Studying the Influence of Silica Fume on Bond Strength of the PCM-Concrete Interface under Shear Stress Condition
Abstract
:1. Introduction
2. Outline of the Experiment
2.1. Materials and Mix Proportion of Substrate Concrete
2.2. Polymer Cement Mortar (PCM)
2.3. Silica Fume
2.4. Primer
2.5. Surface Preparation
2.6. Preparation of the Composite Specimens
2.7. Experimental Procedure
2.8. Microstructure Test
2.9. Statistical Analysis
3. Experimental Results and Discussions
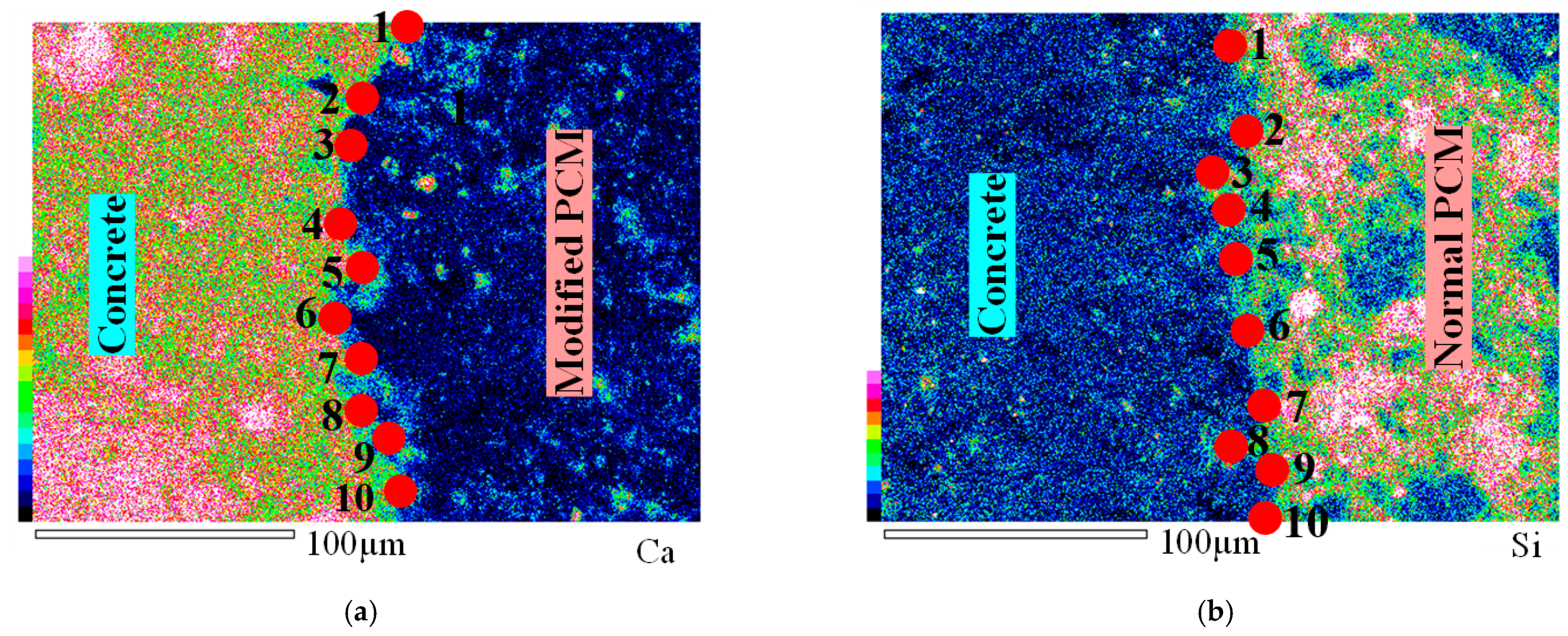
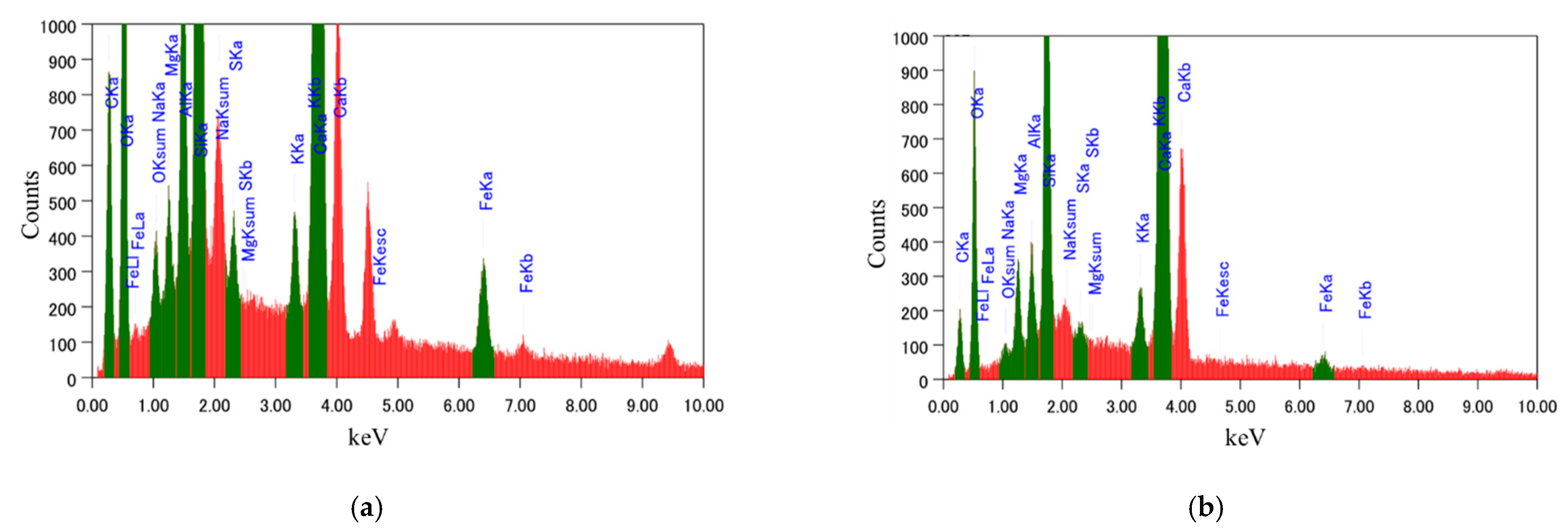
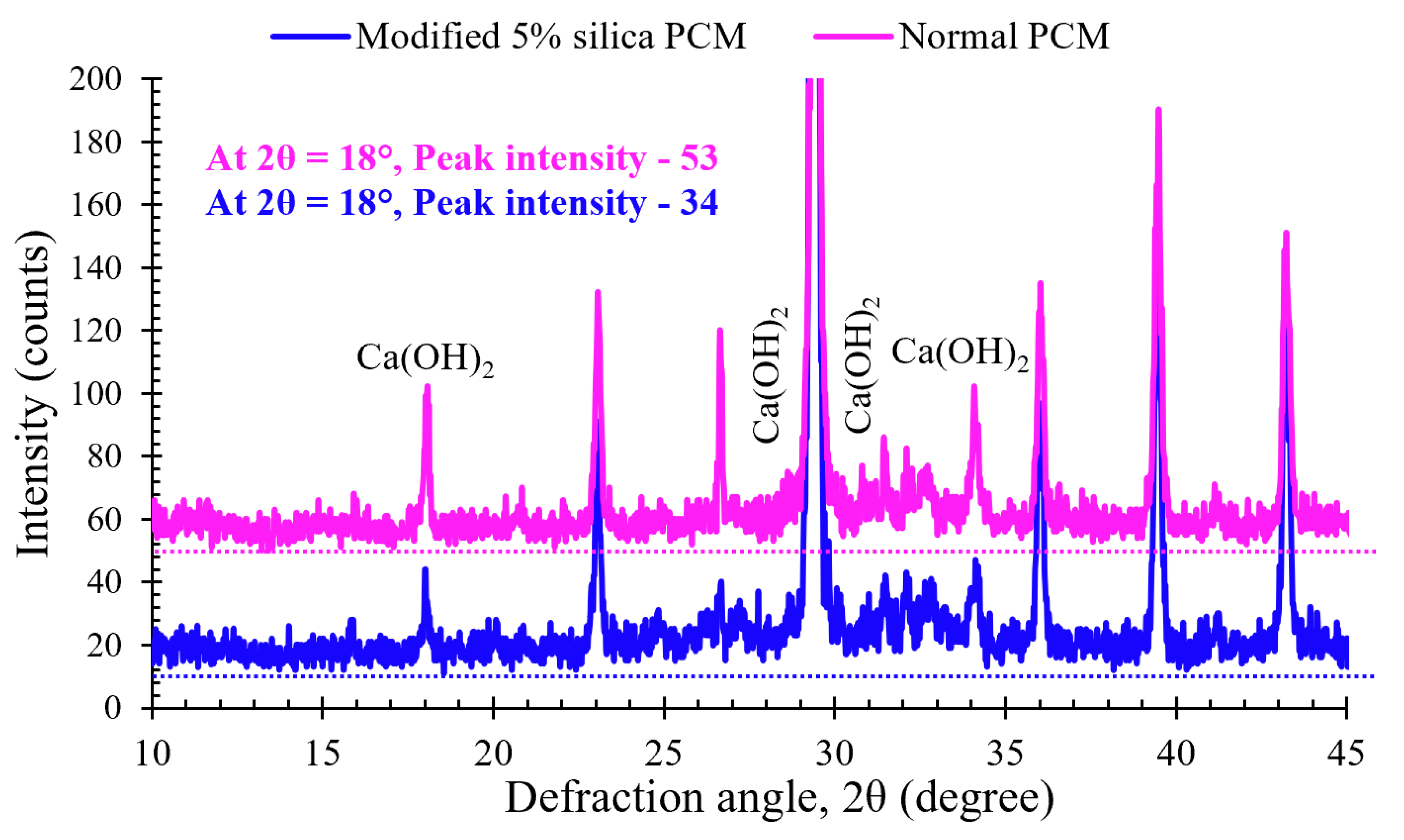
3.1. Microstructural Analysis
3.2. Series-I Testing
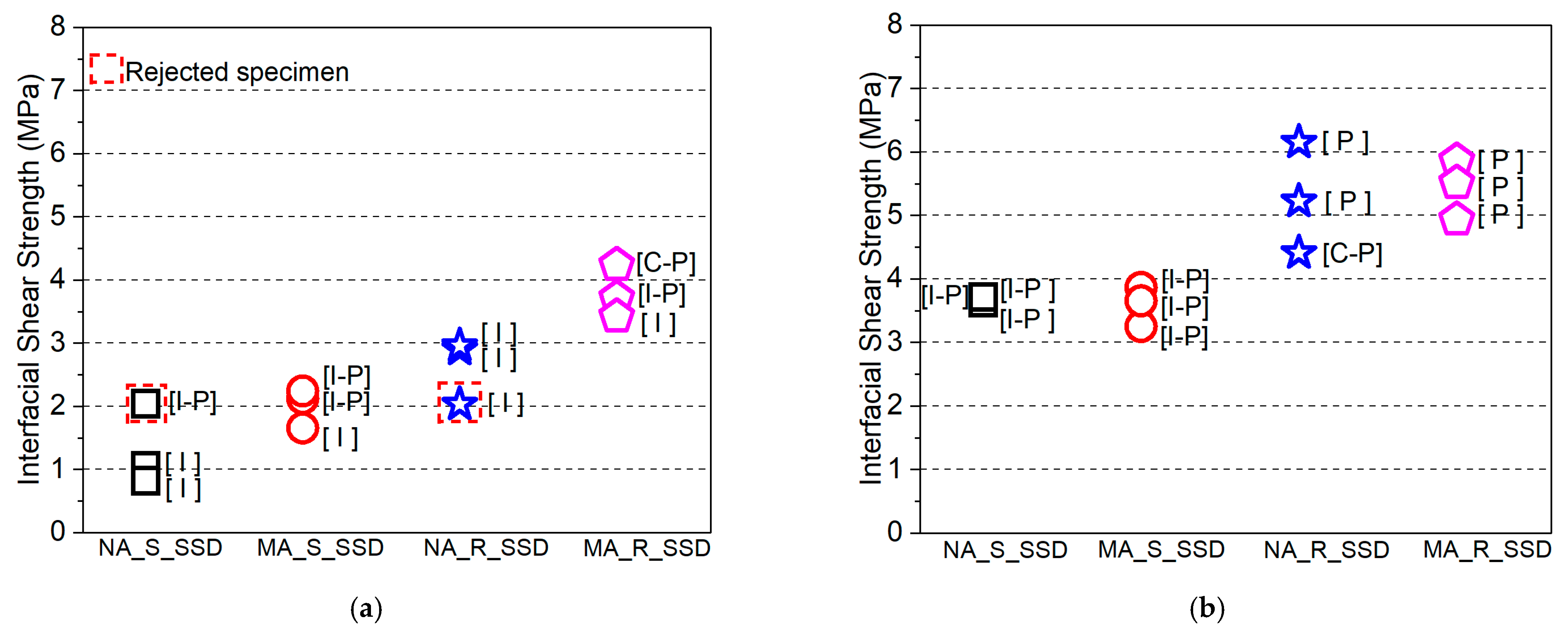
3.2.1. Maximum Stress Capacity
3.2.2. Effect of Surface Roughness Level
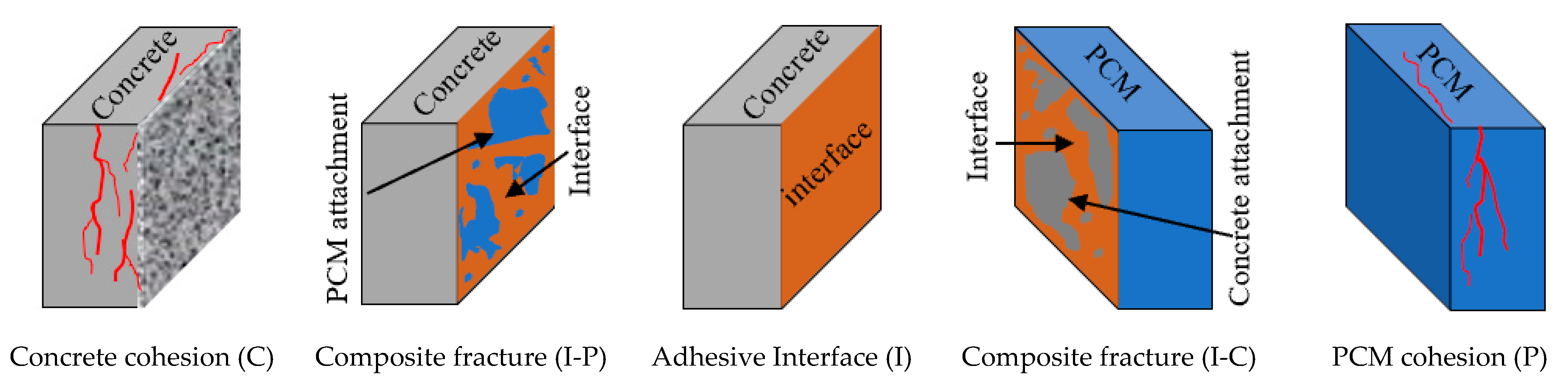
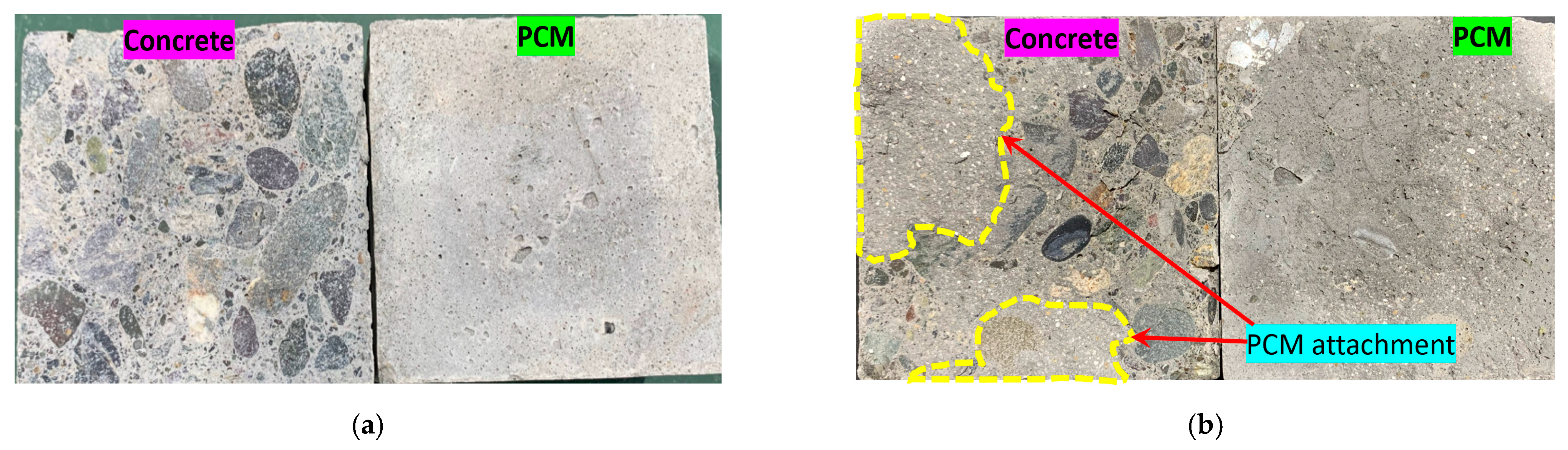
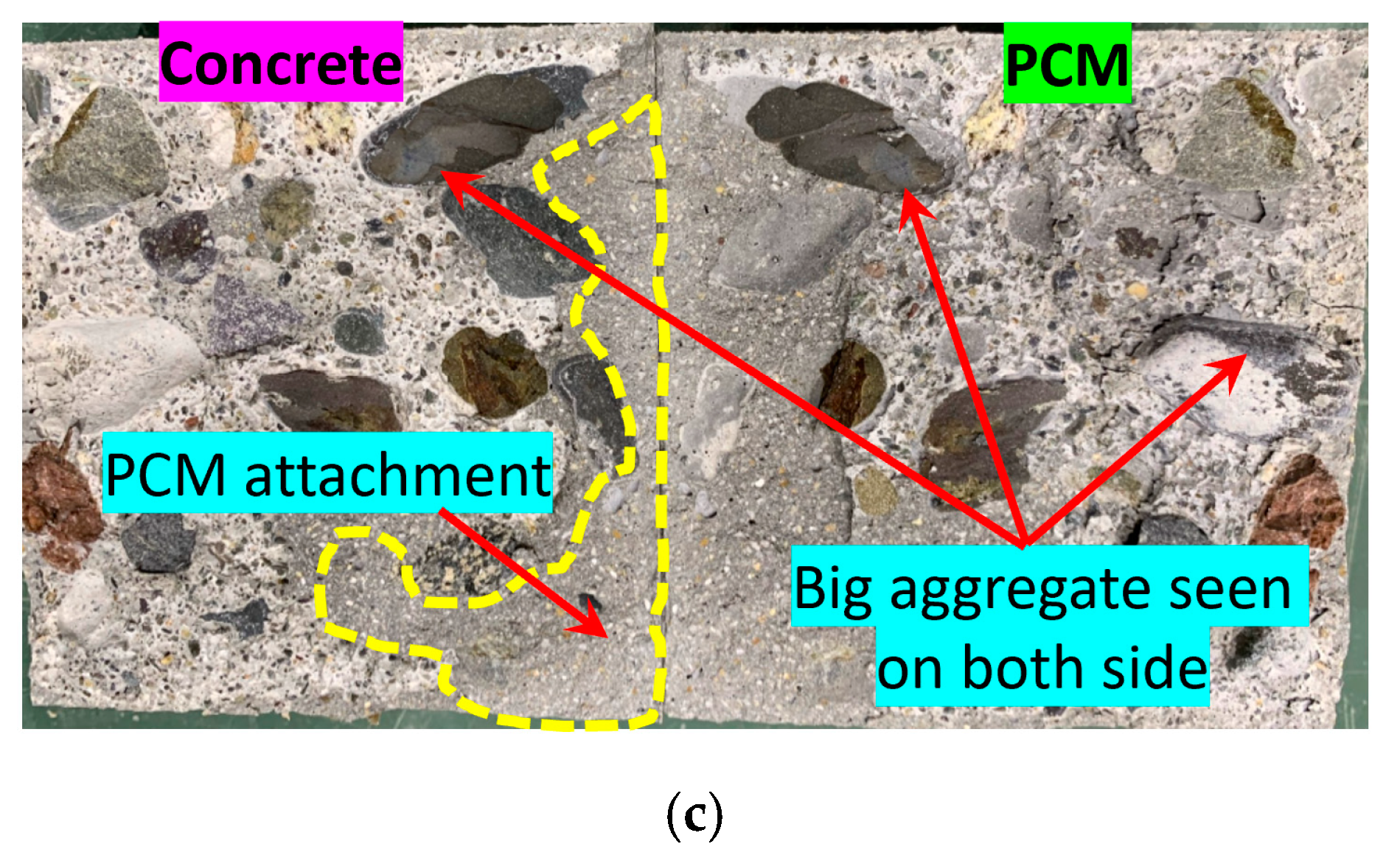
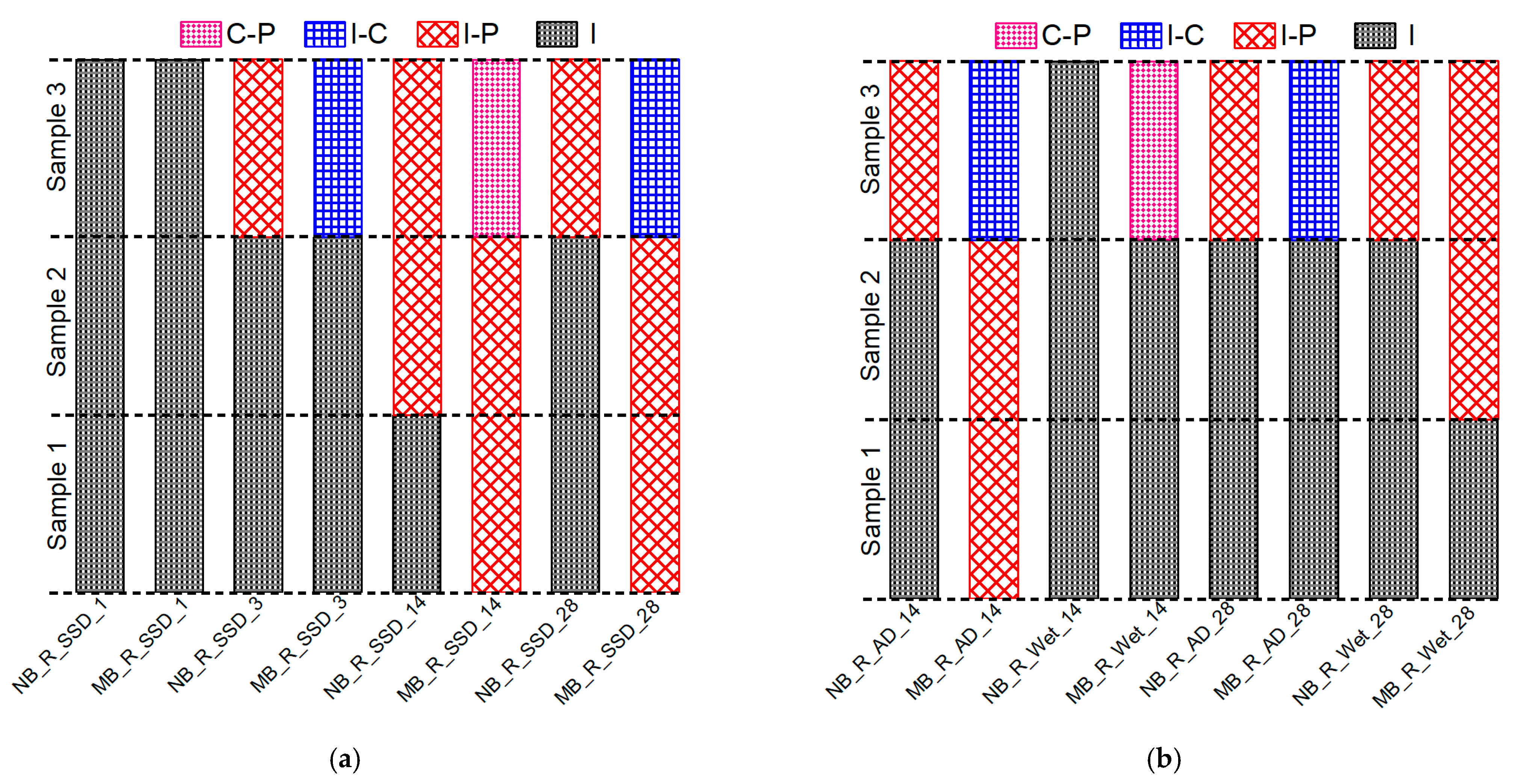
3.2.3. Fracture Modes of the Composite Specimens
3.2.3.1. Definition of the Fracture Mode
3.2.3.2. Observed Fracture Modes in the Composite Specimens
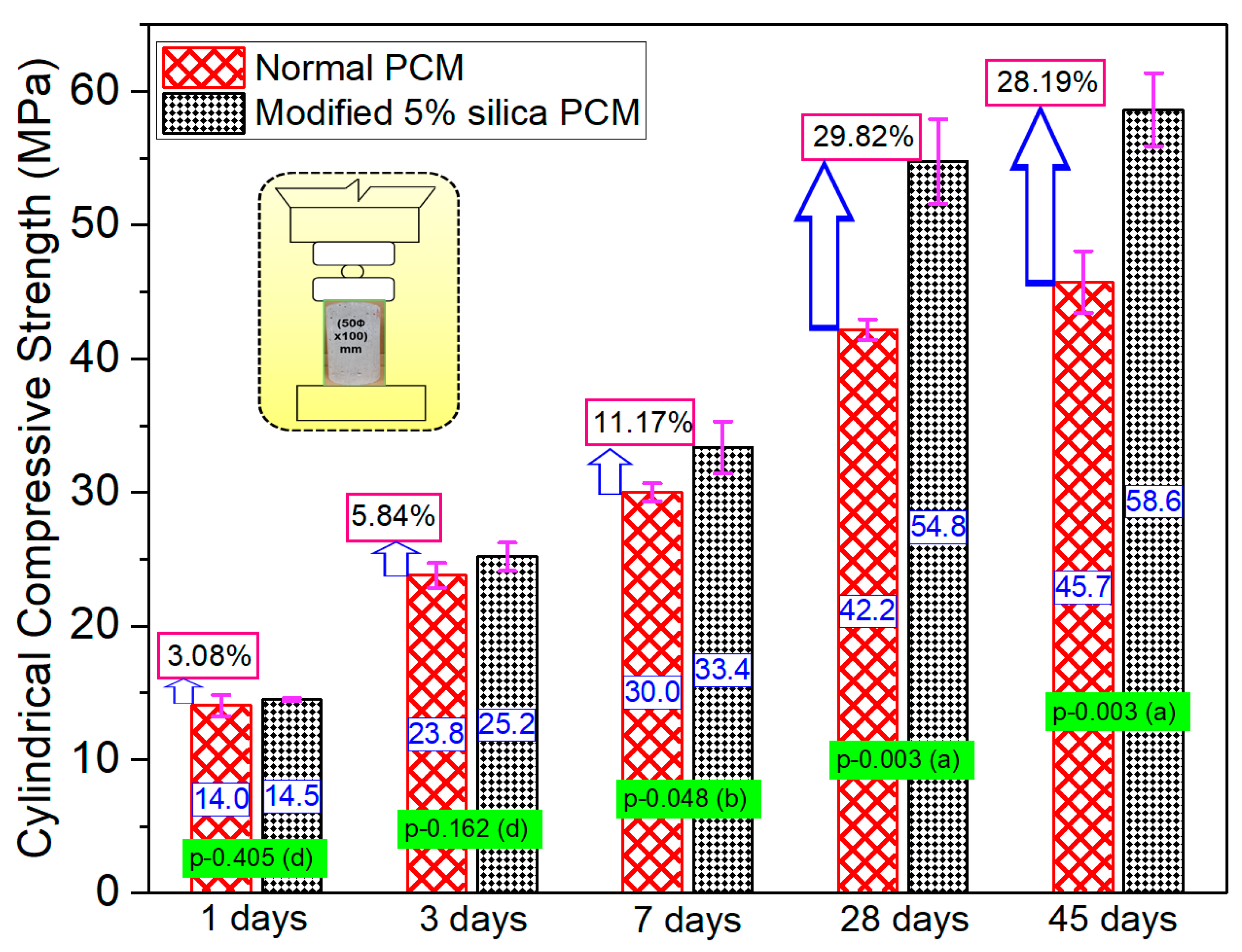
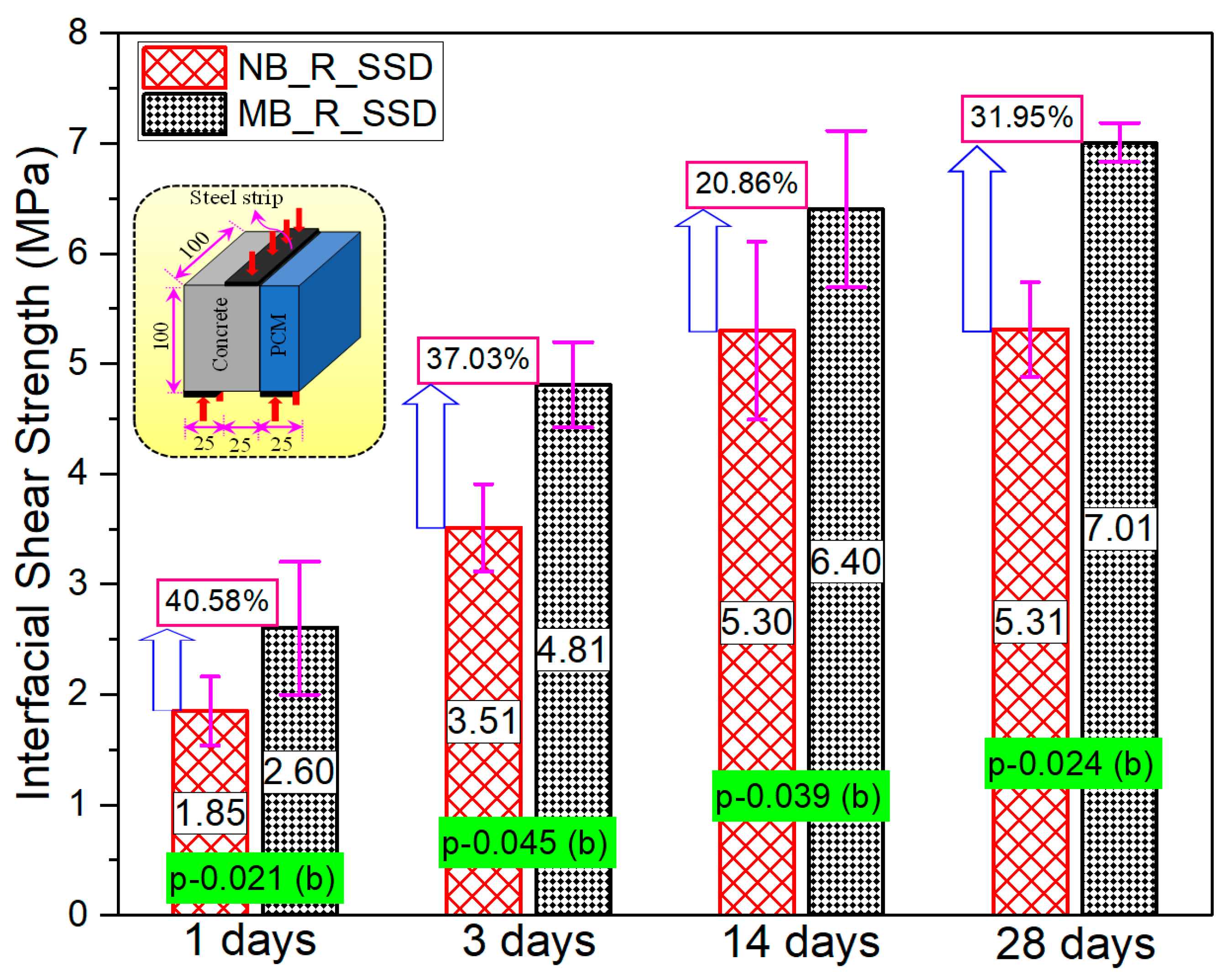
3.3. Series-II (Effect of Silica Fume at Early Ages)
3.3.1. Monolithic Specimen
3.3.2. Composite Specimen
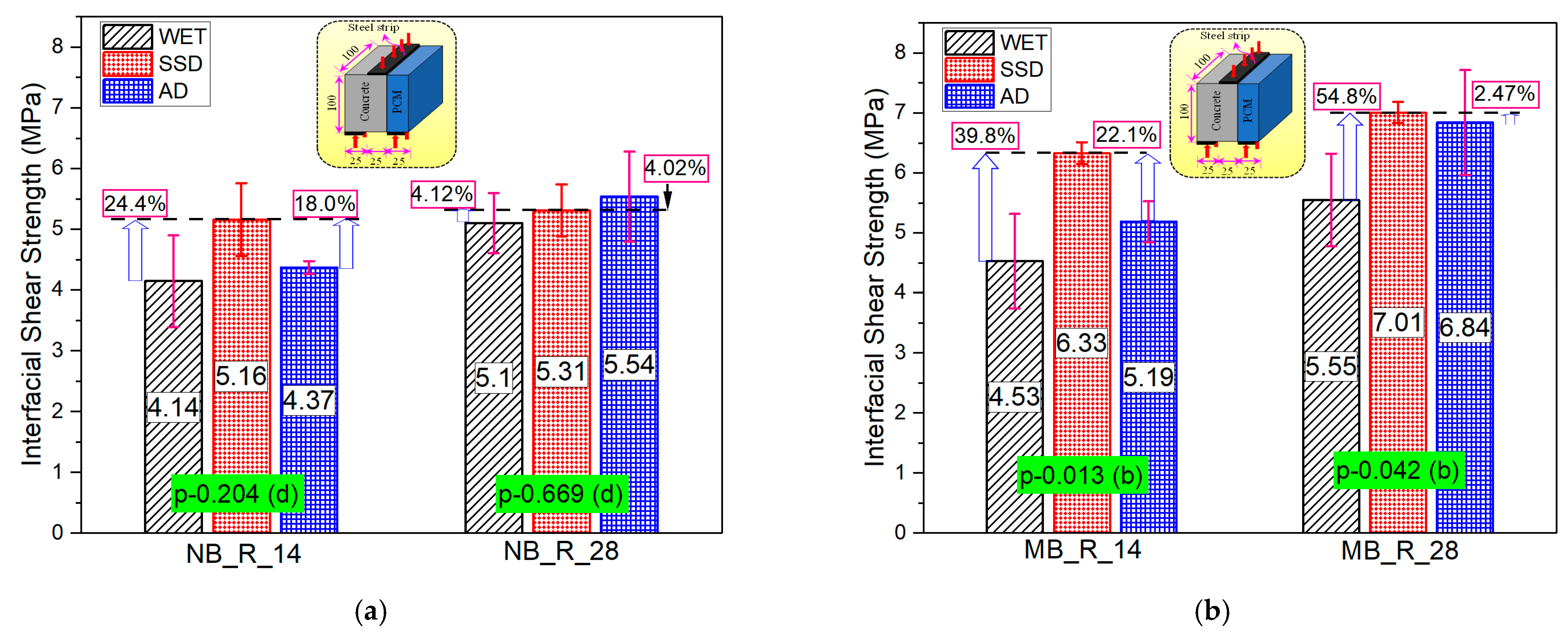
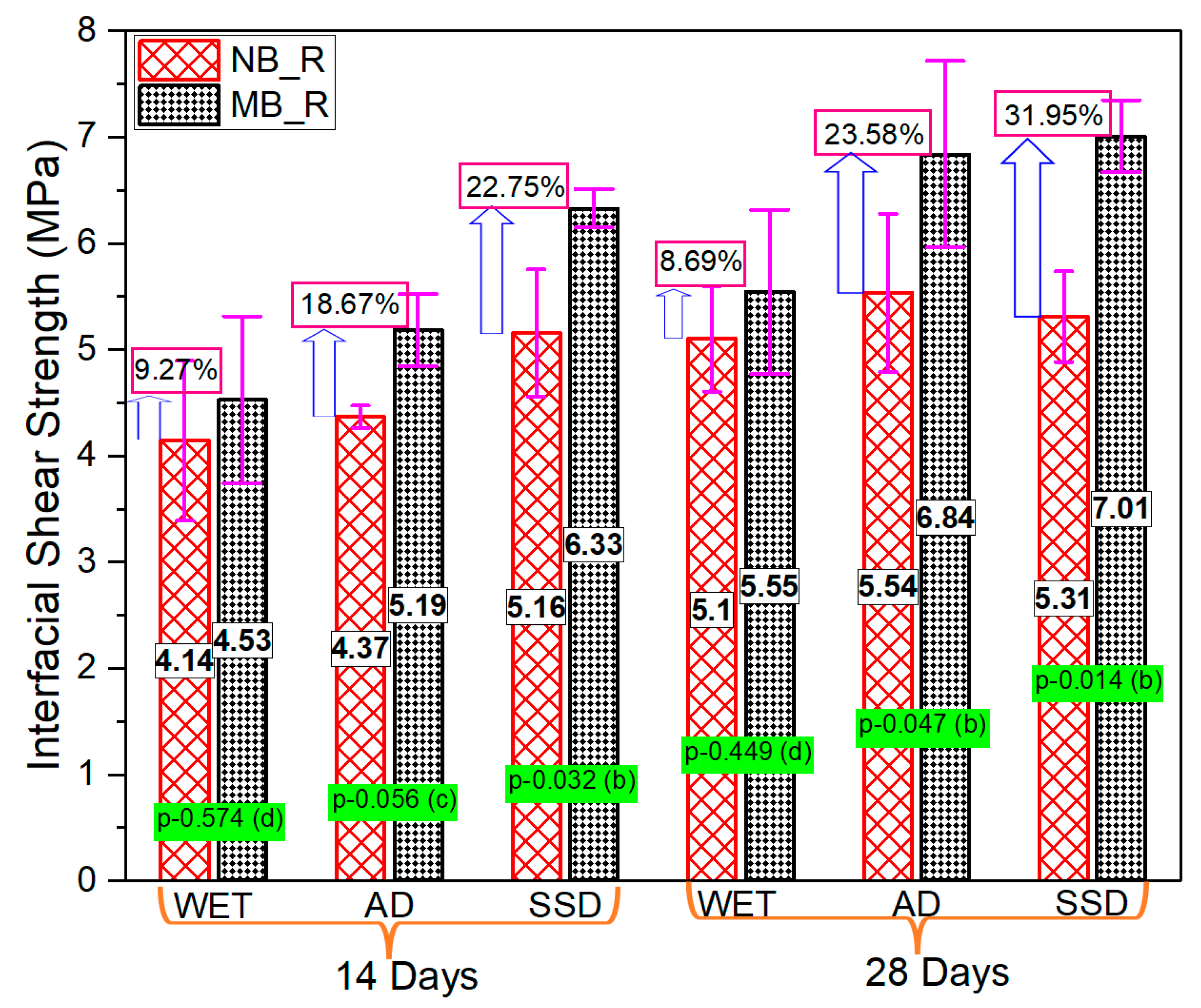
3.4. Series-III (Effect of the Moisture State of the Interface)
4. Conclusions
- In the absence of primer at the substrate concrete interface: (i) The effect of the constituent of the overlay material attached to the concrete substrate on the bond strength is significant. The PCM modified with 5% silica composite specimen showed higher interfacial shear strength than the normal PCM composite specimens. The inclusion of silica fume significantly increased the interfacial shear strength compared to the normal PCM for smooth and rough concrete surfaces. (ii) Surface preparation (texture) significantly affects the bond strength and a better bond performance under shear stress conditions was acquired with a