Synthesis and Structural Characterization of CaO-P2O5-CaF:CuO Glasses with Antitumoral Effect on Skin Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Bioactive Glasses
2.3. Structural Characterization of Bioactive Glasses
2.3.1. Dissolution Tests
2.3.2. pH Evolution
2.3.3. FT-IR
2.3.4. EPR
2.3.5. SEM-EDX
2.4. Assessment of In Vitro Bioactivity Test
2.4.1. Cell Culture
2.4.2. Cytotoxicity Function In Vitro—MTT Assay
3. Results and Discussions
3.1. Structural Characterization
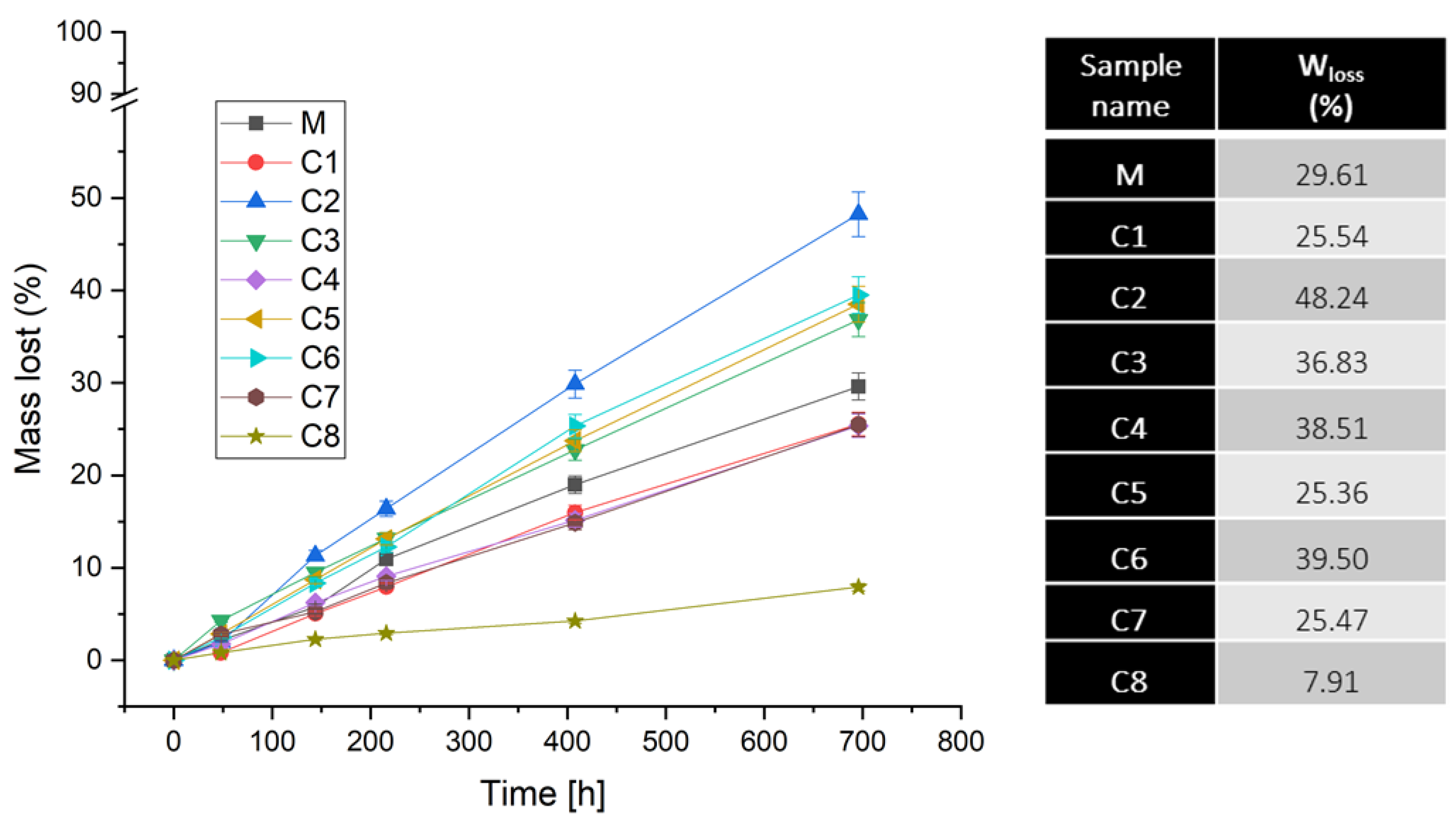
3.1.1. Dissolution Tests
3.1.2. pH Measurements
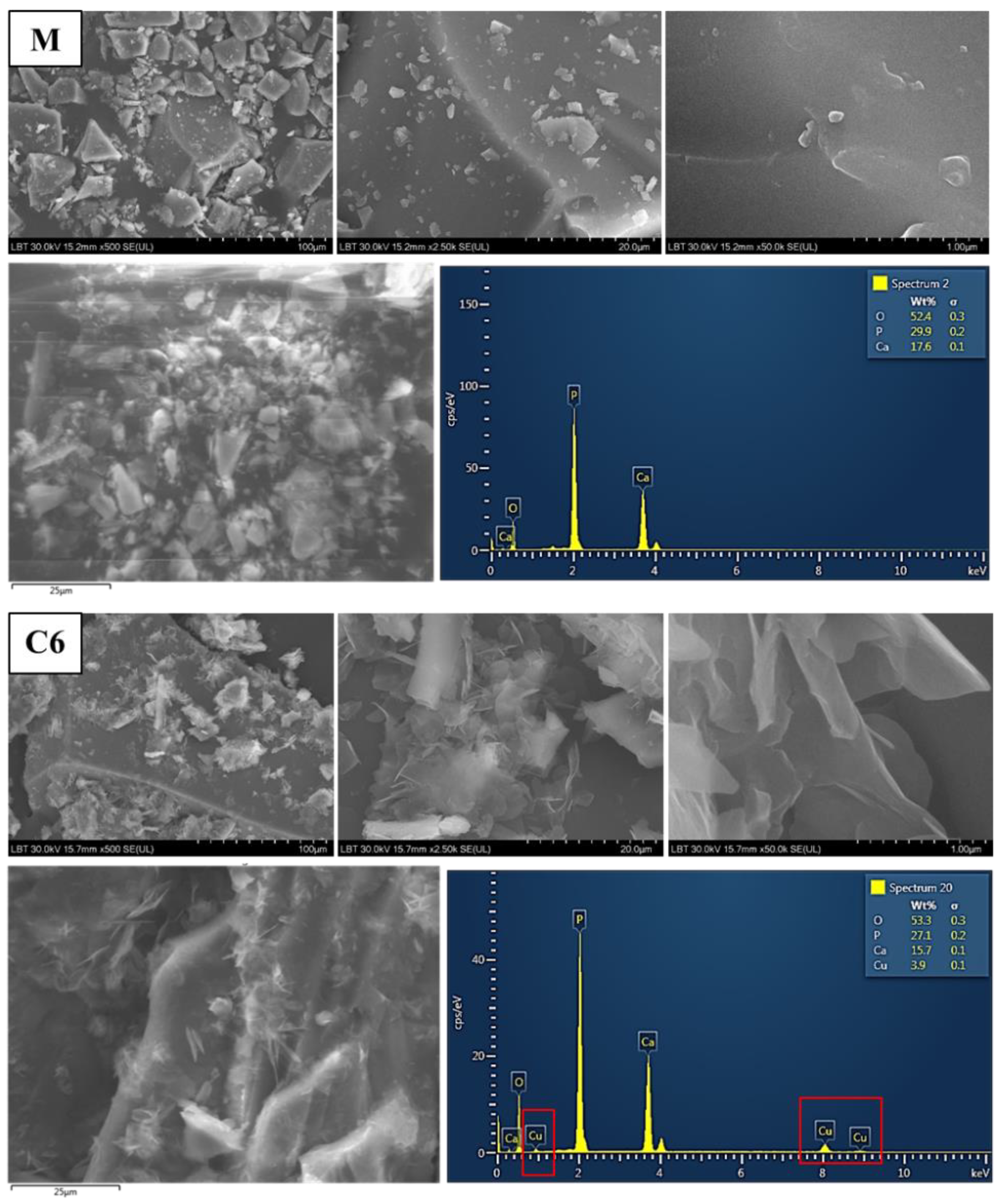
3.1.3. Surface Morphology and EDX
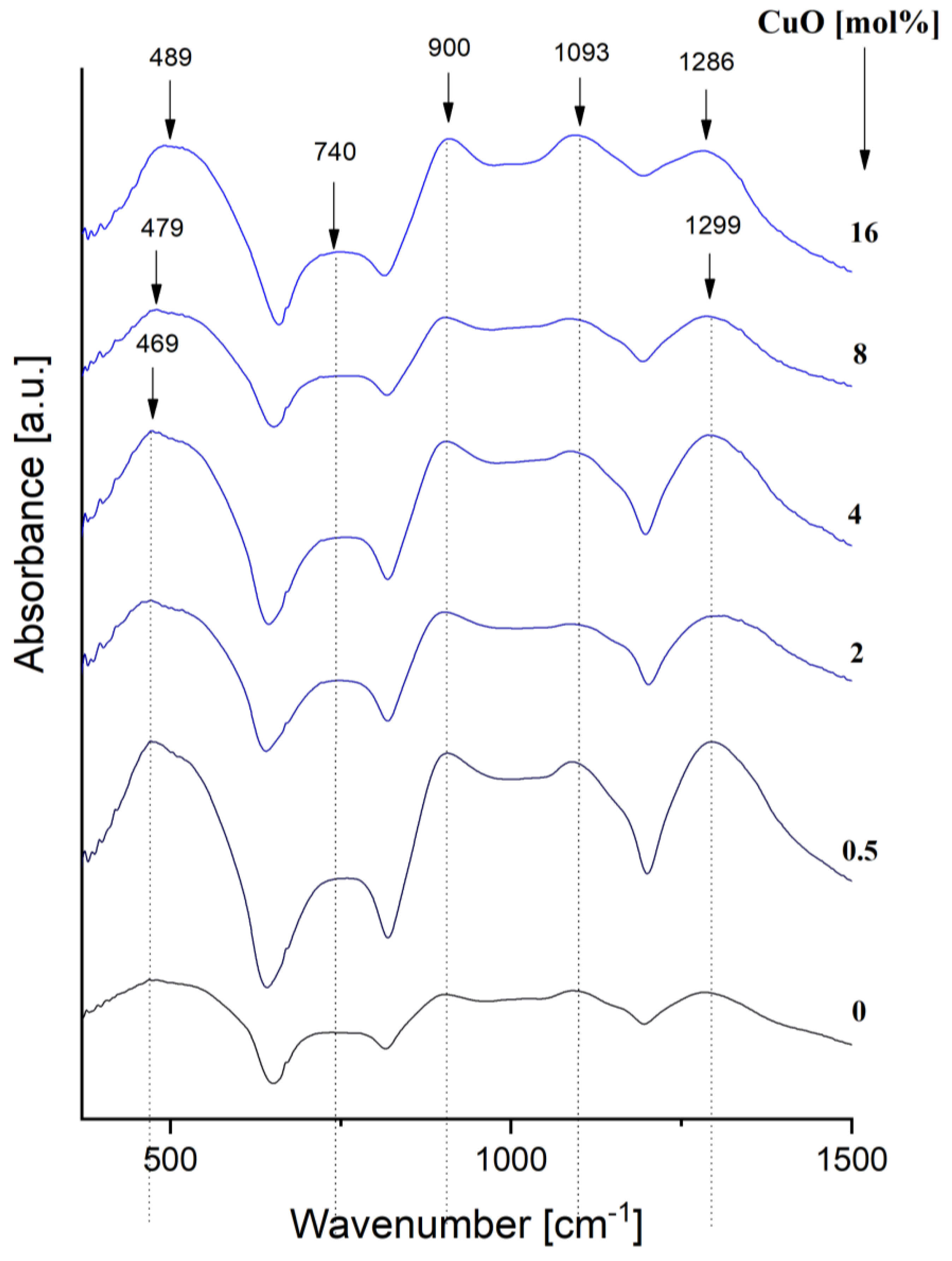
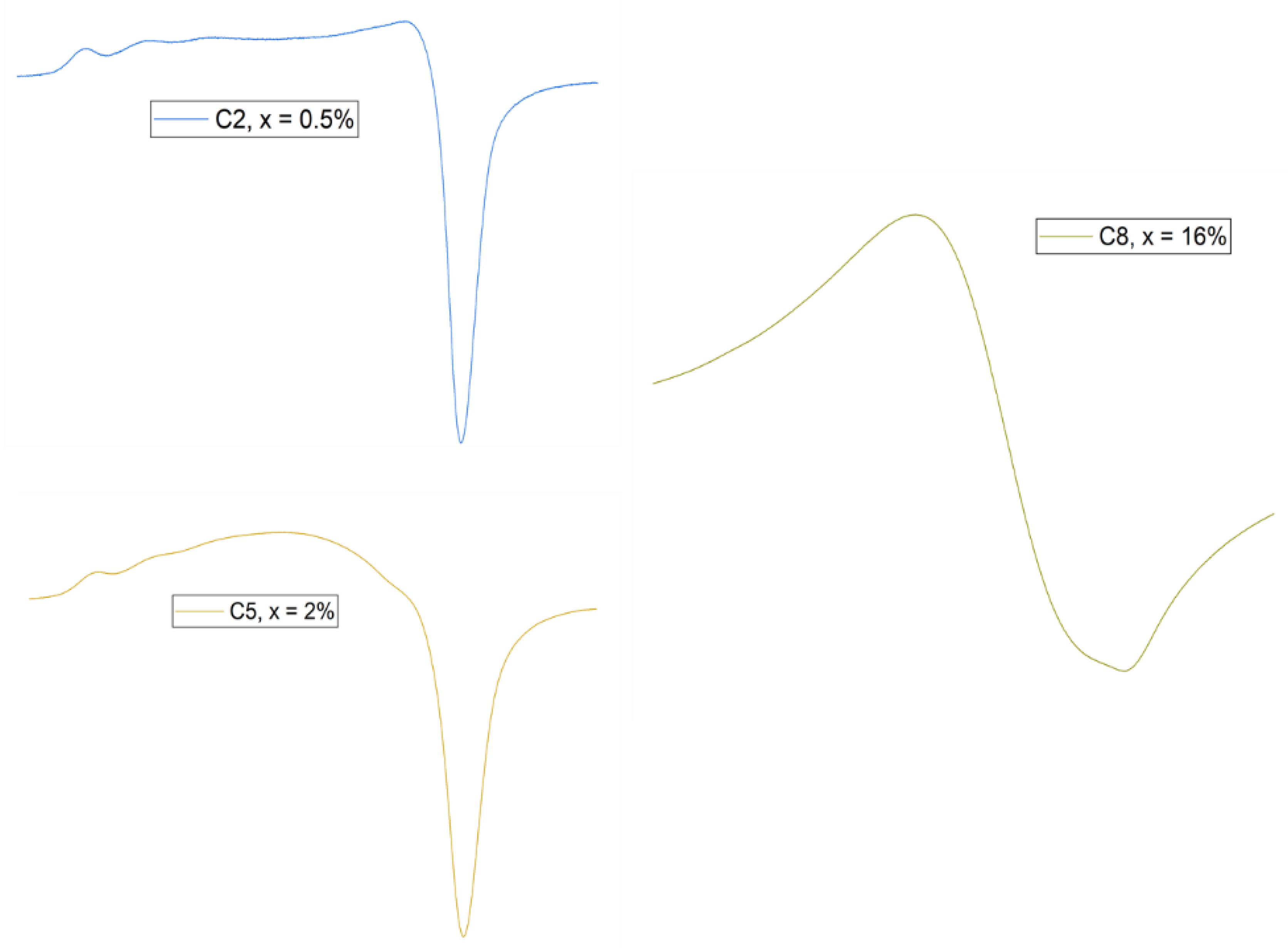
3.1.4. FT-IR
3.1.5. EPR
3.2. In Vitro Biological Evaluation
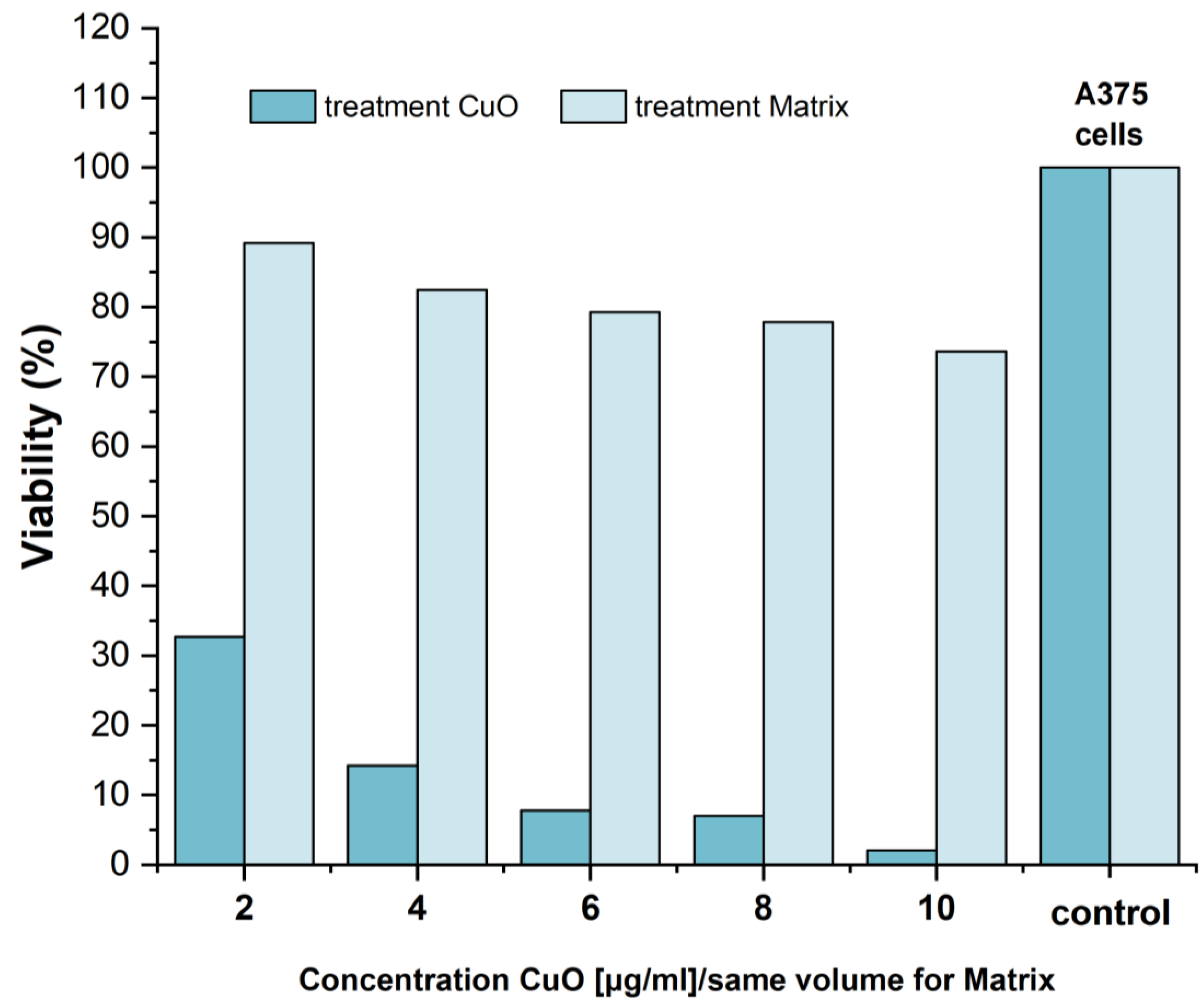
Evaluation of Cytotoxicity-MTT Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Ojeda, W.; Pandey, A.; Alhajj, M.; Oakley, A.M. Anatomy, Skin (Integument). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wang, Y.; Feng, Z.; Yang, M.; Zeng, L.; Qi, B.; Yin, S.; Li, B.; Li, Y.; Fu, Z.; Shu, L.; et al. Discovery of a novel short peptide with efficacy in accelerating the healing of skin wounds. Pharmacol. Res. 2020, 163, 105296. [Google Scholar] [CrossRef] [PubMed]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV Radiation and the Skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simões, M.C.F.; Sousa, J.J.S.; Pais, A.A.C.C. Skin cancer and new treatment perspectives: A review. Cancer Lett. 2015, 357, 8–42. [Google Scholar] [CrossRef] [PubMed]
