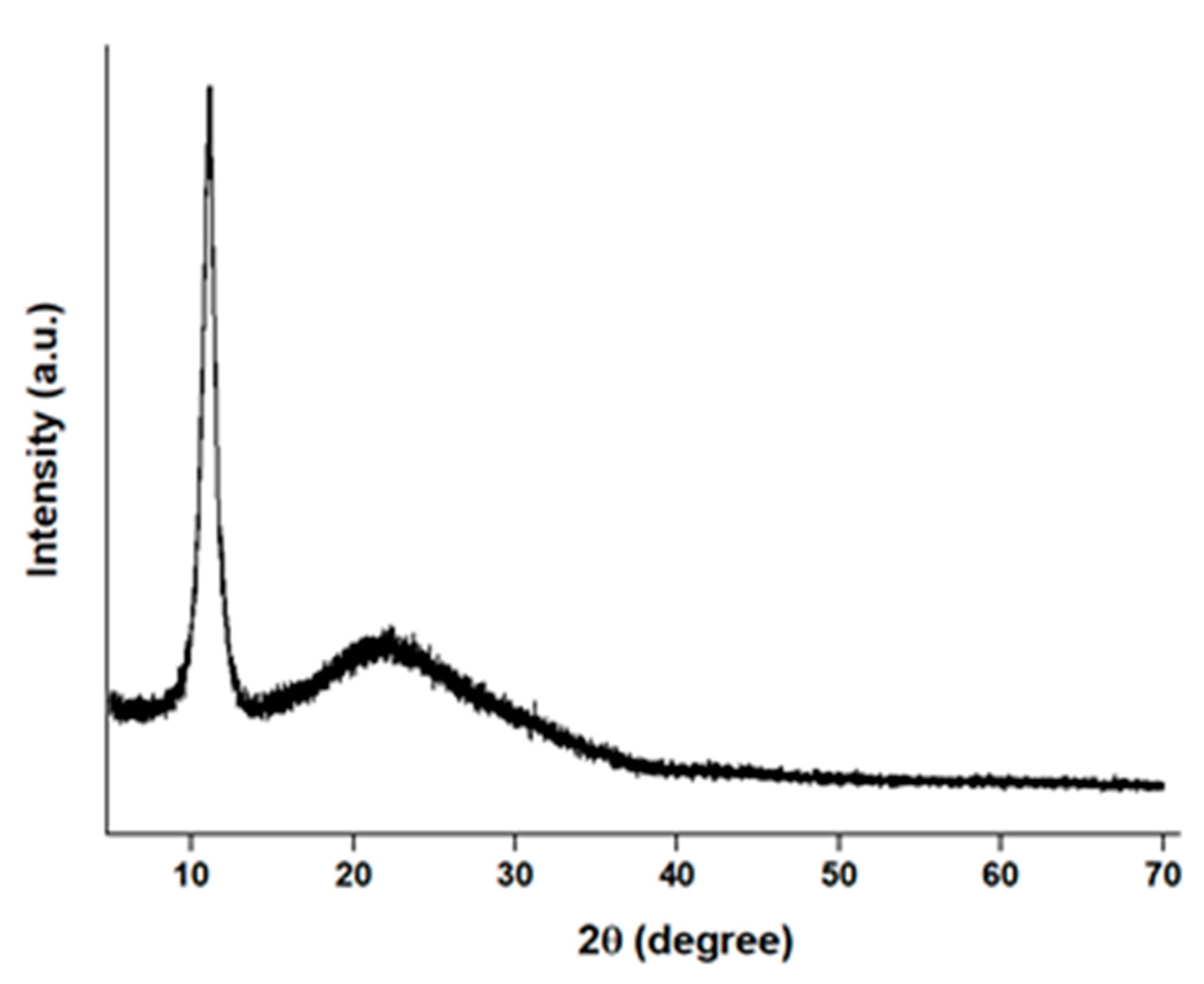
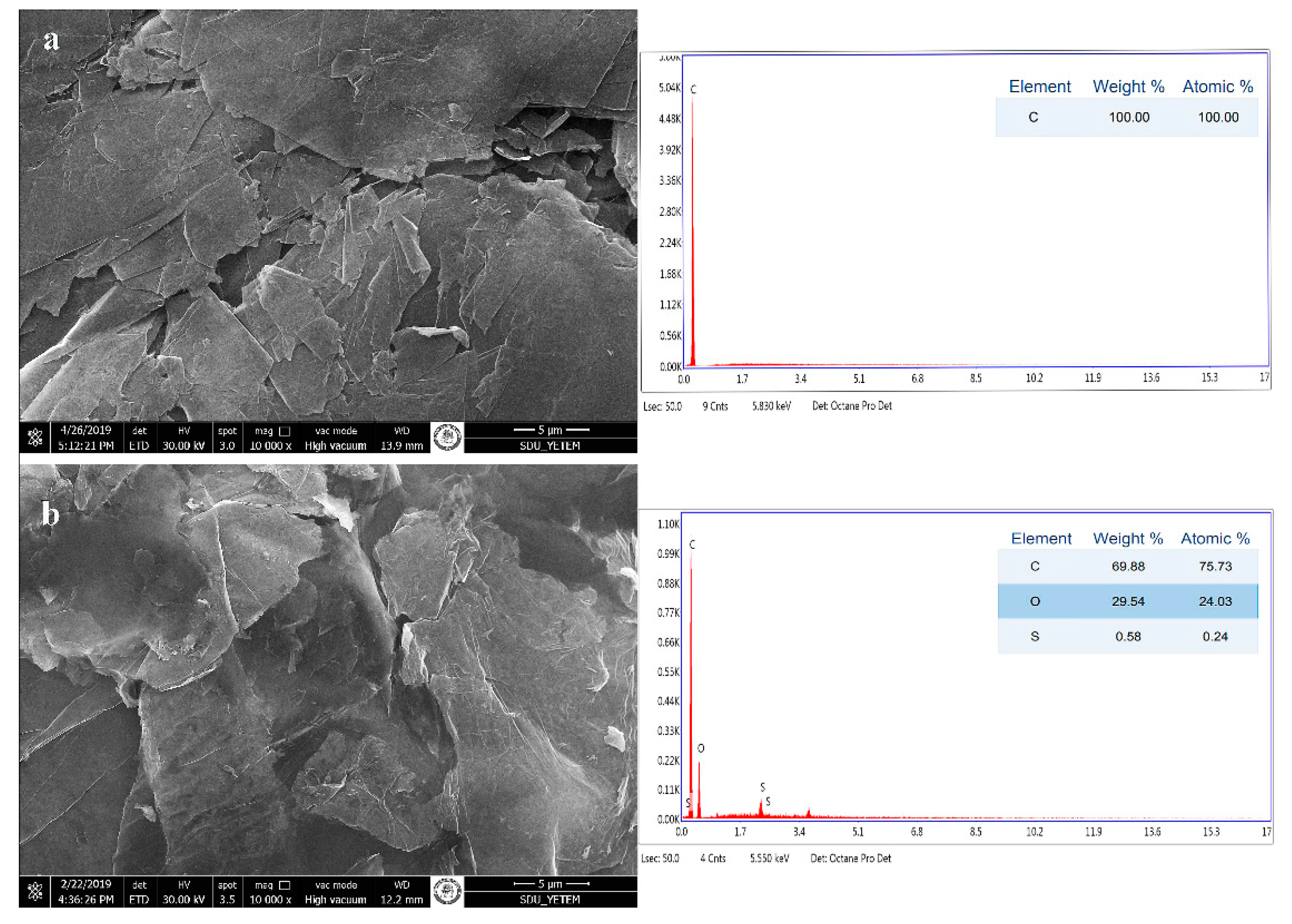
2.5. Characterization Nanocomposite Fabrics
2.6. Assessment of The Data
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- More, C.V.; Alsayed, Z.; Badawi, M.S.; Thabet, A.A.; Pawar, P.P. Polymeric composite materials for radiation shielding: A review. Environ. Chem. Lett. 2021, 19, 2057–2090. [Google Scholar] [CrossRef]
- Crane, G.D.; Abbott, P.V. Radiation shielding in dentistry: An update. Aust. Dent. J. 2016, 61, 277–281. [Google Scholar] [CrossRef]
- Shah, N.; Bansal, N.; Logani, A. Recent advances in imaging technologies in dentistry. World J. Radiol. 2014, 6, 794–807. [Google Scholar] [CrossRef]
- Memon, A.; Rogers, I.; Paudyal, P.; Sundin, J. Dental X-rays and the risk of thyroid cancer and meningioma: A systematic review and meta-analysis of current epidemiological evidence. Thyroid 2019, 29, 1572–1593. [Google Scholar] [CrossRef]
- Rodgers, C.C. Low-dose X-ray imaging may increase the risk of neurodegenerative diseases. Med. Hypotheses 2020, 142, 109726. [Google Scholar] [CrossRef]
- Tsapaki, V. Radiation protection in dental radiology—Recent advances and future directions. Phys. Med. 2017, 44, 222–226. [Google Scholar] [CrossRef]
- Constine, L.S.; Ronckers, C.M.; Hua, C.H.; Olch, A.; Kremer, L.C.M.; Jackson, A.; Bentzen, S.M. Pediatric normal tissue effects in the clinic (PENTEC): An international collaboration to analyze normal tissue radiation dose-volume-response relationships for pediatric cancer patients. Clin. Oncol. 2019, 31, 199–207. [Google Scholar] [CrossRef] [Green Version]
- EzEldeen, M.; Stratis, A.; Coucke, W.; Codari, M.; Politis, C.; Jacobs, R. As Low Dose as Sufficient Quality: Optimization of Cone-beam Computed Tomographic Scanning Protocol for Tooth Autotransplantation Planning and Follow-up in Children. J. Endod. 2017, 43, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.E.; Harris, A.M.P.; van der Merwe, E.J.; Nortje, C.J. The leaded apron revisited: Does it reduce gonadal radiation dose in dental radiology? Oral Surg. Oral Med. Oral Pathol. 1991, 71, 642–646. [Google Scholar] [CrossRef]
- Azman, N.Z.; Siddiqui, S.A.; Low, I.M. Characterisation of micro-sized and nano-sized tungsten oxide-epoxy composites for radiation shielding of diagnostic X-rays. Mater. Sci. Eng. C 2013, 33, 4952–4957. [Google Scholar] [CrossRef] [Green Version]