rough interfacial surface. The interfacial shear strength increased with increasing surface roughness for both the normal PCM and PCM modified with 5% silica cases. (iii) The mixing of silica fume in the PCM tended to shift the pure interfacial fracture mode (I) to a composite fracture (I-P) or (I-C) or mixed fracture (C-P) mode due to higher adhesion of modified PCM overlay with substrate concrete.
- With a primed substrate concrete surface, the interfacial bond performance does not depend on the inclusion of silica fume with PCM as the primer forms a protective layer and hinders the functionality of silica fume.
- There is a high possibility that the bond strength increases significantly with the inclusion of silica fume from the very first day of pouring of overlay mortar due to the predominant reaction of silica compounds with Ca(OH)2 during the early hydration stage.
- The interfacial strength is predominantly influenced by the moistness of the substrate concrete surface, the saturated surface dry interface state of substrate concrete facilitates bond strength development. Interface moisture state exerted a positive influence on the modified 5% silica PCM-concrete bonding performance, while it had no/insignificant impact on the normal PCM-concrete interface.
- The lower C/S ratio observed in the microscopic SEM-EDS test at the modified 5% silica PCM-concrete interface implies high C-S-H content, resulting in high strength. This is mainly due to the transformation of harmful Ca(OH)2 into a large amount of C-S-H, which indicates the possibility of formation of chemical connections at the modified 5% silica PCM-concrete interface.
- A decrease in the Ca(OH)2 content observed qualitatively through XRD analysis and quantitatively through TG-DTA at the modified 5% silica PCM-concrete interface compared to the normal PCM-concrete interface. This suggests an increase in the extend of bond formation between silica compounds and free Ca(OH)2 (modified 5% silica PCM cases) compared to the bond formation in the without-silica fume (normal PCM) cases, thus contributing to the improvement of the interfacial performance in former cases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alam, M.S.; Kanakubo, T.; Yasojima, A. Shear-Peeling Bond Strength between Continuous Fibre Sheet and Concrete. ACI Struct. J. 2012, 109, 75–82. [Google Scholar]
- Alam, M.A.; Ali, S.J.; Zamin, M.J.; Kamal, N.M. Effective method of repairing RC beam using externally bonded steel plate. Appl. Mech. Mater. 2014, 567, 399–404. [Google Scholar] [CrossRef]
- Tidarut, J.; Zhang, D.; Ueda, T. Prediction of the post-peak behavior of reinforced concrete columns with and without FRP-jacketing. Eng. Struct. 2013, 56, 1511–1526. [Google Scholar]
- Bruckner, A.; Ortlepp, R.; Curbach, M. Textile Reinforced Concrete for Strengthening in Bending and Shear. Mater. Struct. 2005, 38, 741–748. [Google Scholar] [CrossRef]
- Triantafillou, T.C. Recent developments in strengthening of concrete structures with advanced composites textile-reinforced mortar (TRM) jacketing. In Proceedings of the International Conference on Structural Composites for Infrastructure Applications, Alexandria, Egypt, 20–23 May 2005. [Google Scholar]
- Wu, H.C.; Sun, P. Fibre-reinforced cement-based composite sheets for structural retrofit. In Proceedings of the International Symposium on Bond Behavior of FRP in Structures (BBFS), Hong Kong, China, 7–9 December 2005. [Google Scholar]
- Taljsten, B.; Blanksvard, T. Mineral based bonding of CFRP to strengthen concrete structures. J. Compos. Constr. 2007, 11, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Bisby, L.; Stratford, T.; Smith, J.; Halpin, S. FRP versus fibre reinforced cementitious mortar systems at elevated temperature. Am. Concr. Inst. 2011, 275, 1–20. [Google Scholar]
- D’Ambrisi, A.; Feo, L.; Focacci, F. Experimental and analytical investigation on bond between Carbon-FRCM materials and masonry. Compos. Part B Eng. 2013, 46, 15–20. [Google Scholar] [CrossRef]