- Samara, E.; Ameerally, P. Skin cancer surgery at the time of the covid-19 pandemic: A single center experience. Adv. Oral Maxillofac. Surg. 2021, 3, 100083. [Google Scholar] [CrossRef]
- Cives, M.; Mannavola, F.; Lospalluti, L.; Sergi, M.C.; Cazzato, G.; Filoni, E.; Cavallo, F.; Giudice, G.; Stucci, L.S.; Porta, C.; et al. Non-Melanoma Skin Cancers: Biological and Clinical Features. Int. J. Mol. Sci. 2020, 21, 5394. [Google Scholar] [CrossRef]
- Loh, L.; Tiwari, P.; Lee, J.; Low, O.-W.; Nallathamby, V.; Lee, H.; Lim, J.; Lim, T.C.; Yap, Y.L. Use of Intraoperative Frozen Section in the Surgical Management of Patients with Nonmelanoma Skin Cancer. J. Ski. Cancer 2021, 2021, 4944570. [Google Scholar] [CrossRef]
- Velasco, M.V.; Souza, M.T.; Crovace, M.C.; Aparecido de Oliveira, A.J.; Zanotto, E.D. Bioactive magnetic glass-ceramics for cancer treatment. Biomed. Glasses 2019, 5, 148–177. [Google Scholar] [CrossRef]
- Stefanic, M.; Peroglio, M.; Stanciuc, A.-M.; Machado, G.C.; Campbell, I.; Kržmanc, M.M.; Alini, M.; Zhang, X. The influence of strontium release rate from bioactive phosphate glasses on osteogenic differentiation of human mesenchymal stem cells. J. Eur. Ceram. Soc. 2018, 38, 887–897. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Ali, A.A.; El-Fiqi, A. Glass-forming compositions and physicochemical properties of degradable phosphate and silver-doped phosphate glasses in the P2O5–CaO–Na2O–Ag2O system. J. Mater. Res. Technol. 2019, 8, 1003–1013. [Google Scholar] [CrossRef]