- Burns, K.M.; Shoag, J.M.; Kahlon, S.S.; Parsons, P.J.; Bijur, P.E.; Taragin, B.H.; Markowitz, M. Lead Aprons Are a Lead Exposure Hazard. J. Am. Coll. Radiol. 2017, 14, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Hazlan, M.H.; Ramli, R.M.; Noor Azman, N.Z. Study of electrospun PVA-based concentrations nanofibre filled with Bi2O3 or WO3 as potential X-ray shielding material. Radiat. Phys. Chem. 2019, 156, 272–282. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.; Mielke, H.; Gulson, B.; Ball, A.S. Lead exposure at firing ranges—A review. Environ. Health 2017, 16, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikeghbal, K.; Zamanian, Z.; Shahidi, S.; Spagnuolo, G.; Soltani, P. Designing and Fabricating Nano-structured and Micro-structured Radiation Shields for Protection against CBCT. Expo. Mater. 2020, 13, 4371. [Google Scholar] [CrossRef] [PubMed]
- Fenga, C.; Gangemi, S.; Di Salvatore, V.; Falzone, L.; Libra, M. Immunological effects of occupational exposure to lead. Mol. Med. Rep. 2017, 15, 3355–3360. [Google Scholar] [CrossRef] [Green Version]
- Low, S.S.; Lim, C.N.; Yew, M.; Chai, W.S.; Low, L.E.; Manickam, S.; Tey, B.T.; Show, P.L. Recent ultrasound advancements for the manipulation of nanobiomaterials and nanoformulations for drug delivery. Ultrason. Sonochem. 2021, 80, 105805. [Google Scholar]
- AbuAlRoos, N.J.; Baharul Amin, N.A.; Zainon, R. Conventional and new lead-free radiation shielding materials for radiation protection in nuclear medicine: A review. Radiat. Phys. Chem. 2019, 165, 108439. [Google Scholar] [CrossRef]
- Derradji, M.; Mehelli, O.; Liu, W.; Fantuzzi, N. Sustainable and Ecofriendly Chemical Design of High Performance Bio-Based Thermosets for Advanced Applications. Front. Chem. 2021, 9, 691117. [Google Scholar] [CrossRef]
- Maghrabi, H.A.; Vijayan, A.; Deb, P.; Wang, L. Bismuth oxide-coated fabrics for X-ray shielding. Text. Res. J. 2016, 86, 649–658. [Google Scholar] [CrossRef]
- Günther, K.; Giebing, C.; Askani, A.; Leisegang, T.; Krieg, M.; Kyosev, Y.; Weide, T.; Mahltig, B. Cellulose/inorganic-composite fibers for producing textile fabrics of high X-ray absorption properties. Mater. Chem. Phys. 2015, 167, 125–135. [Google Scholar] [CrossRef]