- Satoh, K.; Kodama, K. Central peeling failure behavior of polymer cement mortar retrofitting of reinforced concrete beams. J. Mater. Civ. Eng. 2005, 17, 126–136. [Google Scholar] [CrossRef]
- Zhang, D.W.; Ueda, T.; Furuuchi, H. Fracture mechanisms of polymer cement mortar: Concrete interfaces. J. Eng. Mech. 2013, 139, 167–176. [Google Scholar] [CrossRef]
- Rashid, K.; Wang, Y.; Ueda, T. Influence of continuous and cyclic temperature durations on the performance of polymer cement mortar and its composite with concrete. Compos. Struct. 2019, 215, 214–225. [Google Scholar] [CrossRef]
- Hassan, K.E.; Brooks, J.J.; Al-Alawi, L. Compatibility of repair mortars with concrete in a hot-dry environment. Cem. Concr. Compos. 2001, 23, 93–101. [Google Scholar] [CrossRef]
- Chung, D.D.L. Use of polymers for cement-based structural materials. J. Mater. Sci. 2004, 39, 973–2978. [Google Scholar] [CrossRef]
- Mirza, J.; Mirza, M.S.; Lapointe, R. Laboratory and field performance of polymer-modified cement-based repair mortars in cold climates. Constr. Build. Mater. 2002, 16, 365–374. [Google Scholar] [CrossRef]
- Al-Zahrani, M.M.; Maslehuddin, M.; Al-Dulaijan, S.U.; Ibrahim, M. Mechanical properties and durability characteristics of polymer and cement-based repair materials. Cem. Concr. Compos. 2003, 25, 527–537. [Google Scholar] [CrossRef]
- Zhong, S.Y.; Chen, Z.Y. Properties of latex blends and its modified cement mortars. Cem. Concr. Res. 2002, 32, 1515–1524. [Google Scholar] [CrossRef]
- Yang, Z.X.; Shi, X.M.; Creighton, A.T.; Peterson, M.M. Effect of styrene-butadiene rubber latex on the chloride permeability and microstructure of Portland cement mortar. Constr. Build. Mater. 2009, 23, 2283–2290. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.M. Function of styrene-acrylic ester copolymer latex in cement mortar. Mater. Struct. 2010, 43, 443–451. [Google Scholar] [CrossRef]
- Sakai, E.; Sugita, J. Composite Mechanism of Polymer Modified Cement. Cem. Concr. Res. 1995, 25, 127–135. [Google Scholar] [CrossRef]