- Aboulfotoh, N.; Elbashar, Y.; Ibrahem, M.; Elokr, M. Characterization of copper doped phosphate glasses for optical applications. Ceram. Int. 2014, 40, 10395–10399. [Google Scholar] [CrossRef]
- Lakhkar, N.; Abou Neel, E.A.; Salih, V.; Knowles, J.C. Titanium and Strontium-doped Phosphate Glasses as Vehicles for Strontium Ion Delivery to Cells. J. Biomater. Appl. 2011, 25, 877–893. [Google Scholar] [CrossRef]
- Vedeanu, N.; Cozar, O.; Stanescu, R.; Cozar, I.B.; Ardelean, I. Structural investigation of new vanadium–bismuth–phosphate glasses by IR and ESR spectroscopy. J. Mol. Struct. 2013, 1044, 323–327. [Google Scholar] [CrossRef]
- Rao, G.; Shashikala, H.D. Structural, optical and mechanical properties of ternary CaO-CaF2-P2O5 glasses. J. Adv. Ceram. 2014, 3, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.P.; Roy, M.; Mishra, R.K.; Meena, S.S.; Yadav, A.; Kaushik, C.P.; Tyagi, A.K. Structural investigations on Mo, Cs and Ba ions-loaded iron phosphate glass for nuclear waste storage application. J. Alloy Compd. 2021, 850, 156715. [Google Scholar] [CrossRef]
- Joshi, A.C.; Roy, M.; Dutta, D.P.; Mishra, R.K.; Meena, S.S.; Kumar, R.; Bhattacharyya, D.; Alexander, R.; Kaushik, C.P.; Tyagi, A.K. Effect on the structure and stability of iron phosphate glass with Sb and Te-ion loading for nuclear waste storage application. J. Non-Cryst. Solids 2021, 570, 121016. [Google Scholar] [CrossRef]