- Fantuzzi, N.; Bacciocchi, M.; Agnelli, J.; Benedetti, D. Three-phase homogenization procedure for woven fabric composites reinforced by carbon nanotubes in thermal environment. Compos. Struct. 2020, 254, 112840. [Google Scholar] [CrossRef]
- Mehelli, O.; Derradji, M.; Belgacemi, R.; Zegaoui, A.; Khimeche, K.; Fantuzzi, N.; Mouloud, A. Development of highly performant hybrid materials based on phthalonitrile resin for a simultaneous ballistic and nuclear shielding protection. High Perform. Polym. 2020, 33, 217–222. [Google Scholar] [CrossRef]
- Mehelli, O.; Derradji, M.; Habes, A.; Leblalta, N.E.; Belgacemi, R.; Abdous, S.; Izri, Y.; Liu, W. Benzoxazine resin as an interesting building block for advanced neutrons shields. High Perform. Polym. 2021, 33, 1116–1123. [Google Scholar] [CrossRef]
- Magovac, E.; Vončina, B.; Jordanov, I.; Grunlan, J.C.; Bischof, S. Layer-by-Layer Deposition: A Promising Environmentally Benign Flame-Retardant Treatment for Cotton, Polyester, Polyamide and Blended Textiles. Materials 2022, 15, 432. [Google Scholar] [CrossRef]
- Forsman, N.; Lozhechnikova, A.; Khakalo, A.; Johansson, L.S.; Vartiainen, J.; Österberg, M. Layer-by-layer assembled hydrophobic coatings for cellulose nanofibril films and textiles, made of polylysine and natural wax particles. Carbohydr. Polym. 2017, 173, 392–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Woong Han, J.; Kim, E.; Kwon, D.-N.; Park, J.-K.; Kim, J.-H. Enhanced green fluorescent protein-mediated synthesis of biocompatible graphene. J. Nanobiotechnology 2014, 12, 41. [Google Scholar] [CrossRef] [Green Version]
- Shahriary, L.; Athawale, A. Graphene oxide synthesized by using modified Hummers approach. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Anissi, H.; Geibel, M.-A. Intraoral Radiology in General Dental Practices—A Comparison of Digital and Film-Based X-ray Systems with Regard to Radiation Protection and Dose Reduction. RöFo-Fortschr. Geb. R 2014, 186, 762–767. [Google Scholar] [CrossRef] [Green Version]
- Granlund, C.; Thilander-Klang, A.; Ylhan, B.; Lofthag-Hansen, S.; Ekestubbe, A. Absorbed organ and effective doses from digital intra-oral and panoramic radiography applying the ICRP 103 recommendations for effective dose estimations. Br. J. Radiol. 2016, 89, 20151052. [Google Scholar] [CrossRef] [Green Version]