- Brien, J.V.; Mahboub, K.C. Influence of polymer type on adhesion performance of blended cement mortar. Int. J. Adhes. Adhes. 2013, 43, 7–13. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Ueda, T.; Jun, T. Finite element analysis of RC beam strengthened with PCM. In Proceedings of the Japan Concrete Institute, Kanazawa, Japan, 17–19 June 2019; Volume 41. [Google Scholar]
- Zhang, D.; Ueda, T.; Furruchi, H. Concrete cover separation failure of overlay strengthened reinforced concrete beams. Constr. Build. Mater. 2012, 26, 735–745. [Google Scholar] [CrossRef]
- Zhang, D.; Ueda, T.; Furuuchi, H. Intermediate Crack Debonding of Polymer Cement Mortar Overlay-Strengthened RC Beam. J. Mater. Civ. Eng. 2011, 23, 857–865. [Google Scholar] [CrossRef]
- Rashid, K.; Ueda, T.; Zhang, D. Study on Shear Behavior of Concrete-polymer Cement Mortar at Elevated Temperature. Civ. Eng. Dimens. 2016, 18, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Zanotti, C.; Talukdar, S.; Banthia, N. A state-of-the-art on concrete repairs and some thoughts on ways to achieve durability in repairs. In Infrastructure Corrosion and Durability—A Sustainability Study; OMICS Group eBooks: Hyderabad, Telangana, 2014. [Google Scholar]
- Gao, J.M.; Qian, C.X.; Wang, B.; Morino, K. Experimental study on properties of polymer-modified cement mortars with silica fume. Cem. Concr. Res. 2002, 32, 41–45. [Google Scholar] [CrossRef]
- Jiang, C.H.; Zhou, X.B.; Huang, S.S.; Chen, D. Influence of polyacrylic ester and silica fume on the mechanical properties of mortar for repair application. Adv. Mech. Eng. 2017, 9, 1687814016683856. [Google Scholar] [CrossRef]
- Das, K. The effect of Silica Fume on the Properties of Concrete as Defined in Concrete Society Report 74, Cementitious Materials. In Proceedings of the 37th Conference on Our World in Concrete and Structures, Singapore, 29–31 August 2012. [Google Scholar]
- Hayato, S.; Mahmudul, H.M.; Ueda, T.; Katsuichi, M.; Jun, T. A study on the effect of silica fume and surface penetrant on bonding strength for overlaying. J. Asian Concr. Fed. 2019, 5, 1–14. [Google Scholar]