- Karlsson, H. Ylanen, Porous bone implants. In Materials in clinical applications, advances in science and technology 289th Cimtec-World Forum on New Materials; Vincenzini, P., Ed.; Techna: Florence, Italy, 1998; Volume 33, p. 28. [Google Scholar]
- Advances in Calcium Phosphate Biomaterials; Ben-Nissan, B. (Ed.) Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2014; Volume 2, ISBN 978-3-642-53979-4. [Google Scholar]
- Vedeanu, N.; Magdas, A. The influence of some transition metal ions in lead- and calcium-phosphate glasses. J. Alloy Compd. 2012, 534, 93–96. [Google Scholar] [CrossRef]
- Vedeanu, N.; Magdas, A.; Stefan, R. Structural modifications induced by addition of copper oxide to lead-phosphate glasses. J. Non-Cryst. Solids 2012, 358, 3170–3174. [Google Scholar] [CrossRef]
- Magdas, D.A.; Stefan, R.; Toloman, D.; Vedeanu, N.S. Copper ions influence on lead–phosphate glass network. J. Mol. Struct. 2014, 1056, 314–318. [Google Scholar] [CrossRef]
- Vedeanu, N.; Stanescu, R.; Filip, S.; Ardelean, I.; Cozar, O. IR and ESR investigations on V2O5–P2O5–BaO glass system with opto-electronic potential. J. Non Cryst. Solids 2012, 358, 1881–1885. [Google Scholar] [CrossRef]
- Magdas, D.A.; Cozar, O.; Chis, V.; Ardelean, I.; Vedeanu, N. The structural dual role of Fe2O3 in some lead-phosphate glasses. Vib. Spectrosc. 2008, 48, 251–254. [Google Scholar] [CrossRef]
