Preparation and Chromaticity Control of CoTiO3/NiTiO3 Co-Coated TiO2 Composite Pigments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
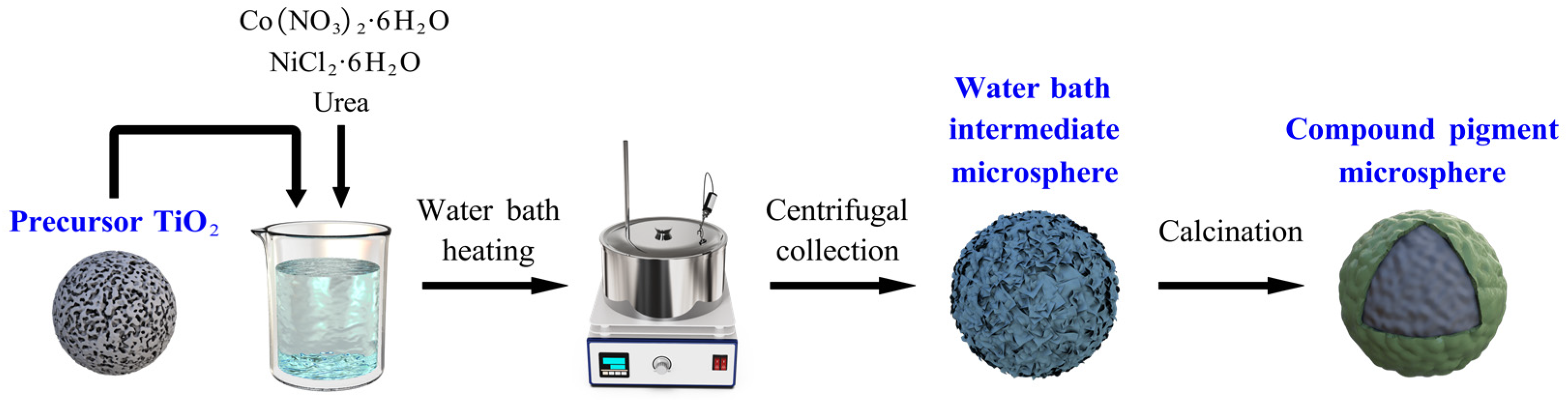
2.2. Synthesis of NiTiO3/CoTiO3 Co-Coated TiO2 Composite Pigments
2.3. Characterization Techniques
3. Results
3.1. X-ray Diffraction
3.2. Raman Spectroscopy
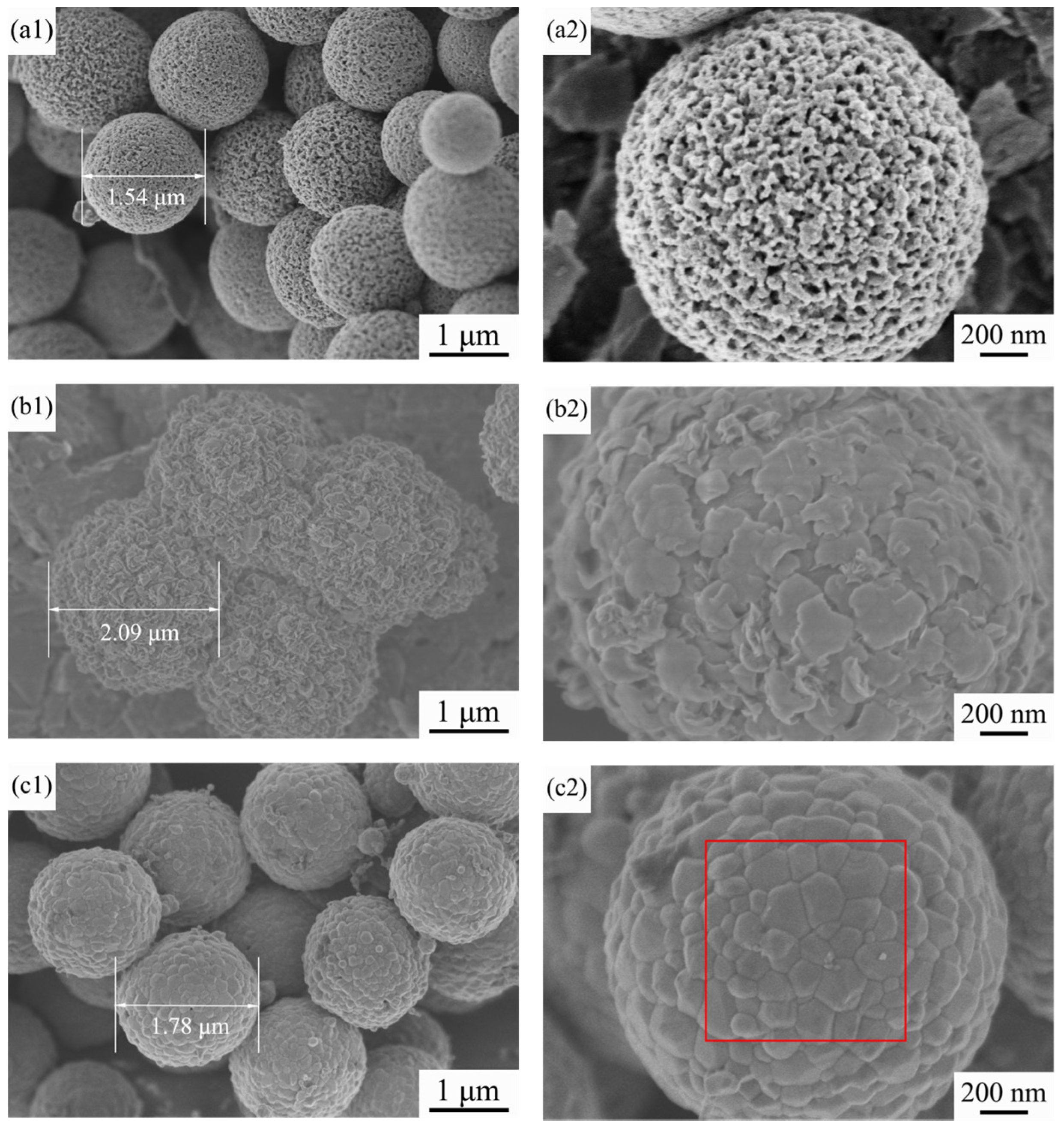
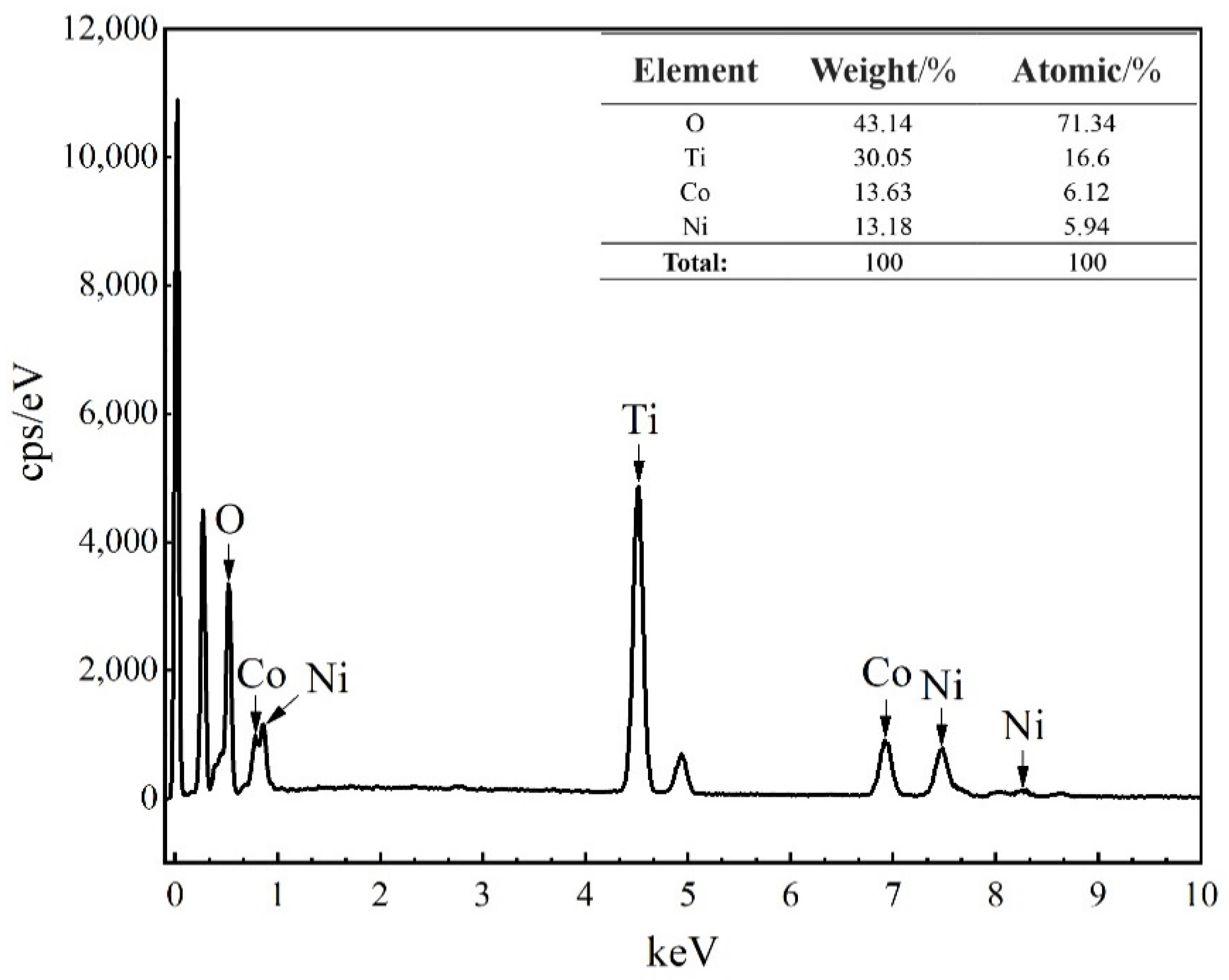
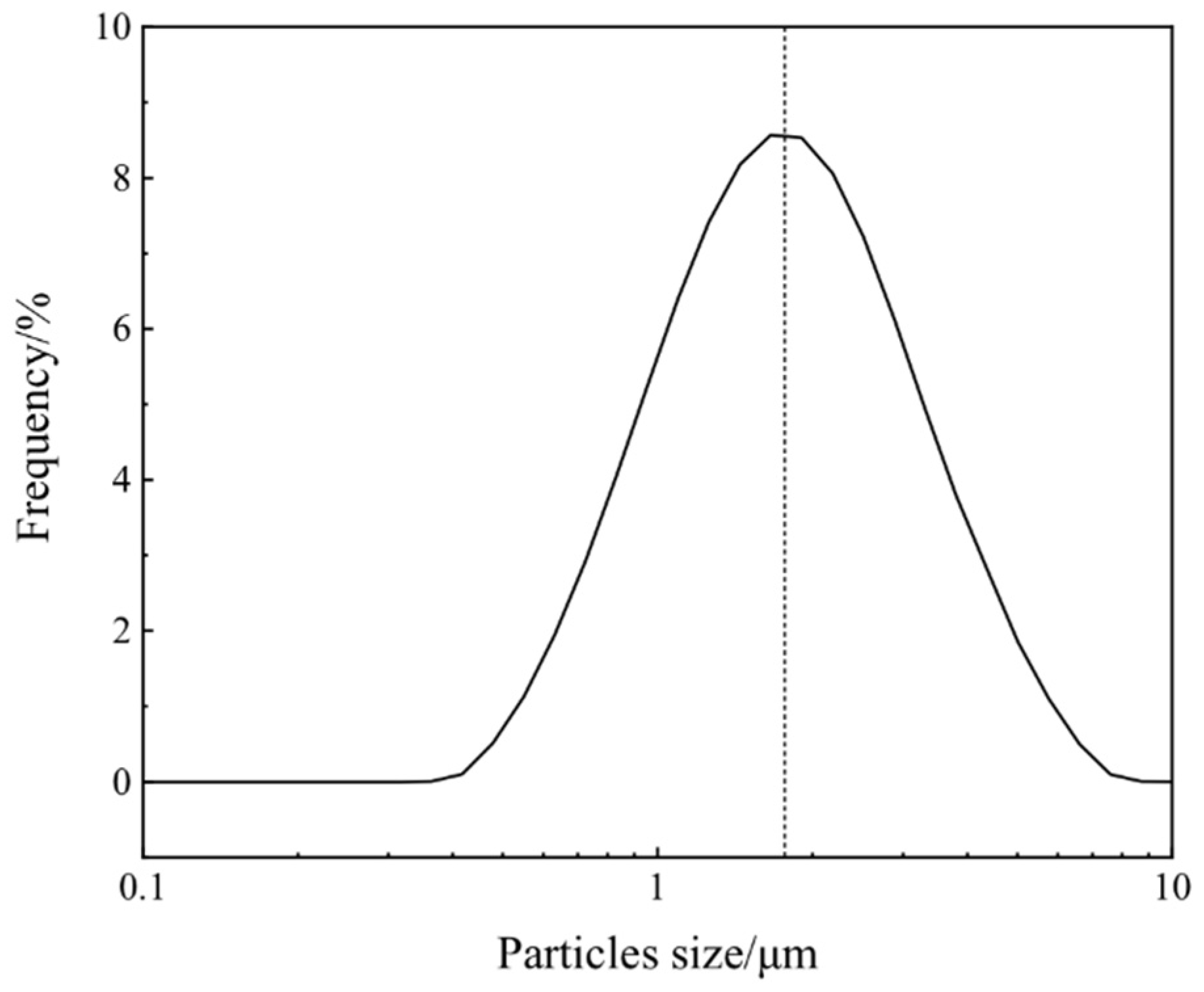
3.3. FE-SEM Observations
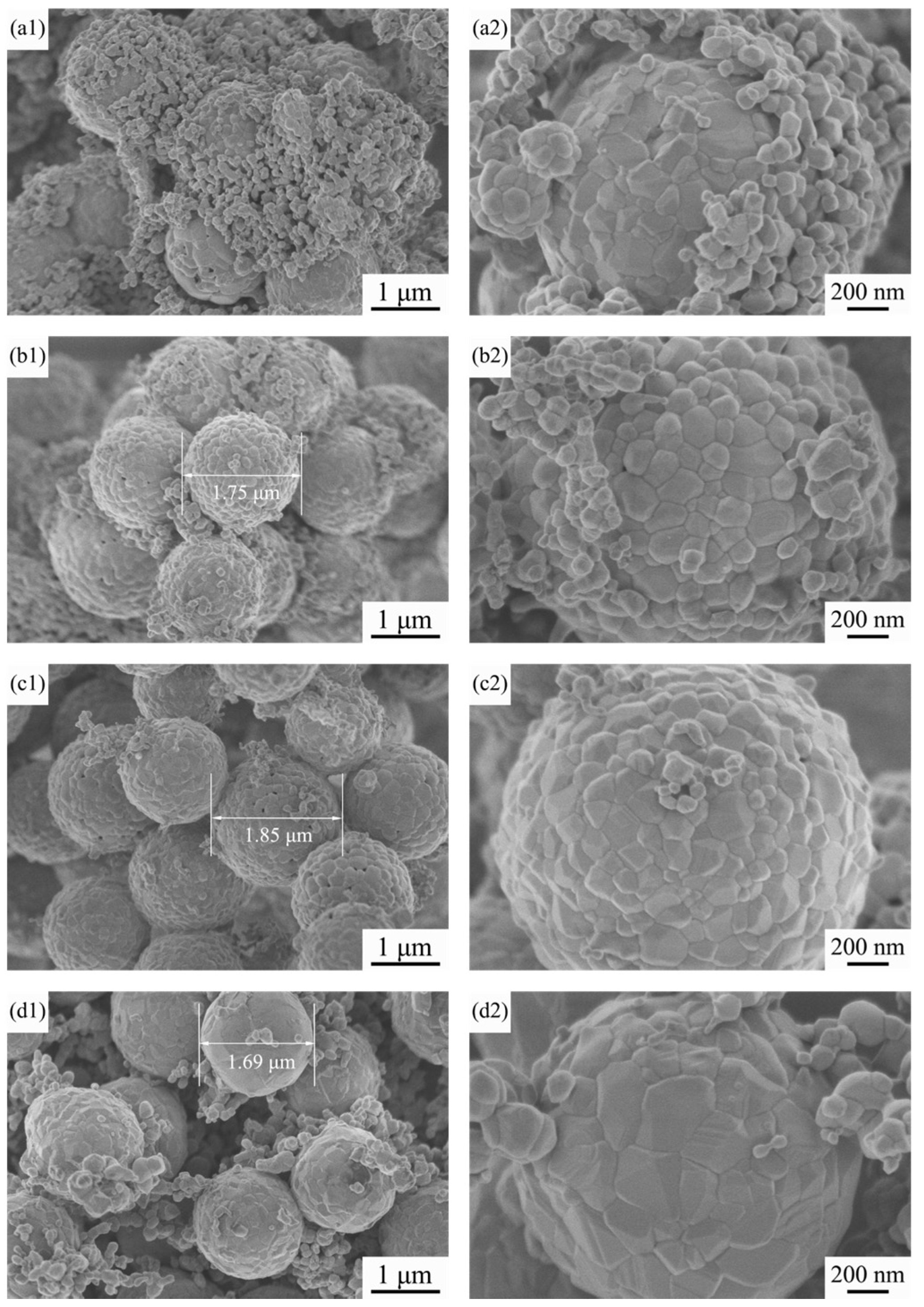
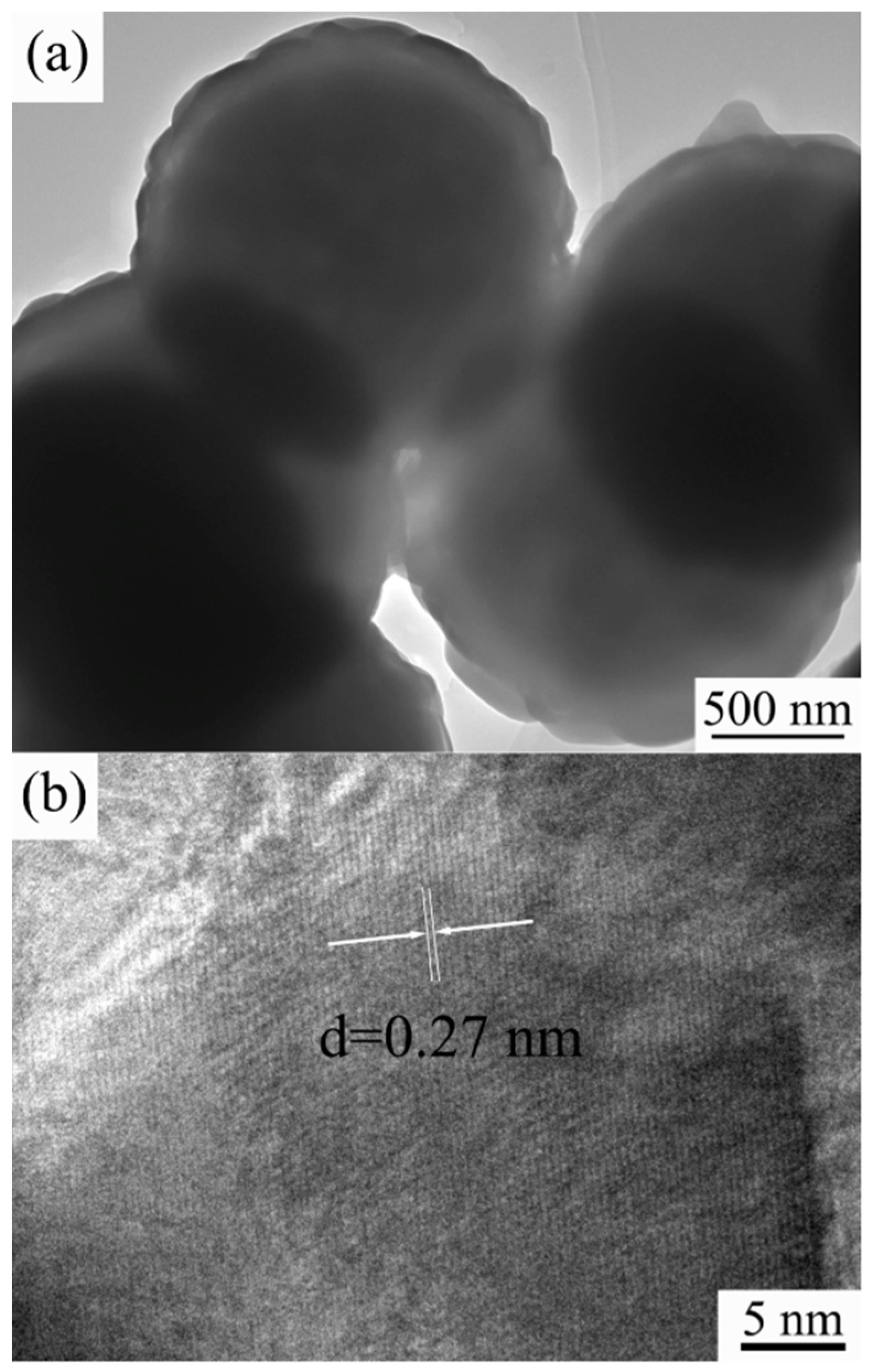
3.4. TEM Images
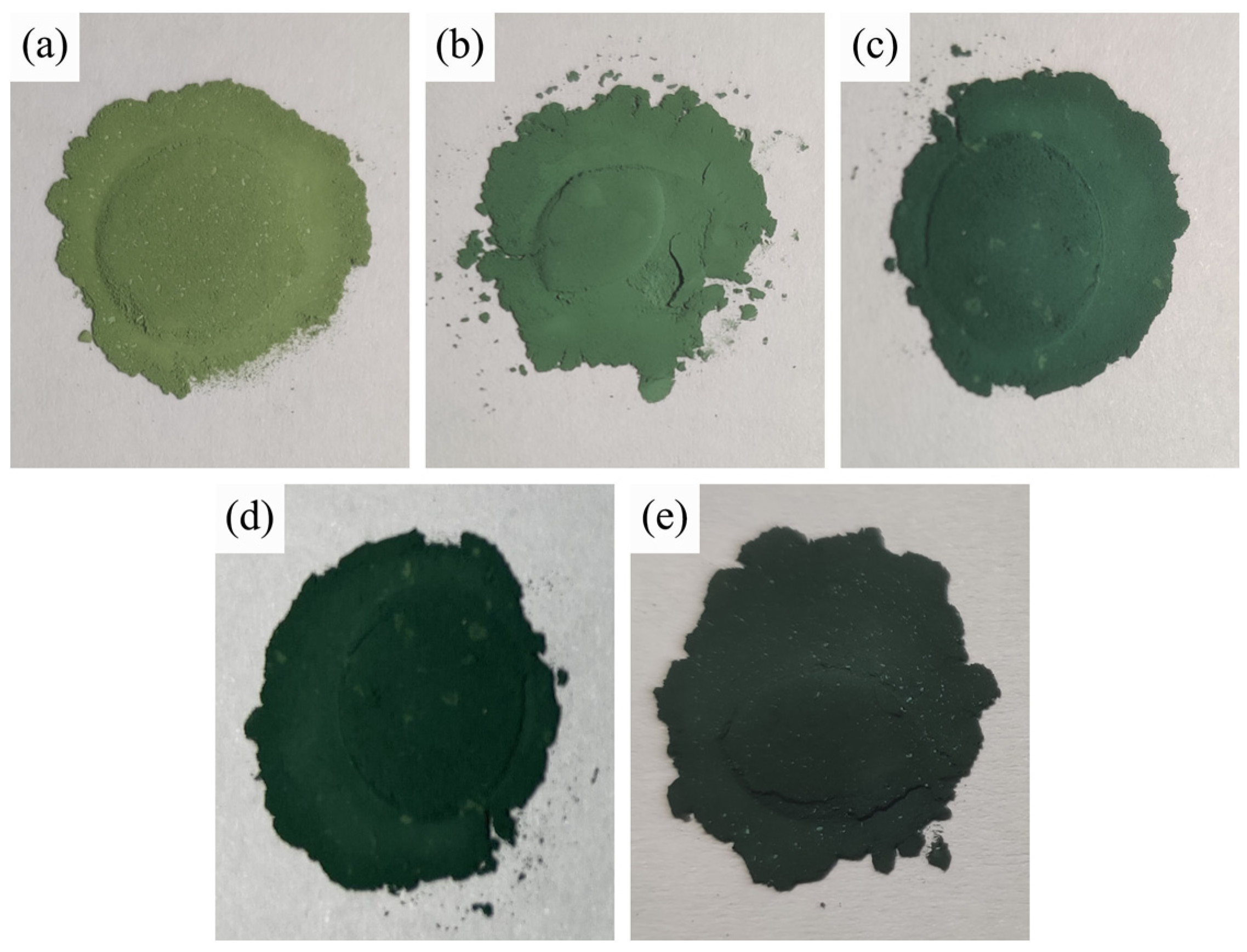
3.5. Colorimetric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, S.J.; Hao, D.; Hou, X.F. Preparation of CaCO3-TiO2 composite particles and their pigment properties. Materials 2018, 11, 1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Sun, S.; Liu, W.; Ding, H.; Zhang, J. Synthesis of perovskite by solid-phase method with metatitanic acid and calcium carbonate and its pigment properties investigation. Materials 2020, 13, 1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Primo, J.O.; Borth, K.W.; Peron, D.C.; Teixeira, V.C.; Galante, D.; Bittencourt, C.; Anaissi, F.J. Synthesis of green cool pigments (CoxZn1-xO) for application in NIR radiation reflectance. J. Alloys Compd. 2019, 780, 17–24. [Google Scholar] [CrossRef]