- Mahmudul, H.M.; Ueda, T.; Koji, M. Enhancement of the concrete-PCM interfacial bonding strength using silica fume. Constr. Build. Mater. 2020, 259, 119774. [Google Scholar]
- Xie, H.; Li, G.; Xiong, G. Microstructure model of the interfacial zone between fresh and old concrete. J. Wuhan Univ. Technol. 2002, 17, 64–68. [Google Scholar]
- Sugawara, S.; Koizumi, T.; Harada, S.; Okazawa, K. Effect of reaction ratio of silica fume on strength development of 150N/mm2 class concrete. Concr. Res. Technol. 2007, 18, 2007. [Google Scholar]
- Paramasivam, P.; Ong, K.C.; Xu, W. Mechanical Behavior of the Interface between Substrate and Repair Material. ACI SP 2002, 206, 71–90. [Google Scholar]
- Saldanha, R.; Julio, E.; da Dias-Costa, D.; Santos, P. A modified slant shear test designed to enforce adhesive failure. Constr. Build. Mater. 2013, 41, 673–680. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Hooton, R.D.; Zhang, X.W. Effects of interface roughness and interface adhesion on new-to-old concrete bonding. Constr. Build. Mater. 2017, 151, 582–590. [Google Scholar] [CrossRef]
- Fowler, D.W. Polymers in Concrete: A Vision for the 21st Century. Cem. Concr. Compos. 1992, 21, 449–452. [Google Scholar] [CrossRef]
- Ueda, T.; Rashid, K.; Qian, Y.; Zhang, D. Effects of Temperature and moisture on concrete-PCM interface performance. Sustain. Civ. Eng. Struct. Constr. Mater. 2017, 171, 71–79. [Google Scholar]
- Ohama, Y. Handbook of Polymer-Modified Concrete and Mortars-Properties and Process Technology; Noyes Publications: Park Ridge, NJ, USA, 1995. [Google Scholar]
- Santos, P.M.D.; Julio, E.N.B.S. Factors affecting bond between new and old concrete. ACI Mater. J. 2011, 108, 449–456. [Google Scholar]
- Li, S.; Geissert, D.G.; Li, S.E.; Frantz, G.C.; Stephens, E.J. Durability and Bond of High-Performance Concrete and Repaired Portland Cement Concrete; University of Connecticut: Storrs, CT, USA, 1997. [Google Scholar]
- Momayez, A.; Ramezanianpour, A.; Rajaie, H.; Ehsani, M.R. Bi-Surface Shear Test for Evaluating Bond between Existing and New Concrete. ACI Mater. J. 2004, 101, 99–106. [Google Scholar]