- Ludlow, J.B.; Timothy, R.; Walker, C.; Hunter, R.; Benavides, E.; Samuelson, D.B.; Scheske, M.J. Effective dose of dental CBCT-a meta analysis of published data and additional data for nine CBCT units. Dentomaxillofac. Radiol. 2015, 44, 20140197. [Google Scholar] [CrossRef] [Green Version]
- Aral, N.; Duch, M.A.; Ardanuy, M. Material characterization and Monte Carlo simulation of lead and non-lead X-ray shielding materials. Radiat. Phys. Chem. 2020, 174, 108892. [Google Scholar] [CrossRef]
- Malekzadeh, R.; Mehnati, P.; Sooteh, M.Y.; Mesbahi, A. Influence of the size of nano- and microparticles and photon energy on mass attenuation coefficients of bismuth–silicon shields in diagnostic radiology. Radiol. Phys. Technol. 2019, 12, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Tekin, H.O.; Sayyed, M.I.; Issa Shams, A.M. Gamma radiation shielding properties of the hematite-serpentine concrete blended with WO3 and Bi2O3 micro and nano particles using MCNPX code. Radiat. Phys. Chem. 2018, 150, 95–100. [Google Scholar] [CrossRef]
- Verdipoor, K.; Alemi, A.; Mesbahi, A. Photon mass attenuation coefficients of a silicon resin loaded with WO3, PbO, and Bi2O3 Micro and Nano-particles for radiation shielding. Radiat. Phys. Chem. 2018, 147, 85–90. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Khatib, A.M.; Badawi, M.S.; Rashad, A.R.; El-Sharkawy, R.M.; Thabet, A.A. Fabrication, characterization and gamma rays shielding properties of nano and micro lead oxide-dispersed-high density polyethylene composites. Radiat. Phys. Chem. 2018, 145, 160–173. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Q.-P.; Zheng, J.; Wu, Y.; Zhao, Y.; Zhou, Y.-L. Co-shielding of neutron and γ-ray with bismuth borate nanoparticles fabricated via a facile sol-gel method. Inorg. Chem. Commun. 2017, 77, 55–58. [Google Scholar] [CrossRef]
- Aghaz, A.; Faghihi, R.; Mortazavi, S.M.J.; Haghparast, A.; Mehdizadeh, S.; Sina, S. Radiation attenuation properties of shields containing micro and Nano WO3 in diagnostic X-ray energy range. Int. J. Radiat. Res. 2016, 14, 127–131. [Google Scholar] [CrossRef]
- Asari Shik, N.; Gholamzadeh, L. X-ray shielding performance of the EPVC composites with micro- or nanoparticles of WO3, PbO or Bi2O3. Appl. Radiat. Isot. 2018, 139, 61–65. [Google Scholar] [CrossRef]