- Wei, G.Y.; Qu, J.K.; Yu, Z.H.; Li, Y.; Guo, Q.; Qi, T. Mineralizer effects on the synthesis of amorphous chromium hydroxide and chromium oxide green pigment using hydrothermal reduction method. Dye. Pigment. 2015, 113, 487–495. [Google Scholar] [CrossRef]
- Yang, H.; Mu, B.; Li, S.; Wang, X.W.; Wang, A.Q. Preparation and coloring mechanism of MAl2O4/CoAl2O4/quartz sand (M = Ca or Ba) composite pigments. Mater. Chem. Phys. 2022, 276, 125413. [Google Scholar] [CrossRef]
- Cao, L.Y.; Fei, X.; Zhao, H.B. Environmental substitution for PbCrO4 pigment with inorganic-organic hybrid pigment. Dye. Pigment. 2017, 142, 100–107. [Google Scholar] [CrossRef]
- Masui, T.; Takeuchi, N.; Nakado, H.; Imanaka, N. Novel environment-friendly green pigments based on rare earth cuprate. Dye. Pigment. 2015, 113, 336–340. [Google Scholar] [CrossRef]
- Li, Z.F.; Du, Y.; Chen, Z.T.; Sun, D.; Zhu, C. Synthesis and characterization of cobalt doped green ceramic pigment from tannery sludge. Ceram. Int. 2015, 41, 12693–12699. [Google Scholar] [CrossRef]
- Zou, J.; Zheng, W. TiO2@CoTiO3 complex green pigments with low cobalt content and tunable color properties. Ceram. Int. 2016, 42, 8198–8205. [Google Scholar] [CrossRef]