- Kan, L.; Zhiqiang, W.; Hongxia, Q.; Chenggong, L.; Hakuzweyezu, T. PCM-Concrete Interfacial Tensile Behavior Using Nano-SiO2 Based on Splitting-Tensile Test. J. Adv. Concr. Technol. 2021, 19, 321–334. [Google Scholar]
- Zhifu, W. Interfacial Shear Bond Strength between Old and New Concrete. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2011. [Google Scholar]
- Afridi, M.; Ohama, Y.; Iqbal, M.Z.; Demura, K. Behavior of Ca(OH)2 in polymer-modifed mortars. Int. J. Cem. Compos. Lightweight Concr. 1989, 11, 235–244. [Google Scholar] [CrossRef]
- Senff, L.; João, A.L.; Victor, M.F.; Dachamir, H.; Wellington, L.R. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Hasan, B.; Nihal, S. Comparative Study of the Characteristics of Nano Silica, Silica Fume and Fly Ash–Incorporated Cement Mortars. Mater. Res. 2014, 17, 570–582. [Google Scholar]
- Shuling, G.; Xiaochong, Z.; Jinli, Q.; Yadong, G.; Guanhua, H. Study on the bonding properties of Engineered Cementitious Composites (ECC) and existing concrete exposed to high temperature. Constr. Build. Mater. 2019, 196, 330–344. [Google Scholar]
- Alireza, V.; Azadeh, J.J.; Islam, M.M.; Atorod, A. Experimental evaluation of concrete-to-UHPC bond strength with correlation to surface roughness for repair application. Constr. Build. Mater. 2020, 238, 117753. [Google Scholar]
- Sahmaran, M.; Yücel, H.E.; Yildirim, G. Investigation of the bond between the concrete substrate and ECC overlays. J. Mater. Civ. Eng. 2013, 26, 167–174. [Google Scholar] [CrossRef]
- Yu, J.T.; Xu, W.L.; Zhang, Y.M. Experiment study on fracture property of ECC-concrete interface. J. Build. Mater. Struct. 2015, 18, 958–963. [Google Scholar]
- Wang, B.; Xu, S.; Liu, F. Evaluation of tensile bonding strength between UHTCC repair materials and concrete substrate. Constr. Build. Mater. 2016, 112, 595–606. [Google Scholar] [CrossRef]
- ASTM C39/C39M-05; Standard Test Method for Compressive Strength of Cylindrical Concrete Specimens. American Standard of Testing Materials: West Conshohocken, PA, USA, 2014.