- Vedeanu, N.; Cozar, O.; Ardelean, I.; Lendl, B.; Magdas, A. Raman spectroscopic study of CuO–V2O5–P2O5–CaO glass system. Vib. Spectrosc. 2008, 48, 259–262. [Google Scholar] [CrossRef]
- Li, K.; Xia, C.; Qiao, Y.; Liu, X. Dose-response relationships between copper and its biocompatibility/antibacterial activities. J. Trace Elem. Med. Biol. 2019, 55, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Ramakrishna, S.; Mozafari, M. Chemistry of biomaterials: Future prospects. Curr. Opin. Biomed. Eng. 2019, 10, 181–190. [Google Scholar] [CrossRef]
- Chaviara, A.T.; Christidis, P.C.; Papageorgiou, A.; Chrysogelou, E.; Hadjipavlou-Litina, D.J.; Bolos, C.A. In vivo anticancer, anti-inflammatory, and toxicity studies of mixed-ligand Cu(II) complexes of dien and its Schiff dibases with heterocyclic aldehydes and 2-amino-2-thiazoline. Crystal structure of [Cu (dien)(Br)(2a-2tzn)](Br)(H2O). J. Inorg. Biochem. 2005, 99, 2102–2109. [Google Scholar] [CrossRef]
- Dam, J.; Ismail, Z.; Kurebwa, T.; Gangat, N.; Harmse, L.; Marques, H.M.; Lemmerer, A.; Bode, M.L.; de Koning, C.B. Synthesis of copper and zinc 2-(pyridin-2-yl)imidazo[1,2-a]pyridine complexes and their potential anticancer activity. Eur. J. Med. Chem. 2017, 126, 353–368. [Google Scholar] [CrossRef]