- Jose, S.; Joshy, D.; Narendranath, S.B.; Periyat, P. Recent advances in infrared reflective inorganic pigments. Sol. Energy Mater. Sol. Cells 2019, 194, 7–27. [Google Scholar] [CrossRef]
- Menon, S.G.; Swart, H.C. Microwave-assisted synthesis of blue-green NiAl2O4 nanoparticle pigments with high near-infrared reflectance for indoor cooling. J. Alloys Compd. 2020, 819, 152991. [Google Scholar] [CrossRef]
- Farha, A.H.; Ibrahim, M.M.; Mansour, S.A. Ga-doped ZnO nanostructured powder for cool-nanopigment in environment applications. Materials 2020, 13, 5152. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.G.; Yu, Y.; Feng, A.H.; Mi, L.; Xiu, H.X.; Yu, Y. Optical properties and radiation stability of SiO2/ZnO composite pigment prepared by co-sintering method. Ceram. Int. 2022, 48, 754–759. [Google Scholar] [CrossRef]
- Shah, K.W.; Huseien, G.F.; Kua, H.W. A State-of-the-art review on core–shell pigments nanostructure preparation and test methods. Micro 2021, 1, 55–85. [Google Scholar] [CrossRef]
- Chen, C.L.; Han, A.J.; Ye, M.Q.; Wang, J.Y.; Chen, X. A new thermal insulation ceramic pigment: Ce-doped Y3Al5O12 compounds combined with high near-infrared reflectance and low thermal conductivity. J. Alloys Compd. 2021, 886, 161257. [Google Scholar] [CrossRef]