- Miura, S. A Study on Interface Shear Behavior of PCM-Strengthened Concrete under High Temperature. Master’s Thesis, Hokkaido University, Hokkaido, Japan, 2017. [Google Scholar]






| W/C (%) | Amount (kg/m3) | Compressive Strength (MPa) | ||||
|---|---|---|---|---|---|---|
| Water | Cement | Sand | Stone | Water Reducer | ||
| 40 | 164 | 411 | 844 | 1045 | 8 | 37.84 |
| Appearance | Specific Surface Area (m2/g) | Relative Density (kg/m3) | Specific Gravity | Average Particle Size (µm) | pH |
|---|---|---|---|---|---|
| Grey ultrafine | 15–30 | 150–700 | 2.2–2.3 | 0.15 | 7–8 |
| Appearance | Main Component | Solid Content (%) | Density (g/cm3) | Stickiness (mPa·s) | pH |
|---|---|---|---|---|---|
| Milky white liquid | Modified Vinyl Acetate-Ethylene Copolymer Emulsion | 45–48 | 1.06 | 800–1200 | 4.5–6.5 |
| Test Type | Specimen Level | Roughness of Interface | Moistness of the Interface | No. of Specimens (Group × Number) | |
|---|---|---|---|---|---|
| Direct single-surface shear | Series-I | NA_S_SSD | Smooth (S) | SSD | 1 × 3 |
| MA_S_SSD | Smooth (S) | SSD | 1 × 3 | ||
| NA_S_SSD_WP | Smooth (S) | SSD | 1 × 3 | ||
| MA_S_SSD_WP | Smooth (S) | SSD | 1 × 3 | ||
| NA_R_SSD | Rough (R) | SSD | 1 × 3 | ||
| MA_R_SSD | Rough (R) | SSD | 1 × 3 | ||
| NA_R_SSD_WP | Rough (R) | SSD | 1 × 3 | ||
| MA_R_SSD_WP | Rough (R) | SSD | 1 × 3 | ||
| Bi-surface shear | Series-II | NB_R_SSD_1 | Rough (R) | SSD | 1 × 3 |
| MB_R_SSD_1 | Rough (R) | SSD | 1 × 3 | ||
| NB_R_SSD_3 | Rough (R) | SSD | 1 × 3 | ||
| MB_R_SSD_3 | Rough (R) | SSD | 1 × 3 | ||
| NB_R_SSD_14 | Rough (R) | SSD | 1 × 3 | ||
| MB_R_SSD_14 | Rough (R) | SSD | 1 × 3 | ||
| NB_R_SSD_28 | Rough (R) | SSD | 1 × 3 | ||
| MB_R_SSD_28 | Rough (R) | SSD | 1 × 3 | ||
| Series-III | NB_R_AD_14 | Rough (R) | AD | 1 × 3 | |
| MB_R_AD_14 | Rough (R) | AD | 1 × 3 | ||
| NB_R_AD_28 | Rough (R) | AD | 1 × 3 | ||
| MB_R_AD_28 | Rough (R) | AD | 1 × 3 | ||
| NB_R_Wet_14 | Rough (R) | Wet | 1 × 3 | ||
| MB_R_Wet_14 | Rough (R) | Wet | 1 × 3 | ||
| NB_R_Wet_28 | Rough (R) | Wet | 1 × 3 | ||
| MB_R_Wet_28 | Rough (R) | Wet | 1 × 3 | ||
| Criteria | Result |
|---|---|
| If F > F0.01 (r − 1, n − r) or p-value < 1% | Highly significant effect (a) |
| If F0.01 (r − 1, n − r) > F > F0.05 (r − 1, n − r) or p-value; 1~5% | Significant effect (b) |
| If F0.05 (r − 1, n − r) > F> F0.1 (r − 1, n − r) or p-value; 5~10% | Little effect (c) |
| If F < F0.10 (r − 1, n − r) or p-value > 10% | No or very little effect (d) |
| Element | Normal PCM | Modified 5% Silica PCM | ||
|---|---|---|---|---|
| Weight% | Atomic% | Weight% | Atomic% | |
| O | 44.38 | 59.86 | 43.55 | 53.67 |
| Fe | 0.82 | 0.32 | 2.36 | 0.83 |
| Mg | 1.46 | 1.30 | 0.66 | 0.54 |
| Al | 1.17 | 0.94 | 2.20 | 1.61 |
| Si | 7.20 | 5.53 | 9.57 | 6.72 |
| S | 0.26 | 0.18 | 0.55 | 0.34 |
| K | 0.74 | 0.41 | 0.55 | 0.28 |
| Ca | 37.53 | 20.21 | 23.95 | 11.78 |
| C | 6.10 | 10.96 | 13.89 | 22.81 |
| Sample | Second Step of TG | Third step of TG | Total mass % of Ca(OH)2 and CaCO3 |
|---|---|---|---|
| Mass % of Ca(OH)2 | Mass % of CaCO3 | ||
| Modified 5% silica PCM | 23.68 | 41.07 | 64.75 |
| Normal PCM | 29.73 | 57.80 | 87.53 |
| Factor | Interfacial Shear Strength (MPa) | SS | DF | MS | F Value | Fx | Level of Significance | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Interface moisture state | AD | 3.80 | 5.36 | 4.43 | 4.992 | 2 | 2.496 | 9.777 | F0.1 = 3.46 | Significant effect (D) |
| SSD | 6.13 | 6.38 | 6.48 | 1.532 | 6 | 0.255 | F0.05 = 5.14 | |||
| Wet | 5.40 | 5.36 | 4.80 | - | - | - | F0.01 = 10.92 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizan, M.H.; Matsumoto, K. Studying the Influence of Silica Fume on Bond Strength of the PCM-Concrete Interface under Shear Stress Condition. Materials 2022, 15, 1473. https://doi.org/10.3390/ma15041473
Mizan MH, Matsumoto K. Studying the Influence of Silica Fume on Bond Strength of the PCM-Concrete Interface under Shear Stress Condition. Materials. 2022; 15(4):1473. https://doi.org/10.3390/ma15041473
Chicago/Turabian StyleMizan, Mahmudul Hasan, and Koji Matsumoto. 2022. "Studying the Influence of Silica Fume on Bond Strength of the PCM-Concrete Interface under Shear Stress Condition" Materials 15, no. 4: 1473. https://doi.org/10.3390/ma15041473
APA StyleMizan, M. H., & Matsumoto, K. (2022). Studying the Influence of Silica Fume on Bond Strength of the PCM-Concrete Interface under Shear Stress Condition. Materials, 15(4), 1473. https://doi.org/10.3390/ma15041473