- Lavanya, M.; Jagadeesh, M.; Haribabu, J.; Karvembu, R.; Rashmi, H.K.; Uma Maheswari Devi, P.; Varada Reddy, A. Synthesis, crystal structure, DNA binding and antitumor studies of β-diketonate complexes of divalent copper, zinc and palladium. Inorg. Chim. Acta 2018, 469, 76–86. [Google Scholar] [CrossRef]
- Arjmand, F.; khursheed, S.; Roisnel, T.; Siddique, H.R. Copper (II)-based halogen-substituted chromone antitumor drug entities: Studying biomolecular interactions with ct-DNA mediated by sigma hole formation and cytotoxicity activity. Bioorg. Chem. 2020, 104, 104327. [Google Scholar] [CrossRef]
- Orlova, M.A.; Spiridonov, V.V.; Orlov, A.P.; Zolotova, N.S.; Lupatov, A.Y.; Trofimova, T.P.; Kalmykov, S.N.; Yaroslavov, A.A. Complexes of carboxymethylcellulose with Cu2+-ions as a prototype of antitumor agent. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127814. [Google Scholar] [CrossRef]
- Benguigui, M.; Weitz, I.S.; Timaner, M.; Kan, T.; Shechter, D.; Perlman, O.; Sivan, S.; Raviv, Z.; Azhari, H.; Shaked, Y. Copper oxide nanoparticles inhibit pancreatic tumor growth primarily by targeting tumor initiating cells. Sci. Rep. 2019, 9, 12613. [Google Scholar] [CrossRef] [Green Version]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper metabolism as a unique vulnerability in cancer. Biochim. Et Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef]
- PBS (Phosphate Buffered Saline) (1X, pH 7.4) Preparation and Recipe | AAT Bioquest. Available online: https://www.aatbio.com/resources/buffer-preparations-and-recipes/pbs-phosphate-buffered-saline (accessed on 30 December 2021).