- Moghtada, A.; Shahrouzianfar, A.; Ashiri, R. Facile synthesis of NiTiO3 yellow nano-pigments with enhanced solar radiation reflection efficiency by an innovative one-step method at low temperature. Dye. Pigment. 2017, 139, 388–396. [Google Scholar] [CrossRef]
- Wang, J.-L.; Li, Y.-Q.; Byon, Y.-J.; Mei, S.-G.; Zhang, G.-L. Synthesis and characterization of NiTiO3 yellow nano pigment with high solar radiation reflection efficiency. Powder Technol. 2013, 235, 303–306. [Google Scholar] [CrossRef]
- Giraldi, T.R.; Dias, J.A.; Baggio, C.M.; Maestrelli, S.C.; Oliveira, J.A. Anatase-to-rutile transition in co-doped TiO2 pigments. J. Sol-Gel Sci. Technol. 2017, 83, 115–123. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis, structural characterisation and optical properties of nanoparticles of MTiO3 (M = Ni and Co) obtained by the chemical method. Int. J. Nanoparticles 2013, 6, 28–37. [Google Scholar] [CrossRef]
- Zou, J.; Chen, Y.P.; Zhang, P. Influence of Crystallite size on color properties and NIR reflectance of TiO2@NiTiO3 inorganic pigments. Ceram. Int. 2021, 47, 12661–12666. [Google Scholar] [CrossRef]
- Saber, N.B.; Mezni, A.; Alrooqi, A.; Altalhi, T. Facile one-pot solvothermal approach to produce inorganic binary TiO2@NiTiO3 and ternary Au-TiO2@NiTiO3 yellow nano-pigment for environmental and energy use. Mater. Res. Express 2021, 8, 045016. [Google Scholar] [CrossRef]