- Zehtabian, M.; Molaiemanesh, Z.; Pirouzan, E.; Sina, S. Design of Light Multi-layered Shields for Use in Diagnostic Radiology and Nuclear Medicine via MCNP5 Monte Carlo Code. Iran. J. Med. Phys. 2015, 12, 223–228. [Google Scholar]
- Bragaglia, M.; Paleari, L.; Lamastra, F.R.; Puglia, D.; Fabbrocino, F.; Nanni, F. Graphene nanoplatelet, multiwall carbon nanotube, and hybrid multiwall carbon nanotube–graphene nanoplatelet epoxy nanocomposites as strain sensing coatings. J. Reinf. Plast. Compos. 2021, 40, 632–643. [Google Scholar] [CrossRef]
- Patle, V.K.; Kumar, R.; Sharma, A.; Dwivedi, N.; Muchhala, D.; Chaudhary, A.; Mehta, Y.; Mondal, D.P.; Srivastava, A.K. Three dimension phenolic resin derived carbon-CNTs hybrid foam for fire retardant and effective electromagnetic interference shielding. Compos. Part C Open Access 2020, 2, 100020. [Google Scholar] [CrossRef]
- Bheema, R.K.; Vuba, K.K.; Etakula, N.; Etika, K.C. Enhanced thermo-mechanical, thermal and EMI shielding properties of MWNT/MAgPP/PP nanocomposites prepared by extrusion. Compos. Part C Open Access 2021, 4, 100086. [Google Scholar] [CrossRef]
- Low, S.S.; Yew, M.; Lim, C.N.; Chai, W.S.; Low, L.E.; Manickam, S.; Tey, B.T.; Show, P.L. Sonoproduction of nanobiomaterials—A critical review. Ultrason. Sonochem. 2022, 82, 105887. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Türkaslan, S.S.; Ugur, Ş.S.; Türkaslan, B.E.; Fantuzzi, N. Evaluating the X-ray-Shielding Performance of Graphene-Oxide-Coated Nanocomposite Fabric. Materials 2022, 15, 1441. https://doi.org/10.3390/ma15041441
Türkaslan SS, Ugur ŞS, Türkaslan BE, Fantuzzi N. Evaluating the X-ray-Shielding Performance of Graphene-Oxide-Coated Nanocomposite Fabric. Materials. 2022; 15(4):1441. https://doi.org/10.3390/ma15041441
Chicago/Turabian StyleTürkaslan, Serhat Süha, Şule Sultan Ugur, Banu Esencan Türkaslan, and Nicholas Fantuzzi. 2022. "Evaluating the X-ray-Shielding Performance of Graphene-Oxide-Coated Nanocomposite Fabric" Materials 15, no. 4: 1441. https://doi.org/10.3390/ma15041441
APA StyleTürkaslan, S. S., Ugur, Ş. S., Türkaslan, B. E., & Fantuzzi, N. (2022). Evaluating the X-ray-Shielding Performance of Graphene-Oxide-Coated Nanocomposite Fabric. Materials, 15(4), 1441. https://doi.org/10.3390/ma15041441