- Zhang, X.; Cresswell, M. Inorganic Controlled Release Technology: Materials Fundamentals of Drug Controlled Release; Butterworth-Heinemann: Oxford, UK, 2015; pp. 17–55. ISBN 978-0-08-099991-3. [Google Scholar]
- Edathazhe, A.B.; Shashikala, H.D. Dissolution studies of Na2O-BaO-CaO-P2O5 glasses in deionized water under semi-dynamic conditions for bioactive applications. Mater. Today Proc. 2018, 5, 21241–21247. [Google Scholar] [CrossRef]
- Oosterbeek, R.N.; Margaronis, K.I.; Zhang, X.C.; Best, S.M.; Cameron, R.E. Non-linear dissolution mechanisms of sodium calcium phosphate glasses as a function of pH in various aqueous media. J. Eur. Ceram. Soc. 2021, 41, 901–911. [Google Scholar] [CrossRef]
- Christie, J.K.; Ainsworth, R.I.; de Leeuw, N.H. Investigating structural features which control the dissolution of bioactive phosphate glasses: Beyond the network connectivity. J. Non-Cryst. Solids 2016, 432, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Tilocca, A. Structural models of bioactive glasses from molecular dynamics simulations. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 465, 1003–1027. [Google Scholar] [CrossRef]
- Naseri, S.; Lepry, W.C.; Nazhat, S.N. Bioactive glasses in wound healing: Hope or hype? J. Mater. Chem. B 2017, 5, 6167–6174. [Google Scholar] [CrossRef] [PubMed]
- Nommeots-Nomm, A.; Hupa, L.; Rohanová, D.; Brauer, D.S. A review of acellular immersion tests on bioactive glasses—Influence of medium on ion release and apatite formation. Int. J. Appl. Glass Sci. 2020, 11, 537–551. [Google Scholar] [CrossRef]
- Möncke, D.; Eckert, H. Review on the structural analysis of fluoride-phosphate and fluoro-phosphate glasses. J. Non-Cryst. Solids X 2019, 3, 100026. [Google Scholar] [CrossRef]
- Moustafa, Y.; El-Egili, K. Infrared Spectra of Sodium Phosphate Glasses. J. Non-Cryst. Solids 1998, 240, 144–153. [Google Scholar] [CrossRef]
- Sułowska, J.; Wacławska, I.; Szumera, M. Effect of copper addition on glass transition of silicate–phosphate glasses. J. Therm. Anal. Calorim. 2012, 109, 705–710. [Google Scholar] [CrossRef] [Green Version]
- El-Egili, K.; Doweidar, H.; Moustafa, Y.M.; Abbas, I. Structure and some physical properties of PbO–P2O5 glasses. Phys. B Condens. Matter 2003, 339, 237–245. [Google Scholar] [CrossRef]
- Schwarz, J.; Tichá, H.; Tichy, L.; Mertens, R. Physical properties of PbO-ZnO-P2O5 glasses I. Infrared and Raman spectra. J. Optoelectron. Adv. Mater. 2004, 6, 737–746. [Google Scholar]
- Pickup, D.M.; Newport, R.J.; Barney, E.R.; Kim, J.-Y.; Valappil, S.P.; Knowles, J.C. Characterisation of phosphate coacervates for potential biomedical applications. J. Biomater. Appl. 2014, 28, 1226–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, M.A.; Khattak, G.D.; Hussain, M.S. X-ray photoelectron spectroscopy, fourier transform infrared spectroscopy and electrical conductivity studies of copper phosphate glasses. J. Non-Cryst. Solids 1995, 185, 101–108. [Google Scholar] [CrossRef]
- Karakassides, M.; Saranti, A.; Koutselas, I. Preparation and Structural Study of Binary Phosphate Glasses with High Calcium and/or Magnesium Content. J. Non-Cryst. Solids 2004, 347, 69–79. [Google Scholar] [CrossRef]
- Cozar, O.; Ardelean, I.; Simon, V.; David, L.; Vedean, N.; Mih, V. EPR studies of Cu2+ and V4+ ions in phosphate glasses. Appl. Magn. Reson. 1999, 16, 473–480. [Google Scholar] [CrossRef]
- Stefan, R.; Karabulut, M.; Popa, A.; Culea, E.; Bolundut, L.; Olar, L.; Pascuta, P. A spectroscopic study of the influence of CuO addition on the ZnO-TeO 2 glass and glass ceramics. J. Non-Cryst. Solids 2018, 498, 430–436. [Google Scholar] [CrossRef]
- Vedeanu, N.; Cozar, O.; Ardelean, I.; Filip, S. Spectroscopic investigation on some calcium-phosphate glasses. J. Optoelectron. Adv. Mater. 2006, 8, 1135. [Google Scholar]