- Thejus, P.K.; Krishnapriya, K.V.; Nishanth, K.G. A cost-effective intense blue colour inorganic pigment for multifunctional cool roof and anticorrosive coatings. Sol. Energy Mater. Sol. Cells 2021, 219, 110778. [Google Scholar] [CrossRef]
- Zhang, L.B.; Wu, P.; Chen, H.; Yuan, L.; Yang, G.; Xie, H.; Liang, D.; Xie, J.; Deng, L. Effect of calcination temperature on visible near-infrared reflectance of aluminum-doped chromium. Mater. Sci. Semicond. Process. 2020, 105, 104672. [Google Scholar] [CrossRef]
- Gabal, M.A.; Angari, Y.M.; Obaid, A.Y. Structural characterization and activation energy of NiTiO3 nanopowders prepared by the co-precipitation and impregnation with calcinations. C. R. Chim. 2013, 16, 704–711. [Google Scholar] [CrossRef]
- He, R.L.; Hocking, R.K.; Tsuzuki, T. Co-doped ZnO nanopowders: Location of cobalt and reduction in photocatalytic activity. Mater. Chem. Phys. 2012, 132, 1035–1040. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Reddy, B.P.; Prakash, B.P.; Park, S.-H. Effects of annealing temperature on phase transformation of CoTiO3 nanoparticles and on their structural, optical, and magnetic properties. J. Supercond. Nov. Magn. 2020, 33, 407–415. [Google Scholar] [CrossRef]
- Sadjadi, M.S.; Zare, K.; Khanahmadzadeh, S.; Enhessari, M. Structural characterization of NiTiO3 nanopowders prepared by stearic acid gel method. Mater. Lett. 2008, 62, 3679–3681. [Google Scholar] [CrossRef]
- Lu, J.; Jiang, Y.; Zhang, Y.; Huang, J.; Xu, Z. Preparation of gas sensing CoTiO3 nanocrystallites using EDTA as the chelating agent in a sol-gel process. Ceram. Int. 2015, 41, 3714–3721. [Google Scholar] [CrossRef]
- Yu, F.; Yang, J.; Ma, J.; Du, J.; Zhou, Y. Preparation of nanosized CoAl2O4 powders by sol-gel and sol-gel-hydrothermal methods. J. Alloys Compd. 2009, 468, 443–446. [Google Scholar] [CrossRef]
- Gabal, M.A.; Hameed, S.A.; Obaid, A.Y. CoTiO3 via cobalt oxalate-TiO2 precursor. Synthesis and characterization. Mater. Charact. 2012, 71, 87–94. [Google Scholar] [CrossRef]
- Ai, S.H.; Zheng, H.D.; Yu, J.C. Preparation and reflectance spectrum modulation of Cr2O3 green pigment by solution combustion synthesis. Materials 2020, 13, 1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, S.; Danaee, I. Effect of preparation method on the anti-corrosive properties of nanocrystalline Zn-CoO ceramic pigments. Mater. Corros. 2011, 62, 405–410. [Google Scholar] [CrossRef]
- Anjana, P.S.; Sebastian, M.T. Synthesis, characterization, and microwave dielectric properties of ATiO3 (A = Co, Mn, Ni) ceramics. J. Am. Ceram. Soc. 2010, 89, 2114–2117. [Google Scholar] [CrossRef]