- Vedeanu, N.S.; Cozar, I.O.; Ardelean, D.A. Magdas The structure of CuO-P2O5-CaF2 glass system. Studia Univ. Babeş-Bolyai. Phys. 2006, 51, 79–84. [Google Scholar]
- Tardito, S.; Marchiò, L. Copper compounds in anticancer strategies. Curr. Med. Chem. 2009, 16, 1325–1348. [Google Scholar] [CrossRef]
- Ganesan, K.; Jothi, V.K.; Natarajan, A.; Rajaram, A.; Ravichandran, S.; Ramalingam, S. Green synthesis of Copper oxide nanoparticles decorated with graphene oxide for anticancer activity and catalytic applications. Arab. J. Chem. 2020, 13, 6802–6814. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, C.; Martoriati, A.; Pelinski, L.; Cailliau, K. Copper Complexes as Anticancer Agents Targeting Topoisomerases I and II. Cancers 2020, 12, 2863. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.A.; De Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.M.L.; Cerchiaro, G. Copper in tumors and the use of copper-based compounds in cancer treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef] [PubMed]



| Sample Glass | P2O5 | CaF2 | CaO | CuO |
|---|---|---|---|---|
| M | 76 | 13.9 | 10 | 0 |
| C1 | 75.9 | 13.9 | 10 | 0.2 |
| C2 | 75.8 | 13.9 | 10 | 0.4 |
| C3 | 75.6 | 13.9 | 10 | 0.5 |
| C4 | 75.5 | 13.8 | 9.9 | 0.7 |
| C5 | 75 | 13.7 | 9.9 | 1.4 |
| C6 | 73.9 | 13.5 | 9.7 | 2.9 |
| C7 | 71.6 | 13.1 | 9.4 | 5.8 |
| C8 | 67 | 12.3 | 8.8 | 11.9 |
| Reagent Chemicals (Chempur) | Amount (mg) | Concentration (M) |
|---|---|---|
| NaCl (mw: 58.4 g/mol) | 8000 | 0.137 |
| KCl (mw: 74.551 g/mol) | 200 | 0.0027 |
| Na2HPO4 (mw: 141.96 g/mol) | 1440 | 0.01 |
| KH2PO4 (mw: 136.086 g/mol) | 245 | 0.0018 |
| Element Wt (%) | ||||
|---|---|---|---|---|
| Sample Glass | O | P | Ca | Cu |
| M | 52.40 | 29.90 | 17.60 | — |
| C1 | 59.35 ± 2.0 | 26.20 ± 0.7 | 14.12 ± 1.3 | 0.30 ± 0.1 |
| C2 | 56.90 ± 1.5 | 27.40 ± 0.9 | 15.15 ± 0.7 | 0.60 ± 0.1 |
| C3 | 57.40 ± 0.5 | 26.00 ± 0.2 | 15.90 ± 0.6 | 0.80 ± 0.2 |
| C4 | 62.25 ± 2.8 | 23.95 ± 1.4 | 12.75 ± 1.2 | 1.05 ± 0.3 |
| C5 | 61.00 ± 2.8 | 24.80 ± 1.4 | 13.10 ± 1.2 | 1.10 ± 0.3 |
| C6 | 55.20 ± 1.9 | 26.55 ± 0.6 | 14.90 ± 0.8 | 3.35 ± 0.6 |
| C7 | 50.65 ± 1.9 | 28.95 ± 0.4 | 15.6 ± 1.2 | 5.00 ± 0.5 |
| C8 | 45.20 | 24.50 | 15.70 | 14.60 |
| x mol% | g‖ | g⊥ | A‖ (10−4 cm−1) | α2 |
|---|---|---|---|---|
| 0.25 | 2.42 | 2.03 | 136 | 0.81 |
| 0.5 | 2.41 | 2.04 | 159 | 0.80 |
| 0.75 | 2.44 | 2.04 | 119 | 0.84 |
| 1 | 2.44 | 2.03 | 109 | 0.84 |
| 2 | 2.42 | 2.03 | 108 | 0.81 |
| 4 | 2.43 | 2.03 | 106 | 0.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vedeanu, N.S.; Lujerdean, C.; Zăhan, M.; Dezmirean, D.S.; Barbu-Tudoran, L.; Damian, G.; Ștefan, R. Synthesis and Structural Characterization of CaO-P2O5-CaF:CuO Glasses with Antitumoral Effect on Skin Cancer Cells. Materials 2022, 15, 1526. https://doi.org/10.3390/ma15041526
Vedeanu NS, Lujerdean C, Zăhan M, Dezmirean DS, Barbu-Tudoran L, Damian G, Ștefan R. Synthesis and Structural Characterization of CaO-P2O5-CaF:CuO Glasses with Antitumoral Effect on Skin Cancer Cells. Materials. 2022; 15(4):1526. https://doi.org/10.3390/ma15041526
Chicago/Turabian StyleVedeanu, Nicoleta Simona, Cristian Lujerdean, Marius Zăhan, Daniel Severus Dezmirean, Lucian Barbu-Tudoran, Grigore Damian, and Răzvan Ștefan. 2022. "Synthesis and Structural Characterization of CaO-P2O5-CaF:CuO Glasses with Antitumoral Effect on Skin Cancer Cells" Materials 15, no. 4: 1526. https://doi.org/10.3390/ma15041526
APA StyleVedeanu, N. S., Lujerdean, C., Zăhan, M., Dezmirean, D. S., Barbu-Tudoran, L., Damian, G., & Ștefan, R. (2022). Synthesis and Structural Characterization of CaO-P2O5-CaF:CuO Glasses with Antitumoral Effect on Skin Cancer Cells. Materials, 15(4), 1526. https://doi.org/10.3390/ma15041526