- Tong, Y.P.; Fu, J.; Chen, Z. Synthesis, characterization, and NIR reflectance of highly dispersed NiTiO3 and NiTiO3/TiO2 composite pigments. J. Nanomater. 2016, 52, 829–883. [Google Scholar] [CrossRef]
- Legodi, M.A.; de Waal, D. The preparation of magnetite, goethite, hematite and maghemite of pigment quality from mill scale iron waste. Dye. Pigment. 2007, 74, 161–168. [Google Scholar] [CrossRef]
- Demarchis, L.; Sordello, F.; Minella, M.; Minero, C. Tailored properties of hematite particles with different size and shape. Dye. Pigment. 2015, 115, 204–210. [Google Scholar] [CrossRef]
- He, X.; Wang, F.; Liu, H.; Li, J.; Niu, L. Synthesis and coloration of highly dispersed NiTiO3@TiO2 yellow pigments with core-shell structure. J. Eur. Ceram. Soc. 2017, 37, 2965–2972. [Google Scholar] [CrossRef]
- Chang, Y.; He, P.; Wei, Z.; Chen, Y.; Wang, H.; Wu, C.; Zhou, Z.; Huang, H.; Kowalska, E.; Dong, S. Three-dimensional monodispersed TiO2 microsphere network formed by a sub-zero sol-gel method. Mater. Lett. 2020, 268, 127592. [Google Scholar] [CrossRef]
- Mathpal, M.C.; Tripathi, A.K.; Singh, M.K.; Gairola, S.P.; Pandey, S.N.; Agarwal, A. Effect of annealing temperature on Raman spectra of TiO2 nanoparticles. Chem. Phys. Lett. 2012, 555, 182–186. [Google Scholar] [CrossRef]
- Lopes, K.P.; Cavalcante, L.S.; Simões, A.Z.; Varela, J.A.; Longo, E.; Leite, E.R. NiTiO3 powders obtained by polymeric precursor method: Synthesis and characterization. J. Alloys Compd. 2009, 468, 327–332. [Google Scholar] [CrossRef]
- Zou, J. Low temperature preparation of Cr-doped rutile pigments with good colour properties. Dye. Pigment. 2013, 97, 71–76. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kothari, A.V.; Gupta, V.K. Preparation and characterization of bi- and trimetallic titanium based oxides. Dye. Pigment. 2011, 89, 149–154. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Guo, W.; Huang, Y.; Chang, Y.; Wei, Z.; Jiang, J.; Boulet, P.; Record, M.-C. Preparation and Chromaticity Control of CoTiO3/NiTiO3 Co-Coated TiO2 Composite Pigments. Materials 2022, 15, 1456. https://doi.org/10.3390/ma15041456
Chen Y, Guo W, Huang Y, Chang Y, Wei Z, Jiang J, Boulet P, Record M-C. Preparation and Chromaticity Control of CoTiO3/NiTiO3 Co-Coated TiO2 Composite Pigments. Materials. 2022; 15(4):1456. https://doi.org/10.3390/ma15041456
Chicago/Turabian StyleChen, Yuan, Wei Guo, Yuan Huang, Ying Chang, Zhishun Wei, Jiuxin Jiang, Pascal Boulet, and Marie-Christine Record. 2022. "Preparation and Chromaticity Control of CoTiO3/NiTiO3 Co-Coated TiO2 Composite Pigments" Materials 15, no. 4: 1456. https://doi.org/10.3390/ma15041456





